Congenital Critical Heart Defect Screening in a Health Area of the Community of Valencia (Spain): A Prospective Observational Study
Abstract
:1. Introduction
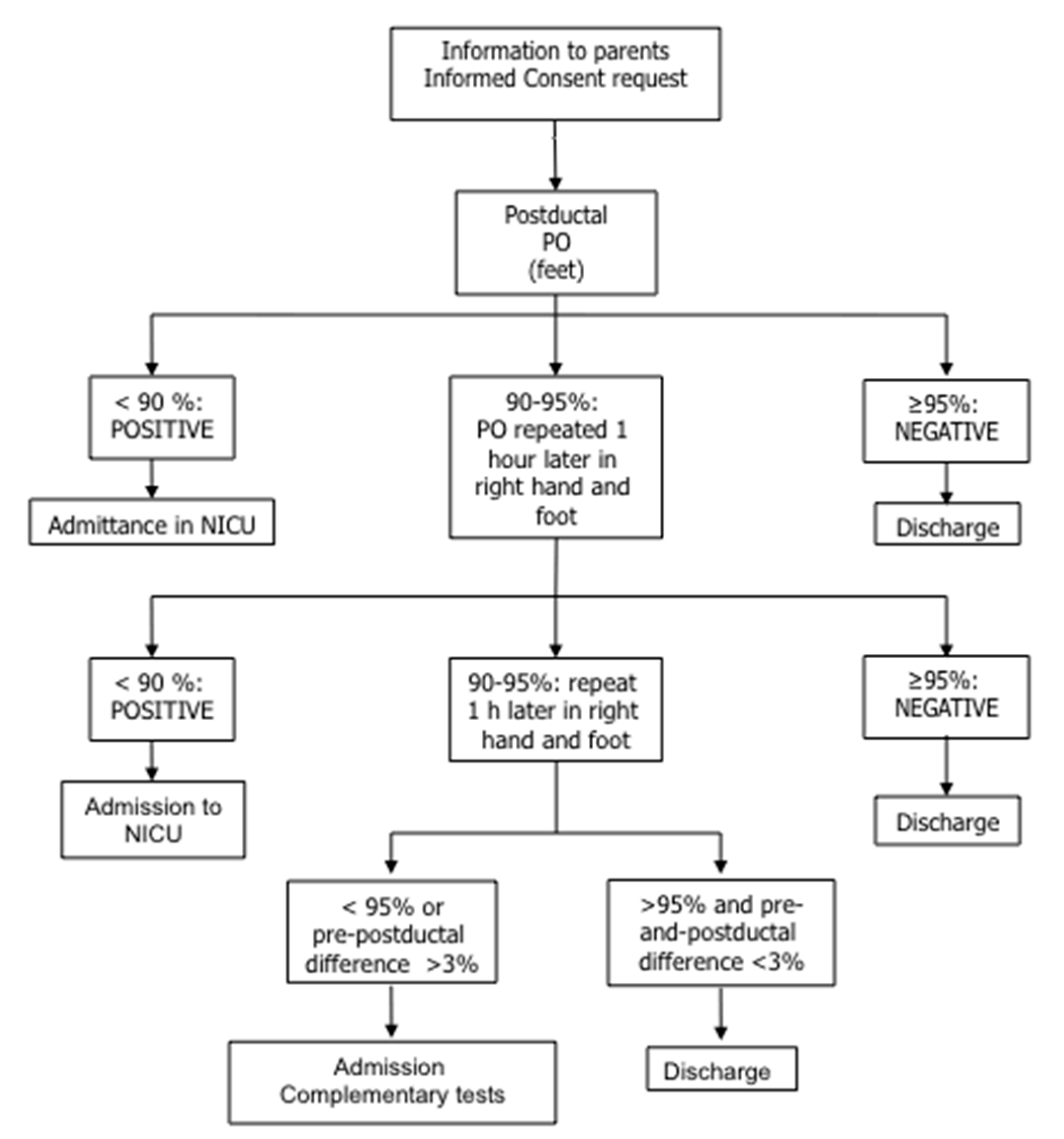
2. Population and Methods
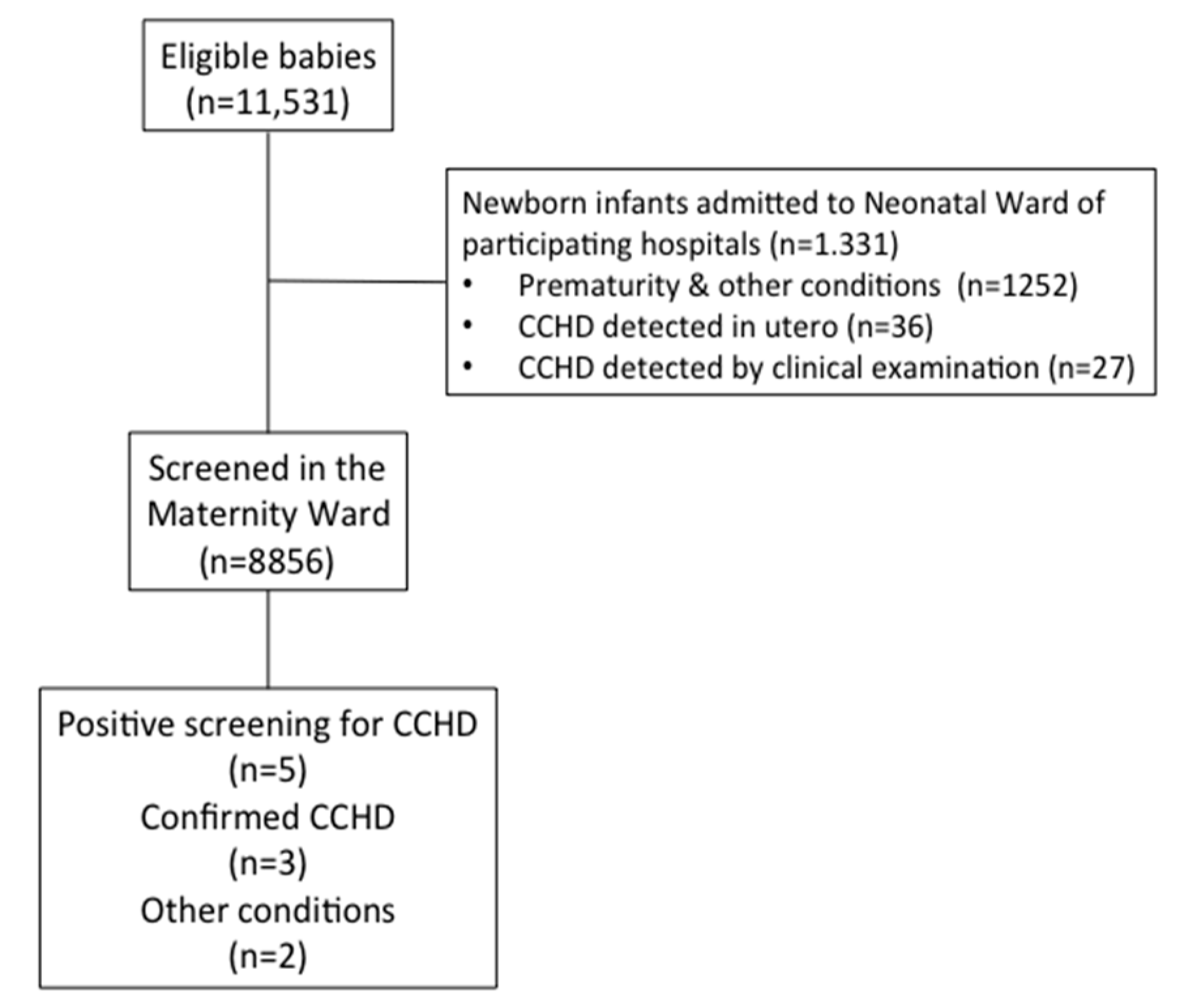
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Schultz, A.H.; Localio, A.R.; Clark, B.J.; Ravishankar, C.; Videon, N.; Kimmel, S.E. Epidemiologic features of the presentation of critical congenital heart disease: Implications for screening. Pediatrics 2008, 121, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, J.; Feng, Q.L.; Gu, H.T.; Wan, G.; Zhang, H.M.; Xie, Y.J.; Li, X.S. Fetal echocardiography for congenital heart disease diagnosis: A meta-analysis, power analysis and missing data analysis. Eur. J. Prev. Cardiol. 2015, 22, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Górska-Kot, A.; Błaz, W.; Pszeniczna, E.; Rusin, J.; Materna-Kiryluk, A.; Homa, E.; Hejda, G.; Franus, J. Trends