A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Recommended Uniform Screening Panel. U.S. Department of Health and Human Services, 2018. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html (accessed on 11 September 2018).
- Gaspar, H. A Practical Guide to Implementing Population Newborn Screening (NBS) for Severe Combined Immunodeficiency (SCID). Int. J. Neonatal Screen. 2017, 3, 29. [Google Scholar] [CrossRef]
- Blom, M.; Bredius, R.G.M.; Weijman, G.; Dekkers, E.H.B.M.; Kemper, E.A.; Van den Akker-van Marle, M.E.; van der Ploeg, C.P.B.; van der Burg, M.; Schielen, P.C.J.I. Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program. Int. J. Neonatal Screen. 2018, 4, 40. [Google Scholar] [CrossRef]
- Public Health England. Criteria for Appraising the Viability, Effectiveness and Appropriateness of a Screening Programme. 2015. Available online: https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes/criteria-for-appraising-the-viability-effectiveness-and-appropriateness-of-a-screening-programme (accessed on 27 October 2016).
- Buckley, R.H.; Schiff, S.E.; Schiff, R.I.; Markert, L.; Williams, L.W.; Roberts, J.L.; Myers, L.A.; Ward, F.E. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N. Engl. J. Med. 1999, 340, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Xu-Bayford, J.; Allwood, Z.; Slatter, M.; Cant, A.; Davies, E.G.; Veys, P.; Gennery, A.R.; Gaspar, H.B. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: The case for newborn screening. Blood 2011, 117, 3243–3246. [Google Scholar] [CrossRef] [PubMed]
- Railey, M.D.; Lokhnygina, Y.; Buckley, R.H. Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis. J. Pediatr. 2009, 155, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.Y.; Logan, B.R.; Griffith, L.M.; Buckley, R.H.; Parrott, R.E.; Dvorak, C.C.; Kapoor, N.; Hanson, I.C.; Filipovich, A.H.; Jyonouchi, S.; et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N. Engl. J. Med. 2014, 371, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.A.; Patel, D.D.; Puck, J.M.; Buckley, R.H. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood 2002, 99, 872–878. [Google Scholar] [CrossRef]
- Clement, M.C.; Mahlaoui, N.; Mignot, C.; Le Bihan, C.; Rabetrano, H.; Hoang, L.; Neven, B.; Moshous, D.; Cavazzana, M.; Blanche, S.; et al. Systematic neonatal screening for severe combined immunodeficiency and severe T-cell lymphopenia: Analysis of cost-effectiveness based on French real field data. J. Allergy Clin. Immunol. 2015, 135, 1589–1593. [Google Scholar] [CrossRef]
- Kubiak, C.; Jyonouchi, S.; Kuo, C.; Garcia-Lloret, M.; Dorsey, M.J.; Sleasman, J.; Zbrozek, A.S.; Perez, E.E. Fiscal Implications of Newborn Screening in the Diagnosis of Severe Combined Immunodeficiency. J. Allergy Clin. Immunol. Pract. 2014, 2, 697–702. [Google Scholar] [CrossRef]
- McGhee, S.A.; Stiehm, E.R.; McCabe, E.R.B. Potential costs and benefits of newborn screening for severe combined immunodeficiency. J. Pediatr. 2005, 147, 603–608. [Google Scholar] [CrossRef]
- Chan, K.; Davis, J.; Pai, S.Y.; Bonilla, F. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol. Genet. Metab. 2011, 104, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.T.J.; Kobrynski, L.; Ojodu, J.; Zarbalian, G.; Grosse, S. Cost-effectiveness/Cost-benefit analysis of newborn screening for severe combined immune deficiency in Washington state. J. Pediatr. 2016, 172, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cost-Effectiveness of Newborn Screening for Severe Combined Immune Deficiency. A Report Prepared for the National Screening Unit; Health Partners Consulting Group, 2014. Available online: https://www.nsu.govt.nz/system/files/resources/cost-effectiveness-newborn-screening-severe-combined-immune-deficiency.pdf (accessed on 15 January 2019).
- Van der Ploeg, C.P.B.; Blom, M.; Bredius, R.G.M.; van der Burg, M.; Schielen, P.C.J.I.; Verkerk, P.H.; Van den Akker-van Marle, M.E. Cost-effectiveness of newborn screening for severe combined immunodeficiency. Eur. J. Pediatr. 2019, 178, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Northern Ireland Statistics and Research Agency. Live Births, 1887 to 2014. 2015. Available online: https://www.nisra.gov.uk/publications/birth-statistics (accessed on 15 November 2016).
- National Records of Scotland. Vital Events-Births. 2015. Available online: https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/general-publications/vital-events-reference-tables (accessed on 15 November 2016).
- Office for National Statistics. Birth Summary Tables-England and Wales 2014. 2015. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales/2017 (accessed on 15 November 2016).
- Gaspar, B. (University College London-Great Ormond Street Institute of Child Health, London, UK). Personal communication–UK incidence estimates, 2015.
