Celebrating 50 Years of Nationwide Newborn Screening in Hungary—Review, Current Situation, and Future Directions
Abstract
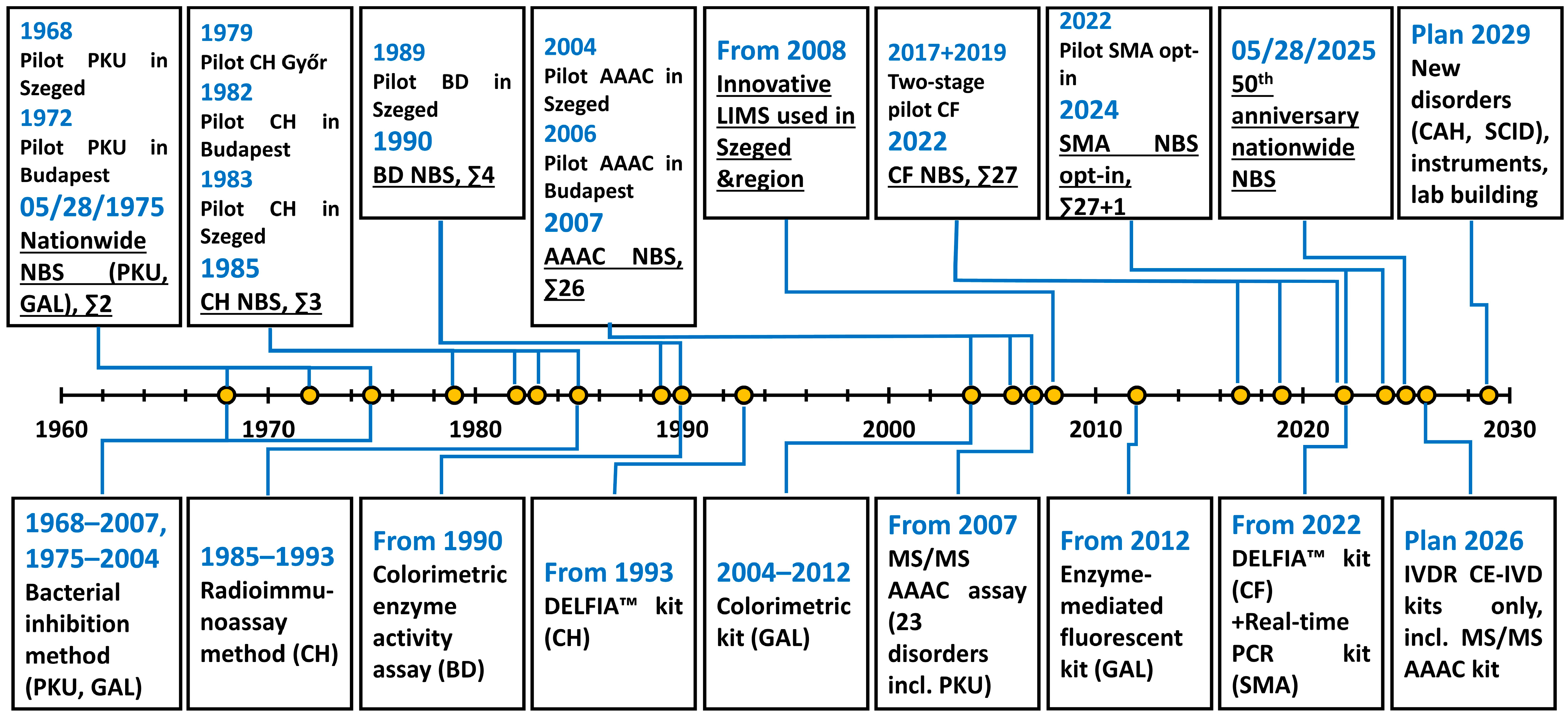
1. Introduction
2. Newborn Screening in Hungary: Implementation, Early Years, and the Era of Traditional Methods (1968–2007)
3. Extended Newborn Screening: Introduction of Tandem Mass Spectrometry and Further Developments (2007–2020)
4. Improvements Since 2020 and the Current Situation of Newborn Screening in Hungary
5. Future Plans and Perspectives
5.1. Extension of the Screening Panel
5.2. Infrastructural and Regulatory Advancements
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAAC | amino acid-acylcarnitine |
| AADC | aromatic L-amino acid decarboxylase |
| CAH | congenital adrenal hyperplasia |
| CDC | Centers for Disease Control and Prevention |
| CE-IVD | Conformité Européenne (European Conformity)—In Vitro Diagnostics |
| CF | cystic fibrosis |
| CH | congenital hypothyroidism |
| DELFIA™ | dissociation-enhanced lanthanide fluorescence immunoassay |
| DBS | dried blood spot |
| ERNDIM | European Research Network for the Evaluation and Improvement of Screening, Diagnosis and Treatment of Inherited Disorders of Metabolism |
| GALT | galactose-1-phosphate uridyltransferase |
| GAMT | guanidinoacetate methyltransferase |
| IEM | inborn error of metabolism |
| IRT | immunoreactive trypsinogen |
| IVDR | Regulation No. 2017/746 of the European Union on in vitro diagnostic medical devices |
| LIMS | laboratory information management system |
| MS/MS | tandem mass spectrometry |
| NBS | newborn screening |
| NSQAP | Newborn Screening Quality Assurance Program |
| PAP | pancreatitis-associated protein |
| PKU | phenylketonuria |
| PT | Proficiency Testing |
| QC | Quality Control |
| RUSP | Recommended Uniform Screening Panel |
| SCID | severe combined immunodeficiency |
| SMA | spinal muscular atrophy |
| UHPLC | ultra-high-performance liquid chromatography |
| X-ALD | X-linked adrenoleukodystrophy |
References
