Harmonization of Newborn Screening Results for Pompe Disease and Mucopolysaccharidosis Type I
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of Participating Laboratories
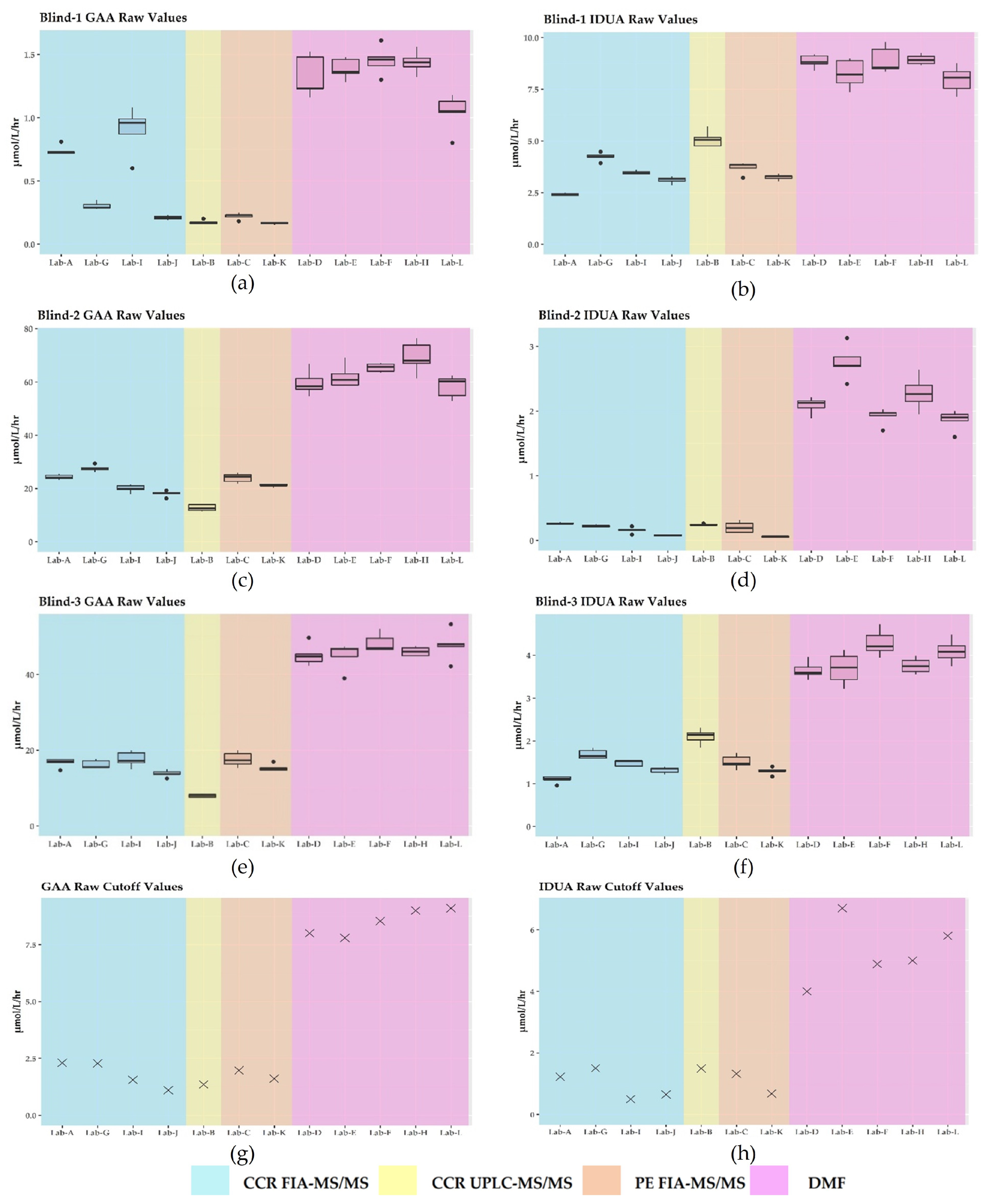
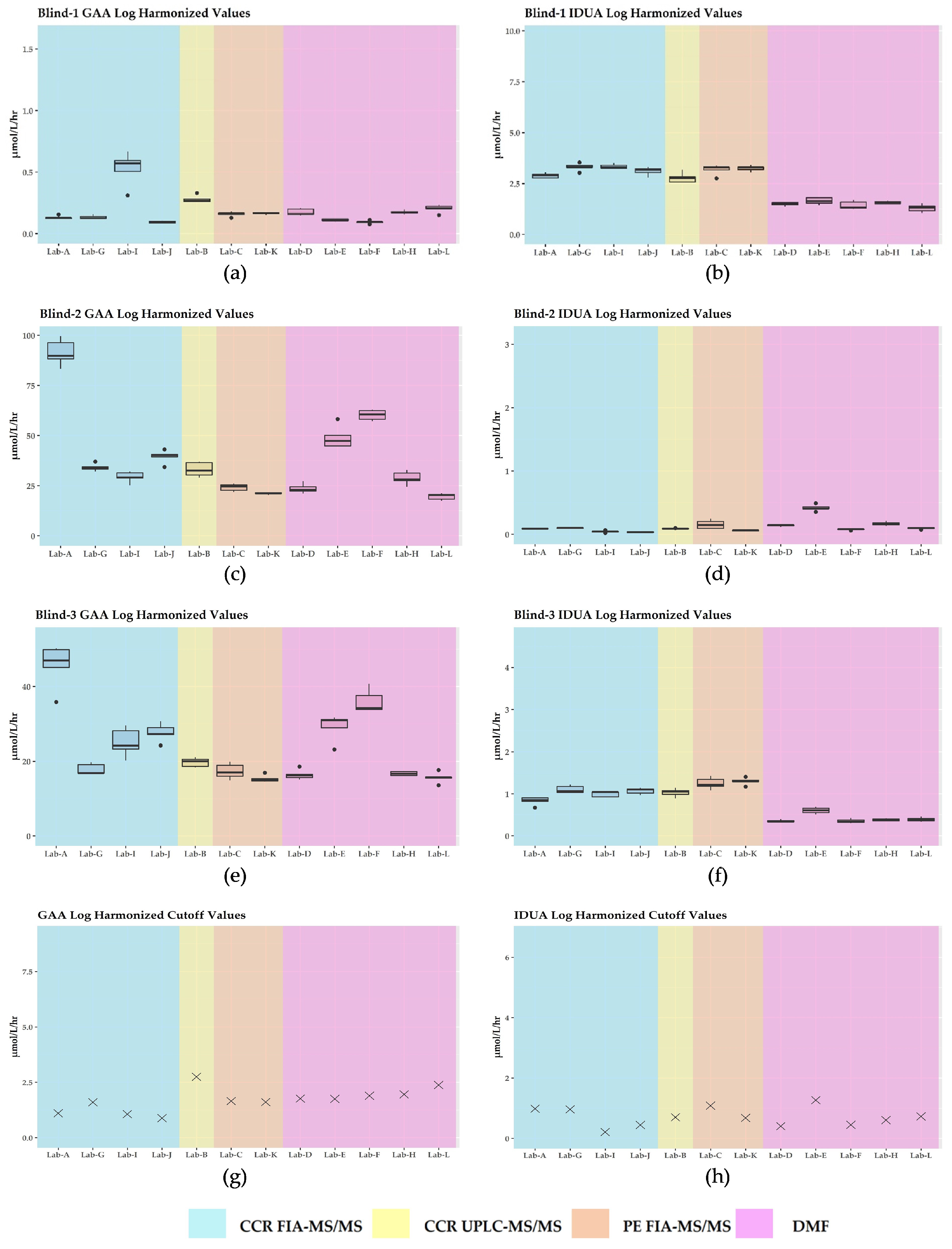
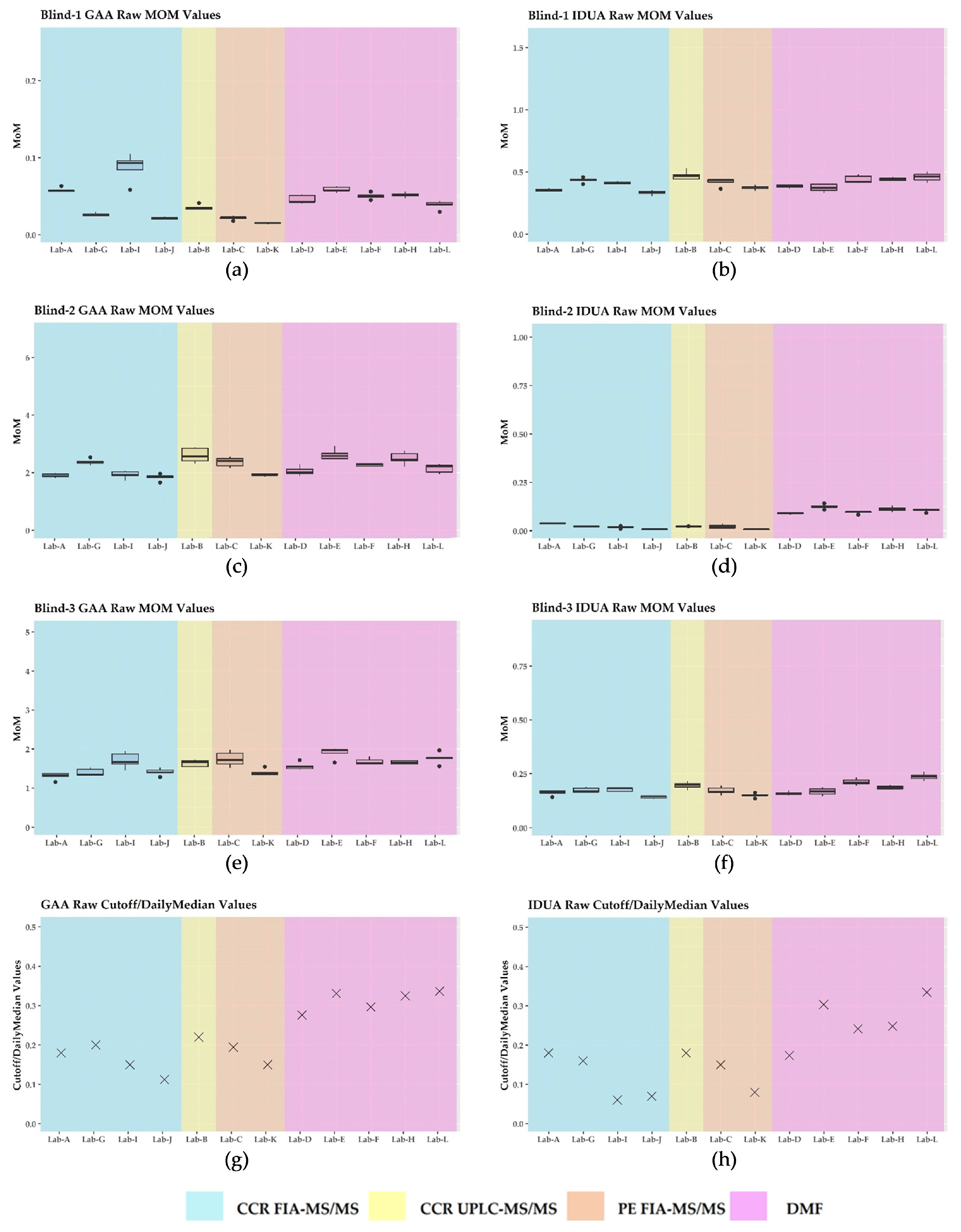
3.2. GAA and IDUA Raw Enzyme Activities and Harmonization
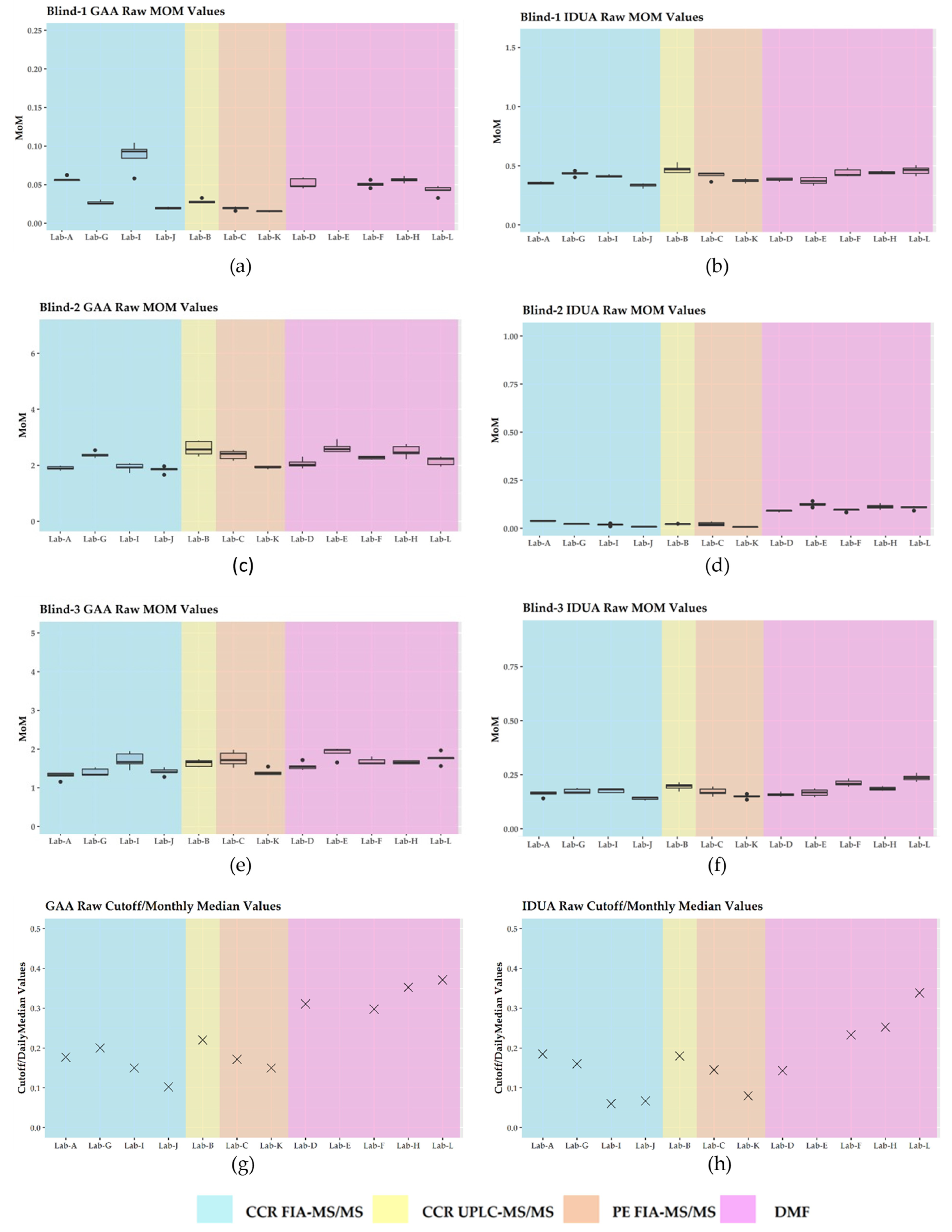
3.3. Harmonization Using Daily Mom and Monthly Mom
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QI | Quality Improvement |
| NBS | Newborn Screening |
| RUSP | Recommended Uniform Screening Panel |
| X-ALD | X-linked adrenal leukodystrophy |
| PD | Pompe disease |
| MPS I | Mucopolysaccharidosis type I |
| GAA | -glucosidase |