- Amatuni, G.S.; Currier, R.J.; Church, J.A.; Bishop, T.; Grimbacher, E.; Nguyen, A.A.C.; Agarwal-Hashmi, R.; Aznar, C.P.; Butte, M.J.; Cowan, M.J.; et al. Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010–2017. Pediatrics 2019, 143, e20182300. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.P.; Rashid, S.; Premachandra, T.; Harvey, K.; Ifederu, A.; Wilson, M.C.; Gaspar, H.B. Screening of neonatal UK dried blood spots using a duplex TREC screening assay. J. Clin. Immunol. 2014, 34, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Van der Spek, J.; Groenwold, R.H.; van der Burg, M.; van Montfrans, J.M. TREC Based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review. J. Clin. Immunol. 2015, 35, 416–430. [Google Scholar] [CrossRef]
- Gaspar, B. (University College London-Great Ormond Street Institute of Child Health, London, UK). Personal communication–Length of stay and other costs estimates, 2016.
- Gaspar, B.; Ladomenou, F. (University College London-Great Ormond Street Institute of Child Health, London, UK). Personal communication–EQ-5D-3L esimates, 2016.
- Department of Health and Social Care. NHS Reference Costs 2014 to 2015. 2015. Available online: https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed on 17 February 2016).
- National Health Service UK Genetic Testing Network. Find A Test. 2016. Available online: http://ukgtn.nhs.uk/find-a-test/ (accessed on 17 February 2016).
- Liu, Z.; Albon, E.; Hyde, C. The Effectiveness and Cost-Effectiveness of Immunoglobulin Replacement Thearpy for Primary Immunodeficiency and Chronic Lymphocytic Leukaemia: A Systmatic Review and Economic Evaluation; West Midlands Health Technology Assessment Collaboration, Department of Public Health and Epidemiology, The University of Birmingham: Birmingham, UK, 2005; Available online: http://www.birmingham.ac.uk/Documents/college-mds/haps/projects/WMHTAC/REPreports/2005/IgRT.pdf (accessed on 17 February 2016).
- Paediatric Formulary Committee. BNF for Children; BMJ Group; Pharmaceutical Press; RCPCH Publications: London, UK; Available online: https://about.medicinescomplete.com/publication/british-national-formulary-for-children/ (accessed on 17 February 2016).
- The Health and Social Care Information Centre. Health Survey for England 2011 Children Trend Tables. 2012. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england (accessed on 17 February 2016).
- Leaviss, J.; Bessey, A.; de la Cruz, C.; Wong, R.; Chilcott, J. Systematic Reviews of Screening for Severe Combined Immunodeficiency (SCID) in the NHS Newborn Blood Spot Screening Programme: Incidence, Screening Test Characteristics and the Effectiveness of Treatments; School of Health and Related Research (ScHARR), University of Sheffield: Sheffield, UK, 2017; Available online: https://legacyscreening.phe.org.uk/scid (accessed on 20 November 2018).
- Chan, A.; Scalchunes, C.; Boyle, M.; Puck, J.M. Early vs. delayed diagnosis of severe combined immunodeficiency: A family perspective survey. Clin. Immunol. 2011, 138, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, H.B.; Qasim, W.; Davies, E.G.; Rao, K.; Amrolia, P.J.; Veys, P. How I treat severe combined immunodeficiency. Blood 2013, 122, 3749–3758. [Google Scholar] [CrossRef]
- Kwan, A.; Church, J.A.; Cowan, M.J.; Agarwal, R.; Kapoor, N.; Kohn, D.B.; Lewis, D.B.; McGhee, S.A.; Moore, T.B.; Stiehm, E.R.; et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: Results of the first 2 years. J. Allergy Clin. Immunol. 2013, 132, 140–150. [Google Scholar] [CrossRef]
- Curtis, L.B.; Burns, A. Unit Costs of Health and Social Care 2015; Personal Social Services Research Unit, University of Kent: Canterbury, UK, 2015; Available online: https://www.pssru.ac.uk/project-pages/unit-costs/ (accessed on 17 February 2016).
- Kwan, A.; Abraham, R.S.; Currier, R.; Brower, A.; Andruszewski, K.; Abbott, J.K.; Baker, M.; Ballow, M.; Bartoshesky, L.E.; Bonilla, F.A.; et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 2014, 312, 729–738. [Google Scholar] [CrossRef]
- Dolan, P. Modeling Valuations for EuroQol Health States. Med. Care 1997, 35, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Neven, B.; Leroy, S.; Decaluwe, H.; Le Deist, F.; Picard, C.; Moshous, D.; Mahlaoui, N.; Debré, M.; Casanova, J.L.; Dal Cortivo, L.; et al. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood 2009, 113, 4114–4124. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.C.; Chinen, J.; Rosenblatt, H.M.; Hanson, I.C.; Brown, B.S.; Paul, M.E.; Abramson, S.L.; Ritz, J.; Shearer, W.T. Long-term outcomes of nonconditioned patients with severe combined immunodeficiency transplanted with HLA-identical or haploidentical bone marrow depleted of T cells with anti-CD6 mAb. J. Allergy Clin. Immunol. 2008, 122, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.C.; Chinen, J.; Rosenblatt, H.M.; Hanson, I.C.; Krance, R.A.; Paul, M.E.; Abramson, S.L.; Noroski, L.M.; Davis, C.M.; Seeborg, F.O.; et al. Outcomes of patients with severe combined immunodeficiency treated with hematopoietic stem cell transplantation with and without preconditioning. J. Allergy Clin. Immunol. 2009, 124, 1062–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slatter, M.A.; Brigham, K.; Dickinson, A.M.; Harvey, H.L.; Barge, D.; Jackson, A.; Bown, N.; Flood, T.J.; Cant, A.J.; Abinun, M.; et al. Long-term immune reconstitution after anti-CD52-treated or anti-CD34-treated hematopoietic stem cell transplantation for severe T-lymphocyte immunodeficiency. J. Allergy Clin. Immunol. 2008, 121, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Mazzolari, E.; Forino, C.; Guerci, S.; Imberti, L.; Lanfranchi, A.; Porta, F.; Notarangelo, L.D. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. J. Allergy Clin. Immunol. 2007, 120, 892–899. [Google Scholar] [CrossRef]
- National Institute of Health and Care Excellence. Methods for the Development of NICE Public Health Guidance, 3rd ed.; National Institute of Health and Care Excellence: London, UK, 2012; Available online: https://www.nice.org.uk/process/pmg4/chapter/introduction (accessed on 27 October 2018).