- Therrell, B.L.; Padilla, C.D.; Borrajo, G.J.C.; Khneisser, I.; Schielen, P.C.J.I.; Knight-Madden, J.; Malherbe, H.L.; Kase, M. Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020–2023). Int. J. Neonatal Screen. 2024, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.E.; Metternick-Jones, S.C.; Lister, K.J. International differences in the evaluation of conditions for newborn bloodspot screening: A review of scientific literature and policy documents. Eur. J. Hum. Genet. 2016, 25, 10–16. [Google Scholar] [CrossRef]
- Guthrie, R.; Susi, A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [CrossRef]
- MacCready, R.A.; Hussey, M.G. Newborn Phenylketonuria Detection Program in Massachusetts. Am. J. Public Health Nations Health 1964, 54, 2075–2081. [Google Scholar] [CrossRef]
- Levy, H.L. Robert Guthrie and the Trials and Tribulations of Newborn Screening. Int. J. Neonatal Screen. 2021, 7, 5. [Google Scholar] [CrossRef]
- Dussault, J.H.; Coulombe, P.; Laberge, C.; Letarte, J.; Guyda, H.; Khoury, K. Preliminary report on a mass screening program for neonatal hypothyroidism. J. Pediatr. 1975, 86, 670–674. [Google Scholar] [CrossRef]
- Millington, D.S.; Kodo, N.; Norwood, D.L.; Roe, C.R. Tandem mass spectrometry: A new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J. Inherit. Metab. Dis. 1990, 13, 321–324. [Google Scholar] [CrossRef]
- Wagner, M.; Tonoli, D.; Varesio, E.; Hopfgartner, G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom. Rev. 2016, 35, 361–438. [Google Scholar] [CrossRef]
- Vincenzi, G.; Petralia, I.T.; Abbate, M.; Vigone, M.C. 50 years of newborn screening for congenital hypothyroidism: Evolution of insights in etiology, diagnosis and management: Transient or permanent congenital hypothyroidism: From milestones to current and future perspectives. Eur. Thyroid. J. 2025, 14, e250019. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Loftus, E.; Thompson, C.E.; Boyle, F.; McNulty, J.; Boruah, R.; Crushell, E.; Howard, C.; Hughes, J.; Monavari, A.A.; et al. Clinical and Developmental Outcomes After 50 Years of Newborn Bloodspot Screening for Classical Galactosaemia in the Republic of Ireland. JIMD Rep. 2025, 66, e70022. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B.; Wiley, V. Fifty years of newborn screening. J. Paediatr. Child Health 2015, 51, 103–107. [Google Scholar] [CrossRef]