| IDUA | -L-iduronidase |
| PPV | Positive Predictive Value |
| GAGs | Glycosaminoglycans |
| CLIR | Collaborative Laboratory Integrated Reports |
| MoM | Multiples of the Median |
| QC | Quality Control |
| PT | Proficiency Testing |
| DMF | Digital Microfluidics |
| MS/MS | Tandem Mass Spectrometry |
| LD | Lysosomal Disorders |
| NSQAP | Newborn Screening Quality Assurance Program |
| CDC | Centers for Disease Control and Prevention |
| NewSTEPs | Newborn Screening Technical Assistance and Evaluation Program |
| CCR | Consumer Contracted Reagents |
| PE | Perkin Elmer Wallac, INC. |
| FIA | Flow Injection Analysis |
| UPLC | Ultra-high-Performance Liquid Chromatography |
| r2 | Coefficient of determination |
| GALC | Galactocerebrosidase |
| QCBP | QC base pool |
Appendix A
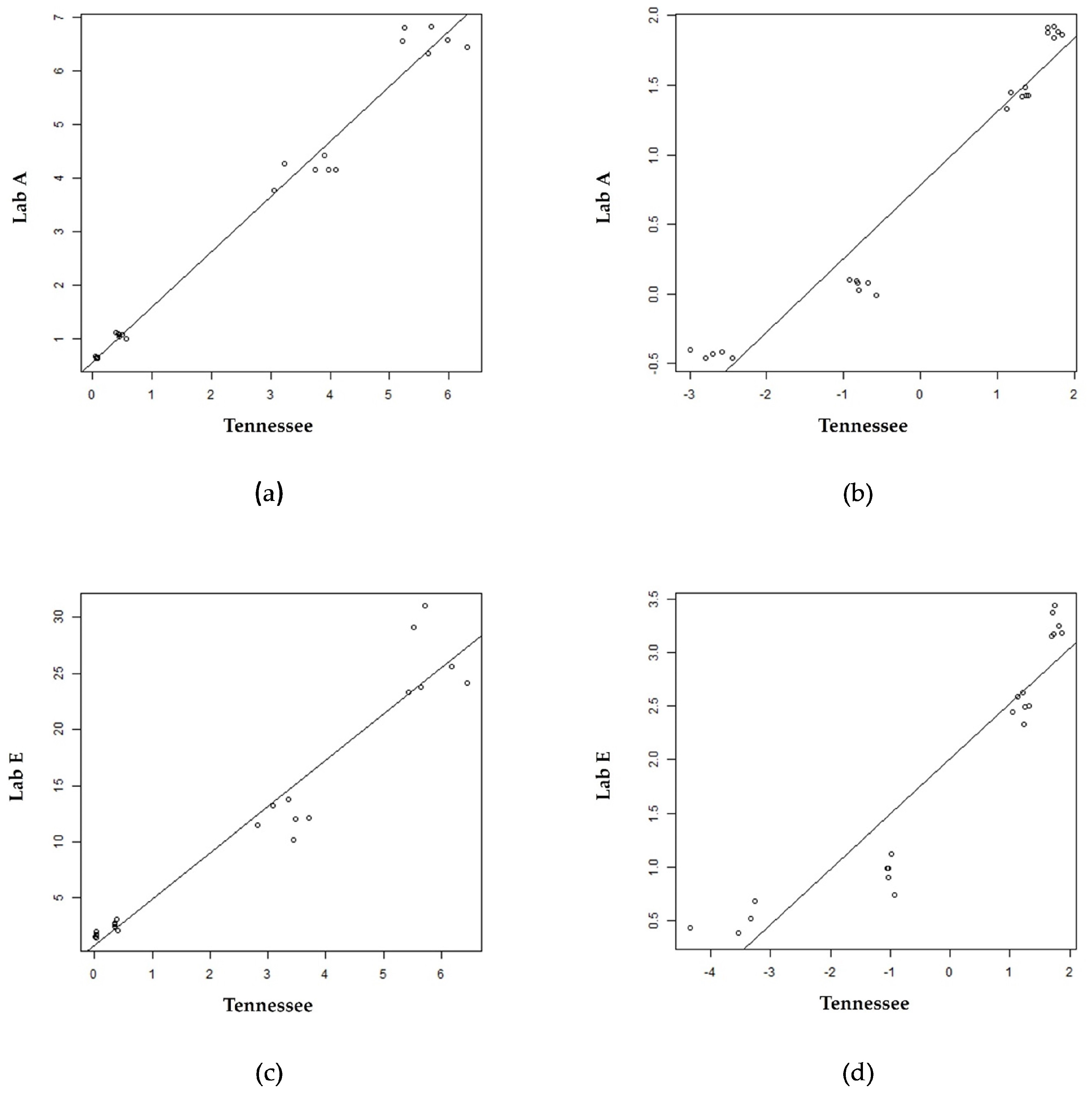
| Laboratory | r2 GAA Raw | r2 IDUA Raw | r2 GAA Log | r2 IDUA Log |
|---|---|---|---|---|
| A | 0.98 | 0.99 | 0.95 | 0.97 |
| B | 0.96 | 0.97 | * 0.99 | * 0.99 |
| C | 0.98 | 0.99 | * 1.00 | * 1.00 |
| D | 0.97 | 0.97 | * 0.99 | 0.96 |
| E | 0.95 | 0.92 | 0.91 | * 0.96 |
| F | 0.96 | 0.97 | 0.90 | 0.90 |
| G | 0.99 | 0.98 | * 0.99 | * 0.99 |
| H | 0.97 | 0.97 | * 0.98 | 0.96 |
| I | 0.98 | 0.98 | 0.97 | * 0.98 |
| J | 0.96 | 0.98 | 0.95 | * 0.98 |
| TN (K) | Reference | Reference | Reference | Reference |
| L | 0.95 | 0.96 | * 0.97 | 0.91 |
| GAA | IDUA | |||
|---|---|---|---|---|
| Laboratory | Blank Signal Average | A QC Pool Average | Blank Signal Average | A QC Pool Average |