- National Institute of Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. 2013. Available online: https://www.nice.org.uk/process/pmg9/chapter/foreword (accessed on 27 October 2018).
- Strong, M.; Oakley, J.E.; Brennan, A. Estimating Multiparameter Partial Expected Value of Perfect Information from a Probabilistic Sensitivity Analysis Sample: A Nonparametric Regression Approach. Med. Decis. Mak. 2014, 34, 311–326. [Google Scholar] [CrossRef]
- Heimall, J.; Logan, B.R.; Cowan, M.J.; Notarangelo, L.D.; Griffith, L.M.; Puck, J.M.; Kohn, D.B.; Pulsipher, M.A.; Parikh, S.; Martinez, C.; et al. Immune reconstitution and survival of 100 SCID patients post–hematopoietic cell transplant: A PIDTC natural history study. Blood 2017, 130, 2718–2727. [Google Scholar] [CrossRef]
- Morillo-Gutierrez, B.; Worth, A.; Valappil, M.; Gaspar, H.B.; Gennery, A.R. Chronic Infection with Rotavirus Vaccine Strains in UK Children with Severe Combined Immunodeficiency. Pediatr. Infect. Dis. J. 2015, 34, 1040–1041. [Google Scholar] [CrossRef]
- Lingen, M.; Albers, L.; Borchers, M.; Haass, S.; Gärtner, J.; Schröder, S.; Goldbeck, L.; von Kries, R.; Brockmann, K.; Zirn, B. Obtaining a genetic diagnosis in a child with disability: Impact on parental quality of life. Clin. Genet. 2016, 89, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; McClaren, B.J.; Archibald, A.D.; Weeks, A.; Langmaid, T.; Ryan, M.M.; Kornberg, A.; Metcalfe, S.A. A mixed methods study of age at diagnosis and diagnostic odyssey for Duchenne muscular dystrophy. Eur. J. Hum. Genet. 2015, 23, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Lawton, S.; Hickerton, C.; Archibald, A.D.; McClaren, B.J.; Metcalfe, S.A. A mixed methods exploration of families’ experiences of the diagnosis of childhood spinal muscular atrophy. Eur. J. Hum. Genet. 2015, 23, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.J.; Comeau, A.M.; Grosse, S.D.; Tanksley, S.; Prosser, L.A.; Ojodu, J.; Botkin, J.R.; Kemper, A.R.; Green, N.S. Evaluating Harms in the Assessment of Net Benefit: A Framework for Newborn Screening Condition Review. Matern. Child Health J. 2016, 20, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.C.; Crowley, T.B.; Jyonouchi, S.; Heimall, J.; Zackai, E.H.; Sullivan, K.E.; McDonald-McGinn, D.M. Identification of 22q11.2 Deletion Syndrome via Newborn Screening for Severe Combined Immunodeficiency. J. Clin. Immunol. 2017, 37, 476. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, M.J.; Dvorak, C.C.; Cowan, M.J.; Puck, J.M. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J. Allergy Clin. Immunol. 2017, 139, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Ungar, W. Economic Evaluation in Child Health; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Steven, K. Valuation of the Child Health Utility 9D Index. Pharmacoeconomics 2012, 30, 729–747. [Google Scholar] [CrossRef] [PubMed]
- Marciano, B.E.; Huang, C.Y.; Joshi, G.; Rezaei, N.; Carvalho, B.C.; Allwood, Z.; Ikinciogullari, A.; Reda, S.M.; Gennery, A.; Thon, V.; et al. BCG vaccination in patients with severe combined immunodeficiency: Complications, risks, and vaccination policies. J. Allergy Clin. Immunol. 2014, 133, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Tuberculosis: The Green Book, Chapter 32. In The Green Book; Public Health England: London, UK, 2018. Available online: https://www.gov.uk/government/publications/tuberculosis-the-green-book-chapter-32 (accessed on 27 October 2018).
- Public Health England. The UK NSC Recommendation on Severe Combined Immunodeficiency. 2017. Available online: https://legacyscreening.phe.org.uk/scid (accessed on 27 October 2018).