- Groselj, U.; Tansek, M.Z.; Battelino, T. Fifty years of phenylketonuria newborn screening—A great success for many, but what about the rest? Mol. Genet. Metab. 2014, 113, 8–10. [Google Scholar] [CrossRef]
- Cornel, M.C.; Rigter, T.; Weinreich, S.S.; Burgard, P.; Hoffmann, G.F.; Lindner, M.; Loeber, J.G.; Rupp, K.; Taruscio, D.; Vittozzi, L. A framework to start the debate on neonatal screening policies in the EU: An Expert Opinion Document. Eur. J. Hum. Genet. 2014, 22, 12–17. [Google Scholar] [CrossRef]
- Newborn Screening in Europe—Expert Opinion Document. EU Tender “Evaluation of Population Newborn Screening Practices for Rare Disorders in Member States of the European Union”. Accepted in Its Final Form on 28.08.2011. Accessible from: International Society for Neonatal Screening Homepage. Available online: https://www.isns-neoscreening.org/guidelines/ (accessed on 30 September 2025).
- Webster, D.; Gaviglio, A.; Khan, A.H.; Baker, M.; Cheillan, D.; Chabraoui, L.; Abdoh, G.; Cabello, J.; Giugliani, R.; Platis, D.; et al. ISNS General Guidelines for Neonatal Bloodspot Screening 2025. Int. J. Neonatal Screen. 2025, 11, 45. [Google Scholar] [CrossRef]
- Scarpa, M.; Bonham, J.R.; Dionisi-Vici, C.; Prevot, J.; Pergent, M.; Meyts, I.; Mahlaoui, N.; Schielen, P.C.J.I. Newborn screening as a fully integrated system to stimulate equity in neonatal screening in Europe. Lancet Reg. Health Eur. 2022, 13, 100311. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Havass, Z.; Soltysiak, J.; Boda, D. Fenilketonuria szűrővizsgálatok Magyarországon. [Screening for phenylketonuria in Hungary]. Orv. Hetil. 1974, 115, 498–504. [Google Scholar]
- Havass, Z.; Soltysiak, J.; Szűts, P.; László, A.; Gyurkovits, K. Az újszülöttkori tömegszűrővizsgálatok 20 éve Magyarországon. [Twenty years of mass screening of newborns in Hungary]. Gyermekgyógy 1988, 39, 372–378. [Google Scholar]
- Havass, Z.; László, A. A galactosaemia újszülöttkori tömegszűrővizsgálatában szerzett tapasztalataink. [Our experience with mass screening of newborns for galactosemia]. Orv. Hetil. 1980, 121, 1767–1770. [Google Scholar]
- Decree of the Ministry of Health No. 5/1975. (V. 28.) on the Examination of the Metabolic Disorders in Newborns. Available online: https://jogkodex.hu/jsz/1975_5_eum_rendelet_3609826 (accessed on 30 September 2025).