in diagnosis and prevalence of critical congenital heart defects in the Podkarpacie province in 2002–2004, based on data from the Polish Registry of Congenital Malformations. J. Appl. Genet. 2006, 47, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Meberg, A.; Andreassen, A.; Brunvand, L.; Markestad, T.; Moster, D.; Nietsch, L.; Silberg, I.E.; Skålevik, J.E. Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Paediatr. 2009, 98, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I. It is time for routine neonatal screening by pulse oximetry. Neonatology 2011, 99, 1–9. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.P.; Kamlin, C.O.; Davis, P.G.; Carlin, J.B.; Morley, C.J. Clinical assessment of infant colour at delivery. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F465–F467. [Google Scholar] [CrossRef] [PubMed]
- Mikrou, P.; Singh, A.; Ewer, A.K. Pulse oximetery screening for critical congenital heart defects: A repeat UK national survey. Arch Dis. Child. Fetal Neonatal. Ed. 2017, 10, F558–F559. [Google Scholar]
- Mahle, W.T.; Martin, G.R.; Beekman, R.H., III; Morrow, W.R. Section on Cardiology and Cardiac Surgery Executive Committee. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics 2012, 129, 190–192. [Google Scholar] [PubMed]
- Riede, F.T.; Wörner, C.; Dähnert, I.; Möckel, A.; Kostelka, M.; Schneider, P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine—Results from a prospective multicenter study. Eur. J. Pediatr. 2010, 169, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Ewer, A.K. Pulse oximetry screening: Do we have enough evidence now? Lancet 2014, 384, 725–726. [Google Scholar] [CrossRef]
- Sebelius, K. Secretary of Health and Human Services Recommendation for Pulse Oximetry Screening; Department of Health and Human Services: Washington, DC, USA, 2011. Available online: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf (accessed on 27 November 2017).
