Umbilical Cord Blood Sampling for Newborn Screening of Pompe Disease and the Detection of a Novel Pathogenic Variant and Pseudodeficiency Variants in an Asian Population
Abstract
1. Introduction
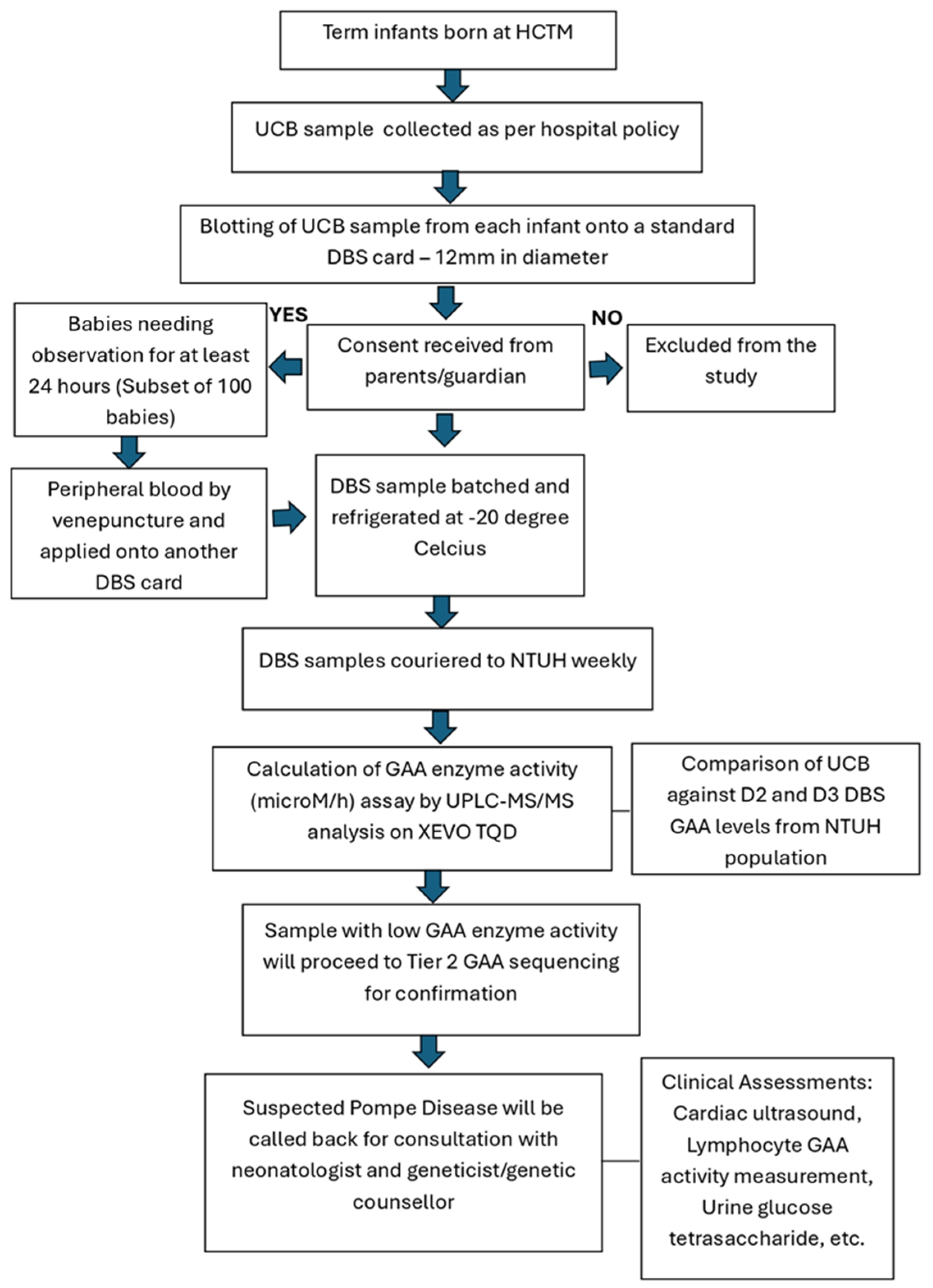
2. Materials and Methods
2.1. Data Collection
2.2. UCB Versus Peripheral Blood Sampling for GAA Levels
2.3. UCB Storage Time on GAA Levels
2.4. Laboratory Assay of DBS for GAA Levels
2.5. UPLC-MS/MS Analysis
2.6. GAA Gene Sequencing
2.7. Statistical Analyses
3. Results
3.1. Descriptive Analyses of UCB GAA Levels
3.2. Demographic Factors Associated with UCB GAA Levels
3.3. UCB and Peripheral Venous Blood GAA Levels
3.4. Comparison of GAA Levels in UCB with Heel-Prick Blood Samples Taken at Day 2 and Day 3
3.5. UCB Sample Storage and DBS Overnight Drying on GAA Levels
3.6. Detection of a Novel Pathogenic Variant and Multiple Pseudodeficiency Alleles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GAA | Acid Alpha-Glucosidase |
| IOPD | Infantile-Onset Pompe Disease |
| ERT | Enzyme Replacement Therapy |
| NBS | Newborn Screening |
| DBS | Dried Blood Spots |
| UCB | Umbilical Cord Blood |
| ICC | Intraclass Correlation Coefficient |
| NTUH | National Taiwan University Hospital |
| LSD | Lysosomal Storage Disease |
| HGMD | Human Gene Mutation Database |
| HCTM | Hospital Canselor Tuanku Muhriz |
| LBW | Low Birth Weight |
| VLBW | Very Low Birth Weight |
| ELBW | Extremely Low Birth Weight |
| TOST | Two-One-Sided-Test |
| LOPD | Late Onset Pompe Disease |
References
- Guo, J.; Kelton, C.M.; Guo, J.J. Recent developments, utilization, and spending trends for Pompe disease therapies. Am. Health Drug Benefits 2012, 5, 182–189. [Google Scholar]
- van der Ploeg, A.T.; Reuser, A.J. Pompe’s disease. Lancet 2008, 372, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Roberts, A.; Plotz, P.H.; Raben, N. Acid alpha-glucosidase deficiency (Pompe disease). Curr. Neurol. Neurosci. Rep. 2007, 7, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Milani-Nejad, S.; Mozaffar, T. Pompe disease: A clinical, diagnostic, and therapeutic overview. Curr. Treat. Options Neurol. 