- Károlyi, G.; Andréka, B. First results of a regional neonatal thyroid screening programme in Hungary. Eur. J. Pediatr. 1984, 143, 76. [Google Scholar] [CrossRef] [PubMed]
- Péter, F.; Blatniczky, L.; Kovács, L.; Tar, A. Experience with neonatal screening for congenital hypothyroidism in Hungary. Endocrinol. Exp. 1989, 23, 143–151. [Google Scholar]
- Szűts, P.; Havass, Z. Hipotireozis szűrővizsgálatok hatása a klinikumra. [The effect of the newborn screening for hypothyroidism on the clinical characteristics]. Gyermekgyógy 1988, 39, 302. [Google Scholar]
- Havass, Z. Neonatal screening for biotinidase deficiency in East Hungary. J. Inherit. Metab. Dis. 1991, 14, 928–931. [Google Scholar] [CrossRef] [PubMed]
- László, A.; Schuler, E.A.; Sallay, E.; Endreffy, E.; Somogyi, C.; Várkonyi, A.; Havass, Z.; Jansen, K.P.; Wolf, B. Neonatal screening for biotinidase deficiency in Hungary: Clinical, biochemical and molecular studies. J. Inherit. Metab. Dis. 2003, 26, 693–698. [Google Scholar] [CrossRef]
- Decree of the Ministry of Welfare No. 51/1997. (XII. 18.) on Health Services for the Prevention and Early Detection of Diseases Available Under Compulsory Health Insurance and on the Certification of Screening Tests. Available online: https://njt.hu/jogszabaly/1997-51-20-3D (accessed on 30 September 2025).
- Karg, E.; Wittmann, G.; Túri, S. Anyagcsere betegségek újszülöttkori szűrése tandem tömegspektrometriával. [Newborn screening for metabolic disorders with tandem mass spectrometry]. Gyermekorv. Továbbképz. 2008, 7, 163–166. [Google Scholar]
- Papp, F.; Rózsa, M.; Wittmann, G.; Baráth, Á.; Monostori, P.; Görög, M.; Gellén, B.; Karg, E.; Túri, S. Anyagcsere betegségek újszülöttkori szűrése és klinikai jellemzőik. [Newborn screening and clinical characteristics of metabolic disorders]. Egészségtudomány 2011, 55, 19–37. [Google Scholar]
- Schuler, Á.; Somogyi, C.; Kiss, E.; Milánkovics, I.; Tőrös, A.; Végh, Z.; Újvári, A.; Csókay, B.; Komory, E.; Fodor, F.; et al. Veleszületett anyagcsere-betegségek nyugat-magyarországi újszülöttkori szűrése és gondozása 1988–2006 között a Budai Gyermekkórházban. Gyermekgyógy 2007, 58, 103–107. [Google Scholar]
- Szabó, E.; Balogh, L.; Szabó, A.; Szatmári, I. Ritka örökletes anyagcsere-betegségek diagnosztikája: Laboratóriumi vizsgálati megközelítések. [Diagnostics of inborn errors of metabolism: Laboratory approaches]. Orv. Hetil. 2017, 158, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Cameron, C.A.; Abdenur, J.E.; Abdulrahman, M.; Adair, O.; Al Nuaimi, S.A.; Åhlman, H.; Allen, J.J.; Antonozzi, I.; Archer, S.; et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: A worldwide collaborative project. Genet. Med. 2011, 13, 230–254. [Google Scholar] [CrossRef]
- Marquardt, G.; Currier, R.; McHugh, D.M.; Gavrilov, D.; Magera, M.J.; Matern, D.; Oglesbee, D.; Raymond, K.; Rinaldo, P.; Smith, E.H.; et al. Enhanced interpretation of newborn screening results without analyte cutoff values. Genet. Med. 2012, 14, 648–655. [Google Scholar] [CrossRef]
- Mørkrid, L.; Rowe, A.D.; Elgstoen, K.B.; Olesen, J.H.; Ruijter, G.; Hall, P.L.; Tortorelli, S.; Schulze, A.; Kyriakopoulou, L.; Wamelink, M.M.; et al. Continuous age- and sex-adjusted reference intervals of urinary markers for cerebral creatine deficiency syndromes: A novel approach to the definition of reference intervals. Clin. Chem. 2015, 61, 760–768. [Google Scholar] [CrossRef]
- CLSI. Dried Blood Spot Specimen Collection for Newborn Screening, 7th ed.; CLSI Standard NBS01; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; Available online: https://clsi.org/shop/standards/nbs01 (accessed on 30 September 2025).