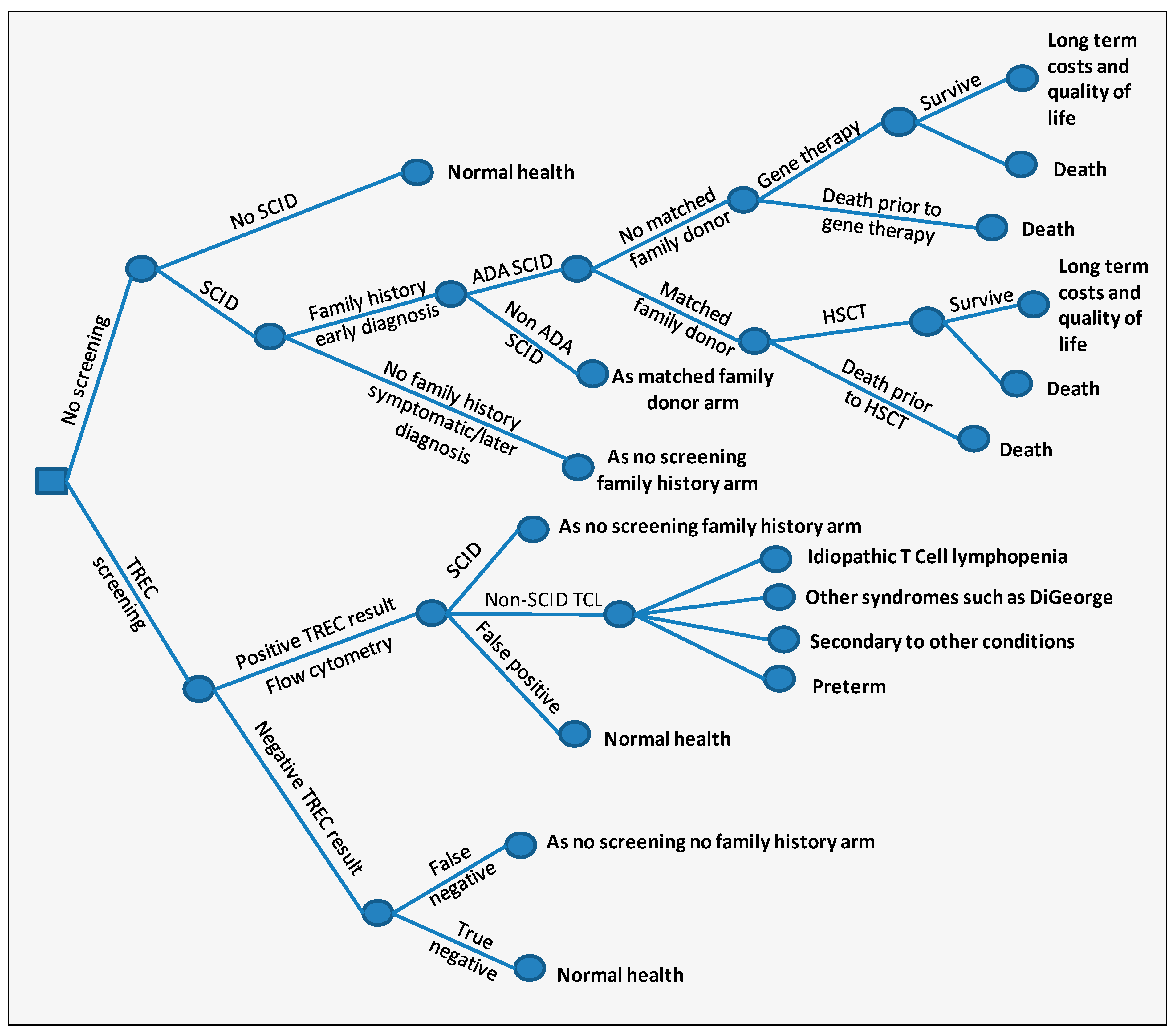
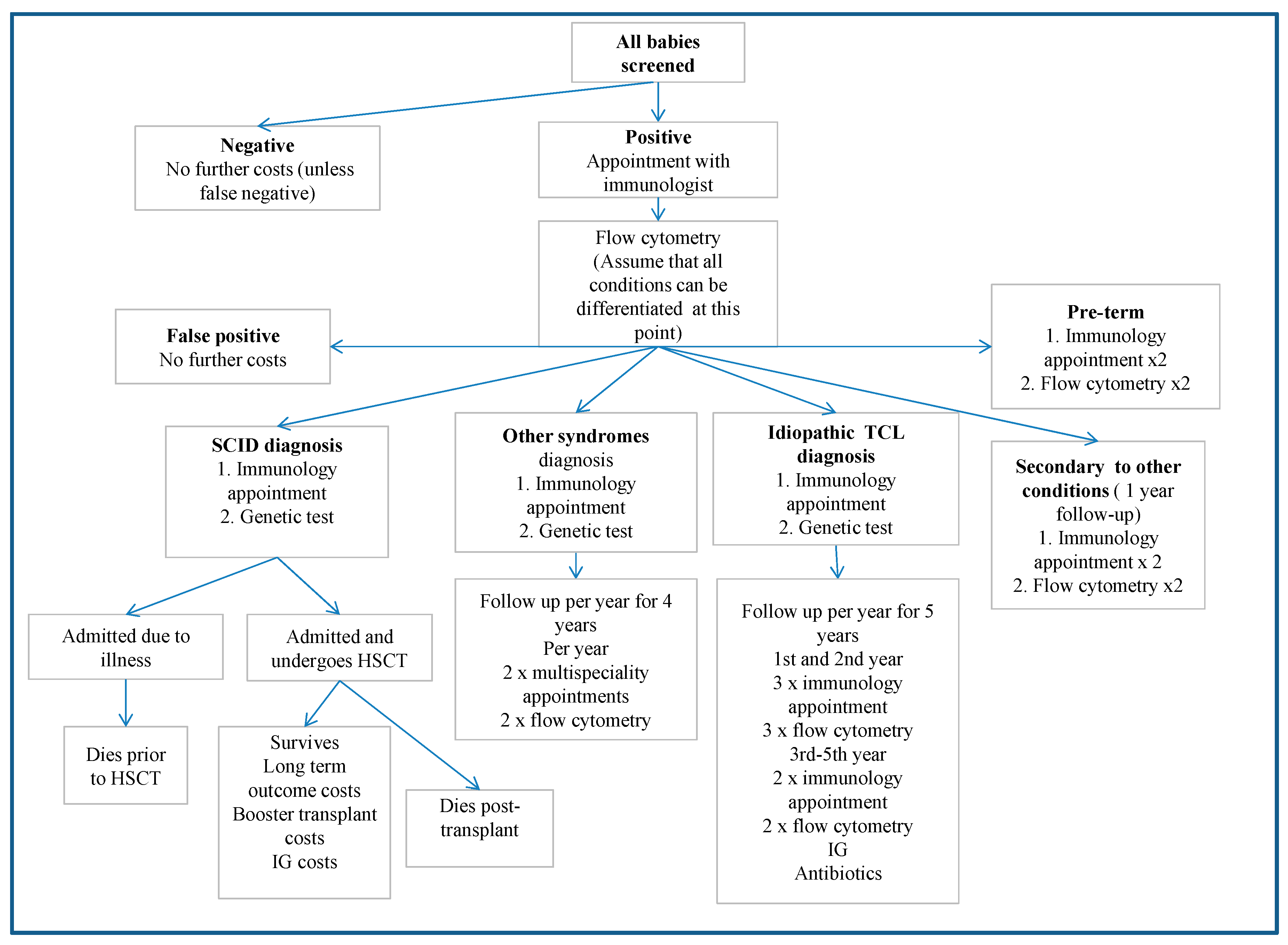
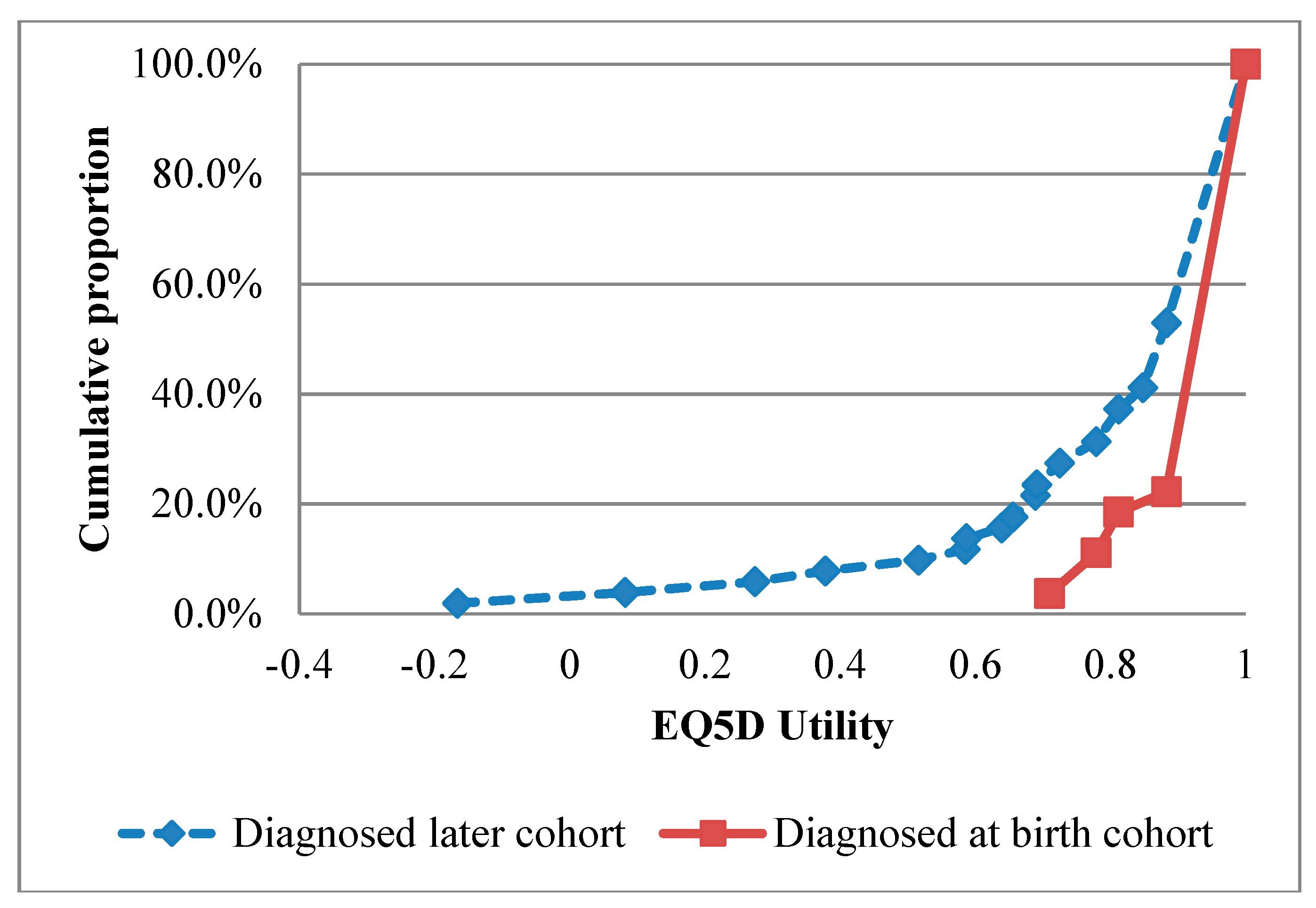
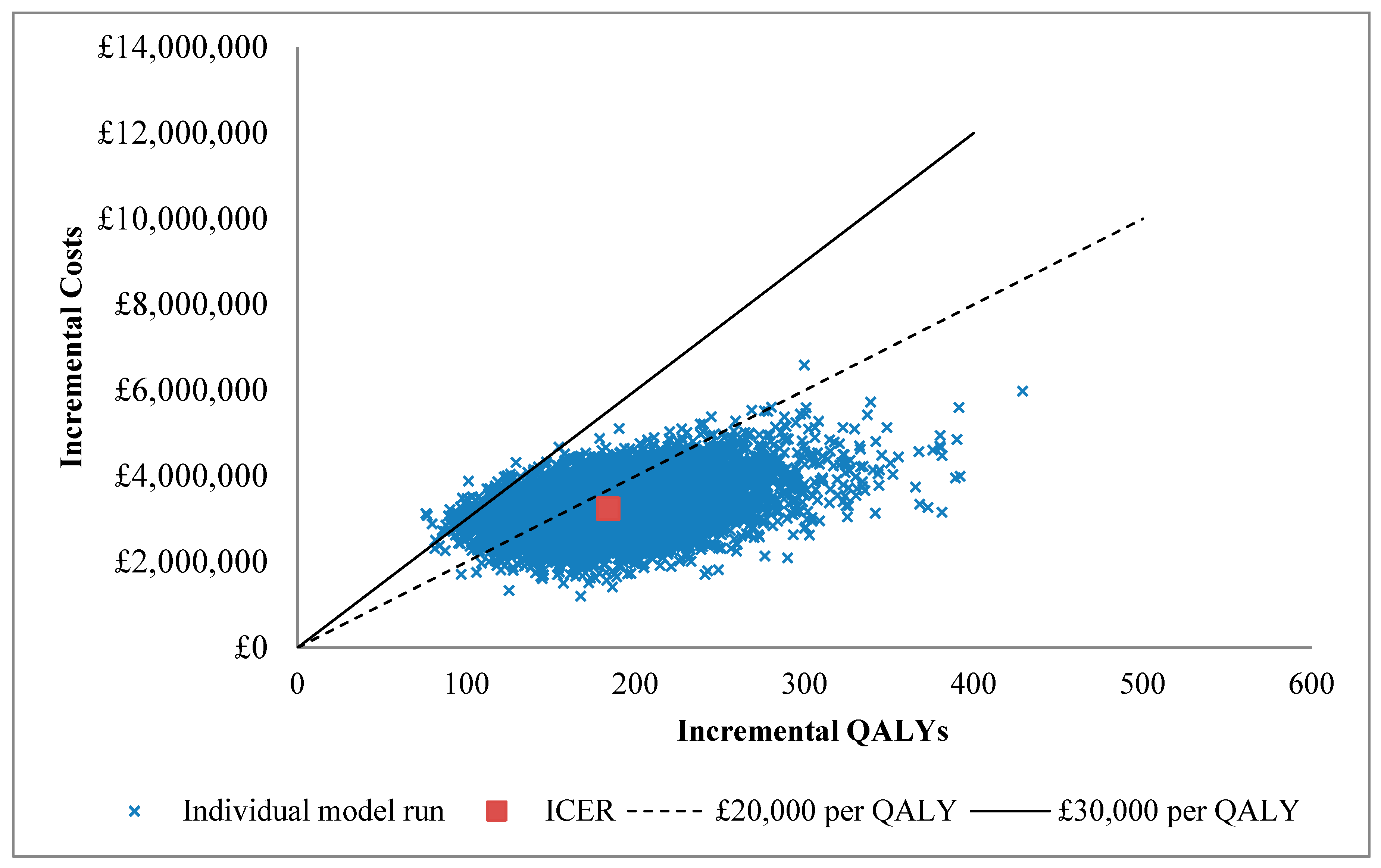
| Parameter | Mean (95% Confidence Interval) | Reference |
|---|---|---|
| Number of births (UK) | 780,835 | [17,18,19] |
| Incidence of SCID | 1:49,000 (1:39,857, 1:61,527) | [20] |
| Incidence of undiagnosed SCID | 1:521,000 (1:167,052, 1:7,236,800) | [20] |
| Incidence of syndromes | 1:45,000 (1:24,390, 1:110,606) | [21] |
| Incidence of secondary conditions | 1:130,000 (1:50,686, 1:782,506) | [21] |
| Incidence of idiopathic TCL | 1:99,000 (1:42,255, 1:432,482) | [21] |
| Incidence of positive TREC in pre-terms | 1:99,000 (1:42,255, 1:432,482) | [21] |
| Presumptive positives (20 copies/µL) | 0.041% (0.0035%, 0.1018%) | [22] |
| Sensitivity for SCID | 0.99 (0.985, 0.998) | [23] |
| Proportion of SCID patients with a family history | 0.30 (0.21, 0.41) | [20] |
| Proportion of SCID that is ADA-SCID | 0.17 (0.1, 0.26) | [20] |
| Proportion of SCID patients with a matched family donor available | 0.25 (0.07, 0.5) | [20] |
| Pre HSCT mortality (late diagnosed) | 35.3% (22.8%, 49.3%) | [6] |
| Pre HSCT mortality (early diagnosed) | 1.68% (0.11%, 7.63%) | [6] |
| HSCT mortality (late diagnosed) | 38.7% (22.4% 56.3%) | [6] |
| HSCT mortality (early diagnosed) | 8.48% (1.79%, 23.4%) | [6] |
| Number of days HSCT | 54.0 | [24] |
| Early diagnosis—Total days non-critical care | 82.6 (50.3, 122.8) | [24] |
| Early diagnosis—Total days critical care | 3.96 (0.15, 8.41) | [24] |