2022, 24, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Steiner, R.D.; Bali, D.; Berger, K.; Byrne, B.J.; Case, L.E.; Crowley, J.F.; Downs, S.; Howell, R.R.; Kravitz, R.M. Pompe disease diagnosis and management guideline. Genet. Med. 2006, 8, 267–288. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Chiang, S.-C.; Zhang, X.K.; Keutzer, J.; Lee, N.-C.; Huang, A.-C.; Chen, C.-A.; Wu, M.-H.; Huang, P.-H.; Tsai, F.-J. Early detection of Pompe disease by newborn screening is feasible: Results from the Taiwan screening program. Pediatrics 2008, 122, e39–e45. [Google Scholar] [CrossRef]
- Taverna, S.; Cammarata, G.; Colomba, P.; Sciarrino, S.; Zizzo, C.; Francofonte, D.; Zora, M.; Scalia, S.; Brando, C.; Curto, A.L. Pompe disease: Pathogenesis, molecular genetics and diagnosis. Aging 2020, 12, 15856–15874. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Hwu, W.-L.; Lee, N.-C. Pompe disease: Early diagnosis and early treatment make a difference. Pediatr. Neonatol. 2013, 54, 219–227. [Google Scholar] [CrossRef]
- Chiang, S.-C.; Chien, Y.-H.; Chang, K.-L.; Lee, N.-C.; Hwu, W.-L. The Timely Needs for Infantile Onset Pompe Disease Newborn Screening—Practice in Taiwan. Int. J. Neonatal Screen. 2020, 6, 30. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Young, S.P.; Changela, M.; Dickerson, G.H.; Zhang, H.; Dai, J.; Peterson, D.; Millington, D.S.; Kishnani, P.S.; Bali, D.S. Screening for Pompe disease using a rapid dried blood spot method: Experience of a clinical diagnostic laboratory. Muscle Nerve 2009, 40, 32–36. [Google Scholar] [CrossRef]
- Leong, Y.H.; Gan, C.Y.; Tan, M.A.F.; Majid, M.I.A. Present status and future concerns of expanded newborn screening in malaysia: Sustainability, challenges and perspectives. Malays. J. Med. Sci. 2014, 21, 63–67. [Google Scholar] [PubMed]
- Yunus, Z.M.; Rahman, S.A.; Choy, Y.S.; Keng, W.T.; Ngu, L.H. Pilot study of newborn screening of inborn error of metabolism using tandem mass spectrometry in Malaysia: Outcome and challenges. J. Pediatr. Endocrinol. Metab. 