- CLSI. Newborn Screening Follow-up and Education, 3rd ed.; CLSI Guideline NBS02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023; Available online: https://clsi.org/shop/standards/nbs02 (accessed on 30 September 2025).
- CLSI. Newborn Screening for Preterm, Low Birth Weight, and Sick Newborns, 2nd ed.; CLSI Guideline NBS03; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; Available online: https://clsi.org/shop/standards/nbs03 (accessed on 30 September 2025).
- CLSI. Newborn Screening by Tandem Mass Spectrometry, 2nd ed.; CLSI Guideline NBS04; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; Available online: https://clsi.org/shop/standards/nbs04 (accessed on 30 September 2025).
- CLSI. Newborn Screening for Congenital Hypothyroidism, 1st ed.; CLSI Guideline NBS10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024; Available online: https://clsi.org/shop/standards/nbs10 (accessed on 30 September 2025).
- Pickens, C.A.; Sternberg, M.; Seeterlin, M.; De Jesús, V.R.; Morrissey, M.; Manning, A.; Bhakta, S.; Held, P.K.; Mei, J.; Cuthbert, C.; et al. Harmonizing Newborn Screening Laboratory Proficiency Test Results Using the CDC NSQAP Reference Materials. Int. J. Neonatal Screen. 2020, 6, 75. [Google Scholar] [CrossRef]
- Mathis, D.; Croft, J.; Chrastina, P.; Fowler, B.; Vianey-Saban, C.; Ruijter, G.J.G. The role of ERNDIM diagnostic proficiency schemes in improving the quality of diagnostic testing for inherited metabolic diseases. J. Inherit. Metab. Dis. 2022, 45, 926–936. [Google Scholar] [CrossRef]
- Decree of the Ministry of Human Resources No. 51/2021. (XII. 10.) on the Amendment of Certain Ministerial Decrees on Health Insurance in Connection with the Introduction of Mandatory Public Health Screening. Available online: https://njt.hu/jogszabaly/2021-51-20-5H (accessed on 30 September 2025).
- Xue, A.; Lénárt, I.; Kincs, J.; Szabó, H.; Párniczky, A.; Balogh, I.; Deák, A.; Monostor, P.B.; Hegedűs, K.; Szabó, A.J.; et al. Neonatal Screening for Cystic Fibrosis in Hungary-First-Year Experiences. Int. J. Neonatal Screen. 2023, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Newborn Screening for Cystic Fibrosis, 2nd ed.; CLSI Guideline NBS05; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; Available online: https://clsi.org/shop/standards/nbs05 (accessed on 30 September 2025).
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Hegedűs, K.; Lénárt, I.; Xue, A.; Monostori, P.B.; Baráth, Á.; Mikos, B.; Udvari, S.; Géresi, A.; Szabó, A.J.; Bereczki, C.; et al. Results of the Hungarian Newborn Screening Pilot Program for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2025, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Newborn Screening for Spinal Muscular Atrophy, 1st ed.; CLSI Guideline NBS13; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025; Available online: https://clsi.org/shop/standards/nbs13 (accessed on 30 September 2025).