| Late diagnosis—Total days non-critical care | 144 (108.6, 184.3) | [24] |
| Late diagnosis—Total days critical care | 8.19 (3.72, 14.4) | [24] |
| QALYs—early diagnosis (1979–2015 cohort, base case values) | 0.95 | [25] |
| QALYs—late diagnosis (1979–2015 cohort, base case values) | 0.82 | [25] |
| QALYs—early diagnosis (2000–2015 cohort) | 0.96 | [25] |
| QALYs—late diagnosis (2000–2015 cohort) | 0.87 | [25] |
| Cost HSCT (early diagnosed) | £128,363 | [24,26] |
| Cost HSCT (late diagnosed) | £231,186 | [24,26] |
| Cost death prior to HSCT | £43,368 | [24,26] |
| Presumptive positive cost | £276 | [24,26] |
| Diagnosis SCID | £711 | [26,27] |
| Diagnosis idiopathic SCID and syndromes | £1,551 | [26,27] |
| Idiopathic SCID follow up (5 years discounted) | £20,142 | [26,28,29,30] |
| Syndromes 4 year follow up | £4,872 | [24,26] |
| Follow up preterm & secondary to other conditions | £533 | [24,26] |
| Screening | 95% CI | No Screening | 95% CI | Incremental | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Outcomes | SCID cases diagnosed symptomatically | 0.2 | (0.1, 0.3) | 11.0 | (8.4, 14.1) | −10.9 | (−13.9, −8.2) |
| SCID cases diagnosed via a family history | 0 | (0, 0) | 4.9 | (3.1, 7.0) | −4.9 | (−3.1, −7.0) | |
| SCID cases not diagnosed | 0 | (0, 0) | 1.5 | (0.1, 4.8) | −1.5 | (−0.1, −4.8) | |
| SCID cases screen detected | 17.2 | (13.5, 21.7) | 0 | (0, 0) | 17.2 | (13.5, 21.7) | |
| ADA SCID | 2.9 | (1.6, 4.7) | 2.9 | (1.6, 4.7) | 0 | (0.0, 0.0) | |
| SCID mortality | 1.7 | (0.6, 4.0) | 8.1 | (5.3, 12.0) | −6.3 | (−9.7, −4) | |