| A | 0.59 | 0.65 | 0.19 | 0.23 |
| B | 0.00 | 0.05 | 0.08 | 0.15 |
| C | 0.00 | 0.13 | 0.00 | 0.08 |
| * D | 0.17 | 0.75 | 0.23 | 1.86 |
| * E | 0.01 | 0.67 | 0.35 | 1.62 |
| * F | 1.57 | 1.29 | 2.19 | 1.98 |
| G | 0.01 | 0.19 | 0.00 | 0.13 |
| * H | 0.18 | 0.85 | 0.23 | 1.61 |
| I | 0.00 | 0.20 | 0.00 | 0.19 |
| J | 0.02 | 0.05 | 0.07 | 0.15 |
| TN (K) | 0.06 | 0.07 | 0.00 | 0.03 |
| * L | 0.92 | 0.21 | 1.61 | 1.82 |
References
- McCandless, S.E.; Wright, E.J. Mandatory newborn screening in the United States: History, current status, and existential challenges. Birth Defects Res. 2020, 110, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Newborn Screening Timeliness Goals. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/newborn-screening-timeliness.html (accessed on 24 February 2021).
- Gabler, E. Lab’s Standards Missed Baby’s Serious Disorder: Uniformity Lacking in States’ Screenings. J. Sentin. 2016. Available online: https://projects.jsonline.com/news/2016/12/29/uniformity-lacking-for-newborn-screening.html (accessed on 24 February 2021).
- Gabler, E. Federal Committee to Study Lack of Uniformity in Newborn Testing. J. Sentin. 2017. Available online: https://www.jsonline.com/story/news/investigations/2017/02/10/federal-committee-study-lack-uniformity-newborn-testing/97703280/ (accessed on 24 February 2021).
- Recommended Uniform Screening Panel. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html (accessed on 5 April 2020).