- Cooper, K.; Nalbant, G.; Sutton, A.; Harnan, S.; Thokala, P.; Chilcott, J.; McNeill, A.; Bessey, A. Systematic Review of Newborn Screening Programmes for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.K.; Greene, C.; Mercer, K.; Taylor, J.; Yazdanpanah, G.; Vogt, R.; Lee, R.; Cuthbert, C.; Cordovado, S. CDC’s Laboratory Activities to Support Newborn Screening for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 51. [Google Scholar] [CrossRef]
- Yusuf, C.; Sontag, M.K.; Miller, J.; Kellar-Guenther, Y.; McKasson, S.; Shone, S.; Singh, S.; Ojodu, J. Development of National Newborn Screening Quality Indicators in the United States. Int. J. Neonatal Screen. 2019, 5, 34. [Google Scholar] [CrossRef]
- Odenwald, B.; Brockow, I.; Hanauer, M.; Lüders, A.; Nennstiel, U. Is Our Newborn Screening Working Well? A Literature Review of Quality Requirements for Newborn Blood Spot Screening (NBS) Infrastructure and Procedures. Int. J. Neonatal Screen. 2023, 9, 35. [Google Scholar] [CrossRef]
- Ruoppolo, M.; Malvagia, S.; Boenzi, S.; Carducci, C.; Dionisi-Vici, C.; Teofoli, F.; Burlina, A.; Angeloni, A.; Aronica, T.; Bordugo, A.; et al. Expanded Newborn Screening in Italy Using Tandem Mass Spectrometry: Two Years of National Experience. Int. J. Neonatal Screen. 2022, 8, 47. [Google Scholar] [CrossRef]
- Németh, K.; Szatmári, I.; Tőkési, V.; Szabó, P.T. Application of Normal-Phase Silica Column in Hydrophilic Interaction Liquid Chromatography Mode for Simultaneous Determination of Underivatized Amino Acids from Human Serum Samples via Liquid Chromatography-Tandem Mass Spectrometry. Curr. Issues Mol. Biol. 2023, 45, 9354–9367. [Google Scholar] [CrossRef] [PubMed]
- Becsei, D.; Kiss, E.; Szatmári, I.; Arató, A.; Reusz, G.; Szabó, A.J.; Bókay, J.; Zsidegh, P. A retrospective analysis of metabolic control in children with PKU in the COVID-19 era. Mol. Genet. Metab. Rep. 2022, 32, 100897. [Google Scholar] [CrossRef] [PubMed]
- Kósa, M.; Galla, Z.; Lénárt, I.; Baráth, Á.; Grecsó, N.; Rácz, G.; Bereczki, C.; Monostori, P. Vitamin B12 (cobalamin): Its fate from ingestion to metabolism with particular emphasis on diagnostic approaches of acquired neonatal/infantile deficiency detected by newborn screening. Metabolites 2022, 12, 1104. [Google Scholar] [CrossRef]
- Monostori, P.; Godejohann, M.; Janda, J.; Galla, Z.; Rácz, G.; Klinke, G.; Szatmári, I.; Zsidegh, P.; Kohlmüller, D.; Kölker, S.; et al. Identification of potential interferents of methylmalonic acid: A previously unrecognized pitfall in clinical diagnostics and newborn screening. Clin. Biochem. 2023, 111, 72–80. [Google Scholar] [CrossRef]
- Monostori, P.; Szabó, P.; Marginean, O.; Bereczki, C.; Karg, E. Concurrent confirmation and differential diagnosis of congenital adrenal hyperplasia from dried blood spots: Application of a second tier LC MS/MS assay in cross border cooperation for newborn screening. Horm. Res. Paediatr. 2015, 84, 311–318. [Google Scholar] [CrossRef]
- Galla, Z.; Rácz, G.; Grecsó, N.; Baráth, Á.; Kósa, M.; Bereczki, C.; Monostori, P. Improved LC-MS/MS method for the determination of 42 neurologically and metabolically important molecules in urine. J. Chromatogr. B 2021, 1179, 122846. [Google Scholar] [CrossRef] [PubMed]
- Polyák, H.; Galla, Z.; Rajda, C.; Monostori, P.; Klivényi, P.; Vécsei, L. Plasma and Visceral Organ Kynurenine Metabolites Correlate in the Multiple Sclerosis Cuprizone Animal Model. Int. J. Mol. Sci. 2025, 26, 976. [Google Scholar] [CrossRef]
- Sárközy, M.; Watzinger, S.; Kovács, Z.Z.A.; Acar, E.; Márványkövi, F.; Szűcs, G.; Lauber, G.Y.; Galla, Z.; Siska, A.; Földesi, I.; et al. Neuregulin-1β Improves Uremic Cardiomyopathy and Renal Dysfunction in Rats. JACC Basic Transl. Sci. 2023, 8, 1160–1176. [Google Scholar] [CrossRef]
- Watson, M.S.; Mann, M.Y.; Lloyd-Puryear, M.A.; Rinaldo, P.; Howell, R.R. Newborn screening: Toward a uniform screening panel and system. Genet. Med. 2006, 8, 1S–252S. [Google Scholar] [CrossRef]
- Recommended Uniform Screening Panel. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp (accessed on 30 September 2025).