- De-Wahl Granelli, A.; Wennergren, M.; Sandberg, K.; Mellander, M.; Bejlum, C.; Inganäs, L.; Eriksson, M.; Segerdahl, N.; Agren, A.; Ekman-Joelsson, B.M.; et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: A Swedish prospective screening study in 39,821 newborns. BMJ 2009, 338, a3037. [Google Scholar] [CrossRef] [PubMed]
- Ewer, A.K.; Middleton, L.J.; Furmston, A.T.; Bhoyar, A.; Daniels, J.P.; Thangaratinam, S.; Deeks, J.J.; Khan, K.S.; PulseOx Study Group. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): A test accuracy study. Lancet 2011, 378, 785–794. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Brown, K.; Zamora, J.; Khan, K.S.; Ewer, A.K. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: A systematic review and meta-analysis. Lancet 2012, 379, 2459–2464. [Google Scholar] [CrossRef]
- Zhao, Q.M.; Ma, X.J.; Ge, X.L.; Liu, F.; Yan, W.L.; Wu, L.; Ye, M.; Zhang, J.; Gao, Y.; Jia, B.; et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: A prospective study. Lancet 2014, 384, 747–754. [Google Scholar] [CrossRef]
- Peterson, C.; Ailes, E.; Riehle-Colarusso, T.; Oster, M.E.; Olney, R.S.; Cassell, C.H.; Fixler, D.E.; Carmichael, S.L.; Shaw, G.M.; Gilboa, S.M.; et al. Late detection of critical congenital heart disease among US infants: Estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014, 168, 361–370. [Google Scholar] [CrossRef] [PubMed]
- De-Wahl Granelli, A.; Meberg, A.; Ojala, T.; Steensberg, J.; Oskarsson, G.; Mellander, M. Nordic pulse oximetry screening—Implementation status and proposal for uniform guidelines. Acta Paediatr. 2014, 103, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Ewer, A.K.; Granelli, A.D.; Manzoni, P.; Sánchez Luna, M.; Martin, G.R. Pulse oximetry screening for congenital heart defects. Lancet 2013, 382, 856–857. [Google Scholar] [CrossRef]
- Manzoni, P.; Martin, G.R.; Luna, M.S.; Mestrovic, J.; Simeoni, U.; Zimmermann, L.; Ewer, A.K. Pulse oximetry screening for critical congenital heart defects: a European consensus statement. Lancet Child Adolesc. Health 2017, 1, 88–90. [Google Scholar] [CrossRef]
- Haas, N.A.; Franke, J. Mangement of a child with cyanosis. Guidelines for the management of congenital heart disease. Cardiol. Young 2017, 27 (Suppl. 3), S3–S4. [Google Scholar]
- Sánchez Luna, M.; Pérez Muñuzuri, A.; Sanz López, E.; Leante Castellanos, J.L.; Benavente Fernández, I.; Ruiz Campillo, C.W.; Sánchez Redondo, M.D.; Vento Torres, M.; Rite Gracia, S. Pulse oximetry screening of critical congenital heart defects in the neonatal period. The Spanish National Neonatal Society recommendation. An. Pediatr. 2017. [Google Scholar] [CrossRef]
| Echocardiography | |||
|---|---|---|---|
| Pulse oximetry | Positive | Negative | Total |
| Positive | 3 | 2 | 5 |
| Negative | 0 | 8851 | 8851 |
| Total | 3 | 8853 | 8856 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubells, E.; Torres, B.; Nuñez-Ramiro, A.; Sánchez-Luna, M.; Izquierdo, I.; Vento, M. Congenital Critical Heart Defect Screening in a Health Area of the Community of Valencia (Spain): A Prospective Observational Study. Int. J. Neonatal Screen. 2018, 4, 3. https://doi.org/10.3390/ijns4010003
Cubells E, Torres B, Nuñez-Ramiro A, Sánchez-Luna M, Izquierdo I, Vento M. Congenital Critical Heart Defect Screening in a Health Area of the Community of Valencia (Spain): A Prospective Observational Study. International Journal of Neonatal Screening. 2018; 4(1):3. https://doi.org/10.3390/ijns4010003
Chicago/Turabian StyleCubells, Elena, Begoña Torres, Antonio Nuñez-Ramiro, Manuel Sánchez-Luna, Isabel Izquierdo, and Máximo Vento. 2018. "Congenital Critical Heart Defect Screening in a Health Area of the Community of Valencia (Spain): A Prospective Observational Study" International Journal of Neonatal Screening 4, no. 1: 3. https://doi.org/10.3390/ijns4010003
APA StyleCubells, E., Torres, B., Nuñez-Ramiro, A., Sánchez-Luna, M., Izquierdo, I., & Vento, M. (2018). Congenital Critical Heart Defect Screening in a Health Area of the Community of Valencia (Spain): A Prospective Observational Study. International Journal of Neonatal Screening, 4(1), 3. https://doi.org/10.3390/ijns4010003