- Orsini, J.J.; Culley, L.; Dorley, M.C.; Haynes, C.A.; Hunt, P.; Koupaei, R.; Manning, A.; Neogi, P.; Dhillon, K.; Roworth, P.; et al. Multi-state harmonization study: Efforts to harmonize the cutoffs used in newborn screening for adrenoleukodystrophy. Int. J. Neonatal Screen. 2020, 6, 75. [Google Scholar]
- Bosfield, K.; Regier, D.S.; Viall, S.; Hicks, R.; Shur, N.; Grant, C.L. Mucopolysaccharidosis type I newborn screening: Importance of second tier testing for ethnically diverse populations. Am. J. Med. Genet. 2020, 185, 134–140. [Google Scholar] [CrossRef]
- Taylor, J.L.; Lee, S. Lessons learned from newborn screening pilots studies. NCJM 2019, 80, 54–58. [Google Scholar] [CrossRef]
- Wasserstein, M.P.; Caggana, M.; Bailey, S.M.; Desnick, R.J.; Edelmann, L.; Estrella, L.; Holzman, I.; Kelly, N.R.; Kornreich, R.; Kupchik, S.G.; et al. The New York pilot newborn screening program for lysosomal storage diseases: Report of the first 65,000 infants. Genet. Med. 2019, 21, 631–640. [Google Scholar] [CrossRef]
- Gelb, M.H. Newborn screening for lysosomal storage diseases: Methodologies, screen positive rates, normalization of datasets, second-tier tests, and post-analysis tools. Int. J. Neonatal Screen. 2018, 4, 23. [Google Scholar] [CrossRef]
- Waggoner, D.J.; Tan, C.A. Expanding newborn screening for lysosomal disorders: Opportunities and challenges. Dev. Disabil. Res. Rev. 2011, 17, 9–14. [Google Scholar] [CrossRef]
- Kelly, N.; Makarem, D.C.; Wasserstein, M.P. Screening of newborns for disorders with high benefit-risk ratios should be mandatory. J. Law Med. Ethics. 2016, 44, 231–240. [Google Scholar] [CrossRef]
- Lisi, E.C.; McCandless, S.E. Newborn screening for lysosomal storage disorders: Views of genetic healthcare providers. J. Genet. Counsel. 2016, 25, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Parini, R.; Broomfield, A.; Cleary, M.A.; De Meirleir, L.; Di Rocco, M.; Fathalla, W.M.; Guffon, N.; Lampe, C.; Lund, A.M.; Scarpa, M.; et al. International working group identifies need for newborn screening for mucopolysaccharidosis type I but states that existing hurdles must be overcome. J. Acta Paediatr. 2018, 107, 2059–2065. [Google Scholar] [CrossRef]
- Hall, P.L.; Sanchez, R.; Hagar, A.F.; Jerris, S.C.; Wittenauer, A.; Wilcox, W.R. Two-tiered newborn screening with post-analytical tools for Pompe Disease and Mucopolysaccharidosis Type I results in performance improvement and future discussion. Int. J. Neonatal Screen. 2020, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.D.; Bainbridge, M.N.; Parad, R.B.; Bhattacharjee, A. Second tier molecular genetic testing in newborn screening for Pompe Disease: Landscape and challenges. Int. J. Neonatal Screen. 2020, 6, 32. [Google Scholar] [CrossRef]
- Keller, R.; Chrastina, P.; Pavlíková, M.; Gouveia, S.; Ribes, A.; Kölker, S.; Blom, H.J.; Baumgartner, M.R.; Bártl, J.; Dionisi-Vici, C.; et al. Newborn screening for homocystinurias: Recent recommendations versus current practice. J. Inherit. Metab. Dis. 2019, 42, 128–139. [Google Scholar] [CrossRef]
- Schmidt, J.L.; Castellanos-Brown, K.; Childress, S.; Bonhomme, N.; Oktay, J.S.; Terry, S.F.; Kyler, P.; Davidoff, A.; Greene, C. The impact of false-positive newborn screening results on families: A qualitative study. Genet. Med. 2012, 14, 76–80. [Google Scholar] [CrossRef]
- Burlina, A.B.; Polo, G.; Rubert, L.; Gueraldi, D.; Cazzorla, C.; Duro, G.; Salviati, L.; Burlina, A.P. Implementation of second-tier tests in newborn screening for lysosomal disorders in North Eastern Italy. Int. J. Neonatal Screen. 2019, 5, 24. [Google Scholar] [CrossRef]
- Washburn, J.; Millington, D.S. Digital microfluidics in newborn screening for mucopolysaccharidoses: A progress report. Int. J. Neonatal Screen. 2020, 6, 78. [Google Scholar] [CrossRef]