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/EU. Available online: https://eur-lex.europa.eu/eli/reg/2017/746/oj/eng (accessed on 30 September 2025).
- El-Maouche, D.; Arlt, W.; Merke, D.P. Congenital adrenal hyperplasia. Lancet 2017, 390, 2194–2210. [Google Scholar] [CrossRef] [PubMed]
- Nordenström, A.; Falhammar, H.; Lajic, S. Current and Novel Treatment Strategies in Children with Congenital Adrenal Hyperplasia. Horm. Res. Paediatr. 2023, 96, 560–572. [Google Scholar] [CrossRef] [PubMed]
| First-Tier Methods | Primary Analytes | Screened Disorders |
|---|---|---|
| MS/MS AAAC | Ala, Arg, Asp, Cit, Glu, Gly, Leu, Met, Orn, Phe, SuAc, Trp, Tyr, Val, C0, C2, C3, C3DC, C4, C4DC, C4OH, C5, C5:1, C5DC, C5OH, C6, C6DC, C8, C10, C10:1, C10:2, C12, C14, C14:1, C14:2, C16, C16:1OH, C16OH, C18, C18:1, C18OH | 3MCC, ASL, BKT, CIT-I, CPT-I, CPT-II, CUD, GA-I, HCYS, HMG, IVA, LCHAD, MADD, MCAD, MCD, MMACBL, MSUD, PA, PKU, SCAD, TYR-I, TYR-II, VLCAD |
| DELFIA™ | TSH, IRT | CH, CF |
| Enzyme-mediated fluorescence | Total galactose | Galactosemia (GALT and galactokinase deficiency) |
| Colorimetric enzyme activity | Biotinidase activity | BD |
| Real-time PCR | SMN1 exon 7 homozygous deletion | SMA |
| Quality Indicator/Category | Threshold/Goal |
|---|---|
| Unsatisfactory specimens | <2% |
| Missing essential information (data fields) | <0.5% (approx. 0% using the LIMS in the Szeged region). |
| Unscreened newborns | Very low, close to 0% (opt-out strategy). |
| Lost to follow-up (no final resolution) | <1% |
| Timeliness (collection) | Main rule: 48–72h, automatically repeated in certain cases (transfusion, preterm newborn, etc.) (fulfilled in >98% of cases). |
| Timeliness (transport) | Next weekday with priority (fulfilled in >95% of cases). |
| Timeliness (reporting) | Within 1 week (fulfilled in >95% of cases). |
| Repeat testing | Typically <1%. |
| Proficiency testing | Acceptance rate was 100% in the last ten years. |
| Missed cases | Six false negatives reported in CF, none reported for other disorders so far (excluding late-onset forms). |
| Parental refusals | Very low, close to 0% (opt-out strategy). |
| Positive predictive value (PPV) | Disorder-specific: from <15% (CF, under revision) to 100% (SMA). |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3MCC (1) | 17 | 7 | 4 | 4 | 8 | 5 | 7 | 5 | 11 | 4 | 72 |
| ASL | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| BKT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CIT-I | 0 | 0 | 0 | 2 | 1 | 0 | 4 | 0 | 0 | 0 | 7 |
| CPT-I | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 4 |
| CPT-II | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CUD (1) | 1 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 10 |
| GA-I | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| HCYS | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 5 |