| Screening outcomes | Non SCID TCL | 31.1 | (16.3, 50.1) | 0 | (0, 0) | 31 | (16.3, 50.1) |
| Pre-term | 8.0 | (1.9, 18.7) | 0 | (0, 0) | 8 | (1.9, 18.7) | |
| Total presumptive positives | 322.1 | (79.5, 852.9) | 0 | (0, 0) | 0 | (79.5, 852.9) | |
| Costs | Direct screening costs | £3.04m | (£2.97m, £3.19m) | £0.00m | (£0.00m, £0.00m) | £3.04m | (£2.97m, £3.19m) |
| Diagnosis and follow up pre-terms | £4,394 | (£1,029.36, £10,311) | £0 | (£0.00m, £0.00m) | £4,394 | (£1,029.36, £10,311) | |
| Diagnosis and follow up non SCID TCL | £0.27m | (£0.12m, £0.51m) | £0.00m | (£0.00m, £0.00m) | £0.27m | (£0.12m, £0.51m) | |
| Diagnosis costs SCID | £14,235 | (£11,112, £17,967) | £13,023 | (£13,023, £15,954) | £1,212 | (£61.7, £3,919) | |
| SCID treatment up and including HSCT | £3.35m | (£2.20m, £4.83m) | £3.63m | (£3.63m, £2.65m) | −£0.28m | (−£1.10m, £0.62m) | |
| SCID long-term costs | £2.03m | (£1.20m, £3.12m) | £1.30m | (£1.30m, £0.78m) | £0.72m | (£0.23m, £1.37m) | |
| Totals | Total costs | £7.30m | (£5.96m, £9.06m) | £3.96m | (£3.96m, £2.92m) | £3.34m | (£2.36m, £4.47m) |
| Total QALYs | 410.1 | (308.3, 527.3) | 226.9 | (226.9, 164.9) | 183.17 | (109.3, 267.2) | |
| ICER | £18,222 | (£12,013, £27,763) |
| Sensitivity Analysis | Screening | No Screening | Incremental | Probability Cost-Effective at Threshold | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | Costs | QALYs | Costs | QALYs | ICER | £20k | £30k | ||
| Base case | £7.30m | 410 | £3.96m | 227 | £3.34m | 183 | £18,222 | 65% | 99% | |