2016, 29, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Umapathysivam, K.; Whittle, A.M.; Ranieri, E.; Bindloss, C.; Ravenscroft, E.M.; van Diggelen, O.P.; Hopwood, J.J.; Meikle, P.J. Determination of acid α-glucosidase protein: Evaluation as a screening marker for Pompe disease and other lysosomal storage disorders. Clin. Chem. 2000, 46, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat. Med. 2012, 31, 3972–3981. [Google Scholar] [CrossRef]
- Kwak, S.G.; Kim, J.H. Central limit theorem: The cornerstone of modern statistics. Korean J. Anesthesiol. 2017, 70, 144–156. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Lee, N.-C.; Chen, P.-W.; Yeh, H.-Y.; Gelb, M.H.; Chiu, P.-C.; Chu, S.-Y.; Lee, C.-H.; Lee, A.-R.; Hwu, W.-L. Newborn screening for Morquio disease and other lysosomal storage diseases: Results from the 8-plex assay for 70,000 newborns. Orphanet J. Rare Dis. 2020, 15, 38. [Google Scholar] [CrossRef]
- Chiang, S.-C.; Chen, P.-W.; Hwu, W.-L.; Lee, A.-J.; Chen, L.-C.; Lee, N.-C.; Chiou, L.-Y.; Chien, Y.-H. Performance of the four-plex tandem mass spectrometry lysosomal storage disease newborn screening test: The necessity of adding a 2nd tier test for Pompe disease. Int. J. Neonatal Screen. 2018, 4, 41. [Google Scholar] [CrossRef]
- Dixon, P.M.; Saint-Maurice, P.F.; Kim, Y.; Hibbing, P.; Bai, Y.; Welk, G.J. A primer on the use of equivalence testing for evaluating measurement agreement. Med. Sci. Sports Exerc. 2018, 50, 837–845. [Google Scholar] [CrossRef]
- Caldwell, A.R. Exploring equivalence testing with the updated TOSTER R package. PsyArXiv 2022. [Google Scholar] [CrossRef]
- Lakens, D.; Scheel, A.M.; Isager, P.M. Equivalence testing for psychological research: A tutorial. Adv. Methods Pract. Psychol. Sci. 2018, 1, 259–269. [Google Scholar] [CrossRef]
- Burlina, A.B.; Polo, G.; Salviati, L.; Duro, G.; Zizzo, C.; Dardis, A.; Bembi, B.; Cazzorla, C.; Rubert, L.; Zordan, R. Newborn screening for lysosomal storage disorders by tandem mass spectrometry in North East Italy. J. Inherit. Metab. Dis. 2018, 41, 209–219. [Google Scholar] [CrossRef]
- Cheah, F.C.; Syed Omar, S.A.; Ng, B.K.; Wan Md Zin, W.N.; Lee, J. (Universiti Kebangsaan Malaysia, Bangi, Malaysia). Unpublished work. 2025.