- Gragnaniello, V.; Gueraldi, D.; Rubert, L.; Manzoni, F.; Cazzorla, C.; Giuliani, A.; Polo, G.; Salviati, L.; Burlina, A. Report of five years of experience in neonatal screening for mucopolysaccharidosis type I and review of the literature. Int. J. Neonatal Screen. 2020, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Tortorelli, S.; Eckerman, J.S.; Orsini, J.J.; Stevens, C.; Hart, J.; Hall, P.L.; Alexander, J.J.; Gavrilov, D.; Oglesbee, D.; Raymond, K.; et al. Moonlighting newborn screening markers: The incidental discovery of a second-tier test for Pompe Disease. Genet. Med. 2017, 20, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Minter Baerg, M.M.; Stoway, S.D.; Hart, J.; Mott, L.; Peck, D.S.; Nett, S.L.; Eckerman, J.S.; Lacey, J.M.; Turgeon, C.T.; Gavrilov, D.; et al. Precision newborn screening for lysosomal disorders. Genet. Med. 2018, 20, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Pickens, C.A.; Sternberg, M.; Seeterlin, M.; De Jesús, V.R.; Morrisey, M.; Manning, A.; Bhakta, S.; Held, P.K.; Mei, J.; Cuthberth, C.; et al. Harmonizing newborn screening laboratory proficiency test results using the CDC NSQAP reference materials. Int. J. Neonatal Screen. 2020, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M. Harmonization in laboratory medicine: The complete picture. Clin. Chem. Lab. Med. 2013, 51, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute (CLSI) Harmonized Terminology Database. Available online: https://htd.clsi.org/listterms.asp?searchdterm=harmonization (accessed on 5 April 2022).
- Donati, M.A.; Pasquini, E.; Spada, M.; Polo, G.; Burlina, A. Newborn screening in mucopolysaccharidoses. Ital. J. Pediatr. 2018, 44, 25–34. [Google Scholar] [CrossRef]
- Polo, G.; Gueraldi, D.; Giuliani, A.; Rubert, L.; Cazzorla, C.; Salviati, L.; Marzollo, A.; Biffi, A.; Burlina, A.P.; Burlina, A.B. The combined use of enzyme activity and metabolite assays as a strategy for newborn screening of mucopolysaccharidosis type I. Clin. Chem. Lab. Med. 2020, 58, 2063–2070. [Google Scholar] [CrossRef]
- Cogley, M.F.; Wiberley-Bradford, A.E.; Mochal, S.T.; Dawe, S.J.; Piro, Z.D.; Baker, M.W. Newborn screening for severe combined immunodeficiency using the multiple of the median values of t-cell receptor excision circles. Int. J. Neonatal Screen. 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.H.; Lukacs, Z.; Ranieri, E.; Schielen, P.C.J.I. Newborn screening for lysosomal storage disorders: Methodologies for measurement of enzymatic activities in dried blood spots. Int. J. Neonatal Screen. 2019, 5, 1. [Google Scholar] [CrossRef]
- Hopkins, P.V.; Campbell, C.; Klug, T.; Rogers, S.; Raburn-Miller, J.; Kiesling, J. Lysosomal storage disorder screening implementation: Findings from the first six months of full population pilot testing in Missouri. Int. J. Pediatr. 2015, 166, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Sista, R.S.; Eckhardt, A.E.; Wang, T.; Graham, C.; Rouse, J.L.; Norton, S.M.; Srinivasan, V.; Pollack, M.G.; Tolun, A.A.; Bali, D.; et al. Digital microfluidic platform for multiplexing enzyme assays: Implications for lysosomal storage disease screening in newborns. Clin. Chem. 2011, 57, 1444–1451. [Google Scholar] [CrossRef]
- Elliott, S.; Buroker, N.; Cournoyer, J.J.; Potier, A.M.; Trometer, J.D.; Elbin, C.; Schermer, M.J.; Kantola, J.; Boyce, A.; Turecek, F.; et al. Pilot study of newborn screening for six lysosomal storage diseases using tandem mass spectrometry. Mol. Genet. Metab. 2016, 118, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Newborn Screening Technical Assistance and Evaluation Program. Available online: https://www.newsteps.org/ (accessed on 24 February 2021).
- De Jesus, V.R.; Zhang, X.K.; Keutzer, J.; Bodamer, O.A.; Mühl, A.; Orsini, J.J.; Caggana, M.; Vogt, R.F.; Hannon, W.H. Development and evaluation of quality control dried blood spot materials in newborn screening for lysosomal storage disorders. Clin. Chem. 2009, 55, 158–164. [Google Scholar] [CrossRef] [PubMed]
- NSQAP. Annual Report Volume 38. Available online: https://www.cdc.gov/labstandards/pdf/nsqap/NSQAP_Annual_Summary_2020-508.pdf (accessed on 9 December 2021).