| Incidence | Halved | £5.49m | 223 | £2.01m | 113 | £3.48m | 110 | £31,647 | 2% | 35% |
| Doubled | £10.97m | 785 | £7.89m | 453 | £3.07m | 332 | £9,266 | 100% | 100% | |
| TREC cut off | Additional false positives | £7.53m | 412 | £3.99m | 227 | £3.54m | 185 | £19,137 | 56% | 98% |
| Increase proportionately | £7.44m | 409 | £3.98m | 227 | £3.46m | 182 | £18,983 | 56% | 98% | |
| Family History | 10% family history | £7.32m | 411 | £4.06m | 183 | £3.26m | 229 | £14,237 | 95% | 100% |
| 50% family history | £7.30m | 410 | £3.89m | 269 | £3.42m | 141 | £24,208 | 14% | 84% | |
| Test cost | £1.50 | £5.75m | 410 | £3.98m | 227 | £1.77m | 183 | £9,674 | 100% | 100% |
| £2.50 | £6.55m | 413 | £3.99m | 228 | £2.56m | 184 | £13,876 | 95% | 100% | |
| £4.50 | £8.10m | 410 | £3.98m | 227 | £4.11m | 183 | £22,471 | 25% | 92% | |
| Discount rate | 1.5% both costs and health benefits | £8.82m | 702 | £5.02m | 391 | £3.80m | 311 | £12,219 | 99% | 100% |
| 1.5% health benefits & 3.5% costs | £7.32m | 703 | £4.00m | 392 | £3.32m | 311 | £10,680 | 100% | 100% | |
| QALYs | 2000–2015 cohort QALYs used | £7.32m | 415 | £3.98m | 235 | £3.34m | 180 | £18,588 | 61% | 98% |
| Pre-transplant mortality | Early diagnosed mortality (OR) | |||||||||
| 8.15%(0.23) | £7.12m | 381 | £3.67m | 206 | £3.45m | 175 | £19,691 | 51% | 97% | |
| 29.40%(0.83) | £6.44m | 294 | £3.48m | 182 | £2.95m | 113 | £26,237 | 12% | 68% | |
| Post-transplant mortality | Early diagnosed mortality (OR) | |||||||||
| 17.42%(0.45) | £7.16m | 369 | £3.68m | 203 | £3.48m | 166 | £20,915 | 39% | 96% | |