- Labrousse, P.; Chien, Y.-H.; Pomponio, R.J.; Keutzer, J.; Lee, N.-C.; Akmaev, V.R.; Scholl, T.; Hwu, W.-L. Genetic heterozygosity and pseudodeficiency in the Pompe disease newborn screening pilot program. Mol. Genet. Metab. 2010, 99, 379–383. [Google Scholar] [CrossRef]
- Lim, J.-A.; Li, L.; Raben, N. Pompe disease: From pathophysiology to therapy and back again. Front. Aging Neurosci. 2014, 6, 177. [Google Scholar] [CrossRef]
- Marques, J.S. The clinical management of pompe disease: A pediatric perspective. Children 2022, 9, 1404. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Scriver, C.R.; Beaudet, A.L.; Sly, W.S.; Valle, D.; Childs, B.; Kinzler, K.W.; Vogelstein, B. The Metabolic and Molecular Bases of Inherited Diseases, 8th ed.; McGraw-Hill Professional: New York, NY, USA, 2000. [Google Scholar]
- Sawada, T.; Kido, J.; Nakamura, K. Newborn screening for Pompe disease. Int. J. Neonatal Screen. 2020, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Chamoles, N.A.; Niizawa, G.; Blanco, M.; Gaggioli, D.; Casentini, C. Glycogen storage disease type II: Enzymatic screening in dried blood spots on filter paper. Clin. Chim. Acta 2004, 347, 97–102. [Google Scholar] [CrossRef]
- AlSaif, S.; Ponferrada, M.B.; AlKhairy, K.; AlTawil, K.; Sallam, A.; Ahmed, I.; Khawaji, M.; AlHathlol, K.; Baylon, B.; AlSuhaibani, A. Screening for glucose-6-phosphate dehydrogenase deficiency in neonates: A comparison between cord and peripheral blood samples. BMC Pediatr. 2017, 17, 159. [Google Scholar] [CrossRef]
- Bhatia, R.; Rajwaniya, D. Congenital hypothyroidism screening in term neonates using umbilical cord blood TSH values. Indian J. Endocrinol. Metab. 2018, 22, 277–279. [Google Scholar] [CrossRef]
- Hussain, S.; Taib, M.N.A.; Zainol, A.R. Audit of Newborn Screening Programme for Congenital Hypothyroidism. Int. Med. J. 2013, 20, 633–634. [Google Scholar]
- Wolff, F.; Cotton, F.; Gulbis, B. Screening for haemoglobinopathies on cord blood: Laboratory and clinical experience. J. Med. Screen. 2012, 19, 116–122. [Google Scholar] [CrossRef]
- Singh, K.C.; Dhillon, P.; Thulaseedharan, T. A retrospective study on newborn screening for metabolic disorders. Bioinformation 2022, 18, 1122–1125. [Google Scholar] [CrossRef]
- Gulbis, B.; Tshilolo, L.; Cotton, F.; Lin, C.; Vertongen, F. Newborn screening for haemoglobinopathies: The Brussels experience. J. Med. Screen. 1999, 6, 11–15. [Google Scholar] [CrossRef]
- Al Juraibah, F.; Alothaim, A.; Al Eyaid, W.; AlMutair, A.N. Cord blood versus heel-stick sampling for measuring thyroid stimulating hormone for newborn screening of congenital hypothyroidism. Ann. Saudi Med. 2019, 39, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Alameer, S.; Althobaiti, E.; Alshaikh, S.; Turjoman, M.; Badriq, F.; AlSofyani, A.; Mujalled, M.; Borai, A. Diagnostic comparison between cord blood and filter paper for the screening of congenital hypothyroidism. J. Clin. Lab. Anal. 2022, 36, e24149. [Google Scholar] [CrossRef] [PubMed]