- NSQAP. Annual Report Volume 39a. Available online: https://www.cdc.gov/labstandards/pdf/nsqap/NSQAP_Annual_Summary_2021_Amended-508.pdf (accessed on 1 September 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- NeoLSD MSMS kit 3093-0020 product insert. Version 13908726-1 (en). 2018.
- Seeker LSD Reagent kit-IDUA, GAA, GBA, GLA product insert. NBS-01-00122 Revision 16.
- Mechtler, T.P.; Metz, T.F.; Müller, H.; Ostermann, K.; Ratschmann, R.; De Jesus, V.R.; Shushan, B.; Di Bussolo, J.M.; Herman, J.L.; Herkner, K.R.; et al. Short-incubation mass spectrometry assay for lysosomal storage disorders in newborn and high-risk population screening. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 908, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.H.; Gelb, M.H. The importance of assay imprecision near the screen cutoff for newborn screening of lysosomal storage diseases. Int. J. Neonatal Screen. 2019, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Chan, M.; Yang, C.; Chiang, C.; Niu, D.; Huang, C.; Gelb, M.H. Mass spectrometry but not fluorometry distinguishes affected and pseudodeficiency patients in newborn screening for Pompe disease. Clin. Chem. 2017, 63, 1271–1277. [Google Scholar] [CrossRef]
- Singh, R.; Chopra, S.; Graham, C.; Langer, M.; Ng, R.; Ullal, A.J.; Pamula, V.K. Emerging approaches for fluorescence-based newborn screening of mucopolysaccharidoses. Int. J. Mol. Sci. 2020, 10, 294. [Google Scholar] [CrossRef]
- Franco, P.G.; Adamo, A.M.; Mathieu, P.; Pérez, M.J.; Setton-Avruj, P.C.; Silvestroff, L. Update on the fluorometric measurement of enzymatic activities for lysosomal storage disorder detection: The example of mps VI. J. Rare Dis. Res. Treat. 2017, 2, 56–61. [Google Scholar]
- Kumar, A.B.; Masi, S.; Ghomashchi, F.; Chennamaneni, N.K.; Ito, M.; Scott, C.R.; Turecek, F.; Gelb, M.H.; Spacil, Z. Tandem mass spectrometry has a larger analytical range than fluorescence assays of lysosomal enzymes: Application to newborn screening and diagnosis of mucopolysaccharidoses types II, IVA, and VI. Clin. Chem. 2015, 61, 1363–1371. [Google Scholar] [CrossRef]
| PT Identifier | 1 GAA (PD) Activity | 1 IDUA (MPS I) Activity |
|---|---|---|
| Blind-1 | Deficient | Normal |
| Blind-2 | Normal | Deficient |
| Blind-3 | Normal | Low |
| Blind-4 | Deficient | Deficient |
| Blind-5 | Low | Low |
| Blind-6 | Normal | Normal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorley, M.C.; Dizikes, G.J.; Pickens, C.A.; Cuthbert, C.; Basheeruddin, K.; Gulamali-Majid, F.; Hetterich, P.; Hietala, A.; Kelsey, A.; Klug, T.; et al. Harmonization of Newborn Screening Results for Pompe Disease and Mucopolysaccharidosis Type I. Int. J. Neonatal Screen. 2023, 9, 11. https://doi.org/10.3390/ijns9010011
Dorley MC, Dizikes GJ, Pickens CA, Cuthbert C, Basheeruddin K, Gulamali-Majid F, Hetterich P, Hietala A, Kelsey A, Klug T, et al. Harmonization of Newborn Screening Results for Pompe Disease and Mucopolysaccharidosis Type I. International Journal of Neonatal Screening. 2023; 9(1):11. https://doi.org/10.3390/ijns9010011
Chicago/Turabian StyleDorley, M. Christine, George J. Dizikes, Charles Austin Pickens, Carla Cuthbert, Khaja Basheeruddin, Fizza Gulamali-Majid, Paul Hetterich, Amy Hietala, Ashley Kelsey, Tracy Klug, and et al. 2023. "Harmonization of Newborn Screening Results for Pompe Disease and Mucopolysaccharidosis Type I" International Journal of Neonatal Screening 9, no. 1: 11. https://doi.org/10.3390/ijns9010011
APA StyleDorley, M. C., Dizikes, G. J., Pickens, C. A., Cuthbert, C., Basheeruddin, K., Gulamali-Majid, F., Hetterich, P., Hietala, A., Kelsey, A., Klug, T., Lesko, B., Mills, M., Moloney, S., Neogi, P., Orsini, J., Singer, D., & Petritis, K. (2023). Harmonization of Newborn Screening Results for Pompe Disease and Mucopolysaccharidosis Type I. International Journal of Neonatal Screening, 9(1), 11. https://doi.org/10.3390/ijns9010011