| 36.77%(0.95) | £6.79m | 283 | £3.58m | 178 | £3.21m | 105 | £30,746 | 3% | 43% | |
| Healthy at Birth Disbenefit (QALY) | Cost per TREC Test £3.50 | |
|---|---|---|
| False-Positive Disbenefit Threshold (Quality-Adjusted Days) | ||
| Discounting 3.5% | Discounting 1.5% | |
| Mean (95% CI) | Mean (95% CI) | |
| 0 | 17 (0, 22) | 171 (1,113, 91) |
| 1 | 6 (0, 14) | 159 (1,069,83) |
| 2 | 0 (0, 6) | 148 (1,025, 75) |
| 3 | 0 (0, 0) | 136 (980, 67) |
| 4 | 0 (0, 0) | 125 (936, 59) |
| 10 | 0 (0, 0) | 55 (672, 11) |
| 20 | 0 (0, 0) | 0 (230, 0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessey, A.; Chilcott, J.; Leaviss, J.; de la Cruz, C.; Wong, R. A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK. Int. J. Neonatal Screen. 2019, 5, 28. https://doi.org/10.3390/ijns5030028
Bessey A, Chilcott J, Leaviss J, de la Cruz C, Wong R. A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK. International Journal of Neonatal Screening. 2019; 5(3):28. https://doi.org/10.3390/ijns5030028
Chicago/Turabian StyleBessey, Alice, James Chilcott, Joanna Leaviss, Carmen de la Cruz, and Ruth Wong. 2019. "A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK" International Journal of Neonatal Screening 5, no. 3: 28. https://doi.org/10.3390/ijns5030028
APA StyleBessey, A., Chilcott, J., Leaviss, J., de la Cruz, C., & Wong, R. (2019). A Cost-Effectiveness Analysis of Newborn Screening for Severe Combined Immunodeficiency in the UK. International Journal of Neonatal Screening, 5(3), 28. https://doi.org/10.3390/ijns5030028