- Momosaki, K.; Kido, J.; Yoshida, S.; Sugawara, K.; Miyamoto, T.; Inoue, T.; Okumiya, T.; Matsumoto, S.; Endo, F.; Hirose, S. Newborn screening for Pompe disease in Japan: Report and literature review of mutations in the GAA gene in Japanese and Asian patients. J. Hum. Genet. 2019, 64, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Vermette, L.; Washburn, J.; Klug, T. Analysis of the effect of demographic variables on lysosomal enzyme activities in the Missouri Newborn Screening Program. Int. J. Neonatal Screen. 2025, 11, 48. [Google Scholar] [CrossRef]
- Schmutz, N.; Henry, E.; Jopling, J.; Christensen, R. Expected ranges for blood neutrophil concentrations of neonates: The Manroe and Mouzinho charts revisited. J. Perinatol. 2008, 28, 275–281. [Google Scholar] [CrossRef]
- Goomber, S.; Huggins, E.; Rehder, C.W.; Cohen, J.L.; Bali, D.S.; Kishnani, P.S. Development of a clinically validated in vitro functional assay to assess pathogenicity of novel GAA variants in patients with Pompe disease identified via newborn screening. Front. Genet. 2022, 13, 1001154. [Google Scholar] [CrossRef]
- Chan, M.-Y.; Jalil, J.A.; Yakob, Y.; Wahab, S.A.A.; Ali, E.Z.; Khalid, M.K.N.M.; Leong, H.-Y.; Chew, H.-B.; Sivabalakrishnan, J.B.; Ngu, L.-H. Genotype, phenotype and treatment outcomes of 17 Malaysian patients with infantile-onset Pompe disease and the identification of 3 novel GAA variants. Orphanet J. Rare Dis. 2023, 18, 231. [Google Scholar] [CrossRef]

| Cord Blood GAA (μM/h) | |||
|---|---|---|---|
| Dataset A (Total Original) | Dataset B (Excluded Samples of Suspect Quality) | Dataset C (Excluded Outlier Values) | |
| n | 4091 | 3987 | 3980 |
| Mean (SD) | 10.04 (5.95) | 10.08 (5.98) | 9.96 (4.05) |
| Median (IQR) | 9.43 (5.25) | 9.47 (5.21) | 9.46 (5.20) |
| Skewness (SE) | 19.50 (0.04) | 19.66 (0.04) | 0.76 (0.04) |
| Kurtosis (SE) | 747.50 (0.08) | 750.30 (0.08) | 0.81 (0.08) |
| Values | Dataset A (n = 4091) | Dataset B (n = 3987) | Dataset C (n = 3980) |
|---|---|---|---|
| Burlina et al. classification: | |||
| -Normal | >2.36 | >2.37 | >2.37 |
| -Borderline risk | 1.89–2.36 | 1.89–2.37 | 1.89–2.37 |
| -High risk | <1.89 | <1.89 | <1.89 |
| NTUH classification: | |||
| -High possibility of Pompe disease | ≤1.54 | ≤1.54 | ≤1.54 |
| n | Mean GAA, μM/h (SD) | p-Value | ||
|---|---|---|---|---|
| Dataset A (complete paired samples) | Cord Blood | 103 | 8.62 (4.93) ^ | <0.001 |
| Peripheral Blood | 103 | 13.21 (9.56) ^ | ||
| Dataset B (excluded poor quality samples) | Cord Blood | 97 | 8.69 (4.81) ^ | <0.001 |
| Peripheral Blood | 97 | 13.23 (9.46) ^ | ||
| Dataset C (excluded outlier values) | Cord Blood | 96 | 9.37 (3.97) | <0.001 |
| Peripheral Blood | 96 | 13.79 (6.10) |
| Subgroups | Time (h) | n | GAA Mean (SD) [μM/h] | p-Value * |
|---|---|---|---|---|
| Samples kept at 4 °C | ||||
| 0-h vs. 24-h | 0 24 | 30 30 | 9.89 (3.16) 10.05 (3.03) | 0.563 |
| 0-h vs. 48-h | 0 48 | 30 30 | 11.32 (5.23) 11.26 (5.25) | 0.804 |
| 0-h vs. 72-h | 0 72 | 30 30 | 8.05 (4.20) ^ 9.46 (5.30) ^ | 0.064 ^^ |
| 0-h vs. 96-h | 0 96 | 30 30 | 9.22 (4.21) ^ 10.05 (4.55) ^ | 0.035 ^^ |
| 48-h vs. 96-h | 48 96 | 30 30 | 9.83 (5.35) ^ 9.01 (5.21) ^ | 0.006 ^^ |
| Drying of DBS | ||||
| <6-h vs. 24-h | <6-h 24-h | 30 30 | 9.88 (3.15) 9.69 (3.17) | 0.282 |
| No | ID | UCB GAA (μM/h) | GAA Pseudodeficiency Alleles * |
|---|---|---|---|