| HMG | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| IVA | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| LCHAD | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 4 |
| MADD | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| MCAD | 5 | 5 | 5 | 4 | 7 | 4 | 10 | 8 | 8 | 12 | 68 |
| MCD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MMACBL (2) | 1 | 1 | 0 | 2 | 1 | 1 | 5 | 11 (3) | 16 (3) | 4 | 42 |
| MSUD | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 4 |
| PA | 1 | 0 | 1 | 0 | 3 | 0 | 3 | 1 | 0 | 1 | 10 |
| PKU (4) | 15 | 16 | 15 | 14 | 18 | 17 | 14 | 16 | 15 | 17 | 157 |
| SCAD | 17 | 6 | 5 | 2 | 4 | 2 | 11 | 11 | 2 | 3 | 63 |
| TYR-I | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 6 |
| TYR-II | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VLCAD | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 7 |
| BD | 6 | 5 | 2 | 4 | 7 | 8 | 0 | 1 | 4 | 5 | 42 |
| CF (5) | – | – | – | – | – | – | – | 17 | 26 | 16 | 59 (5) |
| CH | 33 | 33 | 32 | 27 | 52 | 37 | 31 | 25 | 23 | 29 | 322 |
| GAL (6) | 8 | 12 | 16 | 9 | 8 | 13 | 27 | 16 | 17 | 10 | 136 |
| SMA (7) | – | – | – | – | – | – | – | 0 | 9 | 10 | 19 (7) |
| Total patients | 107 | 91 | 87 | 70 | 116 | 92 | 119 | 112 | 138 | 115 | 1,047 |
| Total births | 91,690 | 93,063 | 91,577 | 89,807 | 89,193 | 92,338 | 93,039 | 88,491 | 88,225 | 77,500 | 891,923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monostori, P.; Szatmári, I.; Baráth, Á.; Bókay, J.; Csenki, M.; Galla, Z.; Gellén, B.; Grecsó, N.; Gyüre, E.; Halász, Z.; et al. Celebrating 50 Years of Nationwide Newborn Screening in Hungary—Review, Current Situation, and Future Directions. Int. J. Neonatal Screen. 2025, 11, 99. https://doi.org/10.3390/ijns11040099
Monostori P, Szatmári I, Baráth Á, Bókay J, Csenki M, Galla Z, Gellén B, Grecsó N, Gyüre E, Halász Z, et al. Celebrating 50 Years of Nationwide Newborn Screening in Hungary—Review, Current Situation, and Future Directions. International Journal of Neonatal Screening. 2025; 11(4):99. https://doi.org/10.3390/ijns11040099
Chicago/Turabian StyleMonostori, Péter, Ildikó Szatmári, Ákos Baráth, János Bókay, Marianna Csenki, Zsolt Galla, Balázs Gellén, Nóra Grecsó, Eszter Gyüre, Zita Halász, and et al. 2025. "Celebrating 50 Years of Nationwide Newborn Screening in Hungary—Review, Current Situation, and Future Directions" International Journal of Neonatal Screening 11, no. 4: 99. https://doi.org/10.3390/ijns11040099
APA StyleMonostori, P., Szatmári, I., Baráth, Á., Bókay, J., Csenki, M., Galla, Z., Gellén, B., Grecsó, N., Gyüre, E., Halász, Z., Hegedűs, K., Kincs, J., Kiss, E., Kósa, M., Lénárt, I., Pálmay, A., Rácz, G., Szabó, H., Szabó, L., ... Bereczki, C. (2025). Celebrating 50 Years of Nationwide Newborn Screening in Hungary—Review, Current Situation, and Future Directions. International Journal of Neonatal Screening, 11(4), 99. https://doi.org/10.3390/ijns11040099