| 1 | UKM-3006 | 0.85 | c.1726G>A (p.Gly576Ser) Hom. c.2065G>A (p.Glu689Lys) Hom. |
| 2 | UKM-3108 | 1.47 | c.841C>T(p.Arg281Trp) Het. c.1726G>A (p.Gly576Ser) Het. c.2065G>A (p.Glu689Lys) Het. |
| 3 | UKM-0413 | 1.54 | c.1987C>T (p.Gln663Ter) Het. c.1726G>A (p.Gly576Ser) Het. c.2065G>A (p.Glu689Lys) Hom. |
| 4 | UKM-3917 | 1.55 | c.1726G>A (p.Gly576Ser) Het. c.2065G>A (p.Glu689Lys) Het. |
| 5 | UKM-0154 | 1.57 | c.1062C>G (p.Tyr354Ter) Het. c.1726G>A (p.Gly576Ser) Het. c.2065G>A (p.Glu689Lys) Het. |
| 6 | UKM-3649 | 1.62 | c.1726G>A (p.Gly576Ser) Het. c.2065G>A (p.Glu689Lys) Het. |
| 7 | UKM-3383 | 1.68 | c.1726G>A (p.Gly576Ser) Het. c.2065G>A (p.Glu689Lys) Het. |
| 8 | UKM-2219 | 1.74 | c.913G>A(p.Gly305Arg) Het. c.1726G>A (p.Gly576Ser) Het. c.2065G>A (p.Glu689Lys) Het. |
| 9 | UKM-1334 | 1.83 | c.1726G>A (p.Gly576Ser) Hom. c.2065G>A (p.Glu689Lys) Hom. |
| 10 | UKM-2501 | 1.89 | c.1726G>A (p.Gly576Ser) Hom. c.2065G>A (p.Glu689Lys) Hom. |
| 11 | UKM-0812 | 1.91 | c.1726G>A (p.Gly576Ser) Het. c.2065G>A (p.Glu689Lys) Het. |
| 12 | UKM-2791 | 2.00 | c.1726G>A (p.Gly576Ser) Hom. c.2065G>A (p.Glu689Lys) Hom. |
| No | ID | GAA Level (μM/h) | GAA (Likely) Pathogenic Alleles * | Interpretation |
|---|---|---|---|---|
| 1 | UKM-2617 | 0.38 (from UCB) 0.53 (Repeat, from venous blood sample) | c.1123C>T (p.Arg375Cys) Het. c.2005_2010del (p.Pro669_Phe670del) Het. | Pompe disease |
| 2 | Father | 2.50 | c.1123C>T (p.Arg375Cys) Het. | Pompe disease carrier |
| 3 | Mother | 2.90 | c.2005_2010del (p.Pro669_Phe670del) Het. | Pompe disease carrier |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheah, F.-C.; Syed Omar, S.A.; Lee, J.; Ang, Z.J.; Gopal, A.R.; Wan Md Zin, W.N.; Ng, B.K.; Chiang, S.-C.; Chien, Y.-H. Umbilical Cord Blood Sampling for Newborn Screening of Pompe Disease and the Detection of a Novel Pathogenic Variant and Pseudodeficiency Variants in an Asian Population. Int. J. Neonatal Screen. 2025, 11, 74. https://doi.org/10.3390/ijns11030074
Cheah F-C, Syed Omar SA, Lee J, Ang ZJ, Gopal AR, Wan Md Zin WN, Ng BK, Chiang S-C, Chien Y-H. Umbilical Cord Blood Sampling for Newborn Screening of Pompe Disease and the Detection of a Novel Pathogenic Variant and Pseudodeficiency Variants in an Asian Population. International Journal of Neonatal Screening. 2025; 11(3):74. https://doi.org/10.3390/ijns11030074
Chicago/Turabian StyleCheah, Fook-Choe, Sharifah Azween Syed Omar, Jasmine Lee, Zheng Jiet Ang, Anu Ratha Gopal, Wan Nurulhuda Wan Md Zin, Beng Kwang Ng, Shu-Chuan Chiang, and Yin-Hsiu Chien. 2025. "Umbilical Cord Blood Sampling for Newborn Screening of Pompe Disease and the Detection of a Novel Pathogenic Variant and Pseudodeficiency Variants in an Asian Population" International Journal of Neonatal Screening 11, no. 3: 74. https://doi.org/10.3390/ijns11030074
APA StyleCheah, F.-C., Syed Omar, S. A., Lee, J., Ang, Z. J., Gopal, A. R., Wan Md Zin, W. N., Ng, B. K., Chiang, S.-C., & Chien, Y.-H. (2025). Umbilical Cord Blood Sampling for Newborn Screening of Pompe Disease and the Detection of a Novel Pathogenic Variant and Pseudodeficiency Variants in an Asian Population. International Journal of Neonatal Screening, 11(3), 74. https://doi.org/10.3390/ijns11030074






