Seasonal Fluctuations and Stability of Adenosine in Dried Blood Spots for Neonatal Screening
Abstract
1. Introduction
2. Materials and Methods
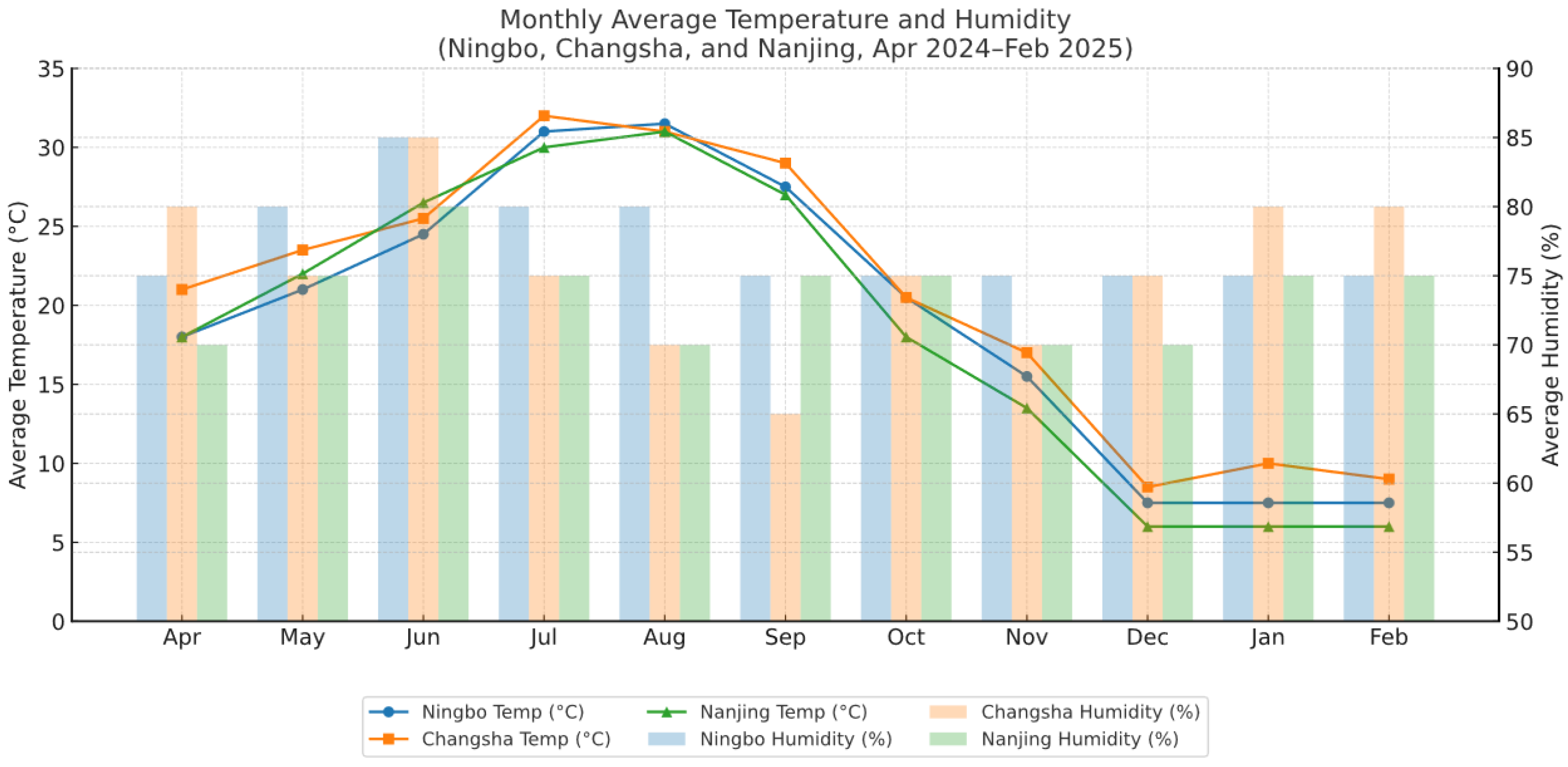
2.1. Monthly Variations in ADO Levels in Neonatal Screening
2.2. Short-Term Stability Studies of ADO in DBSs
2.3. Statistical Analyses
2.4. Ethics
3. Results
3.1. Analysis of ADO Quality Control in Neonatal Screening
3.2. Assessment of Monthly Changes in ADO Levels in Neonatal Screening
3.3. Analysis of Positive Cases and Positivity Rates Under Different ADO Reference Intervals
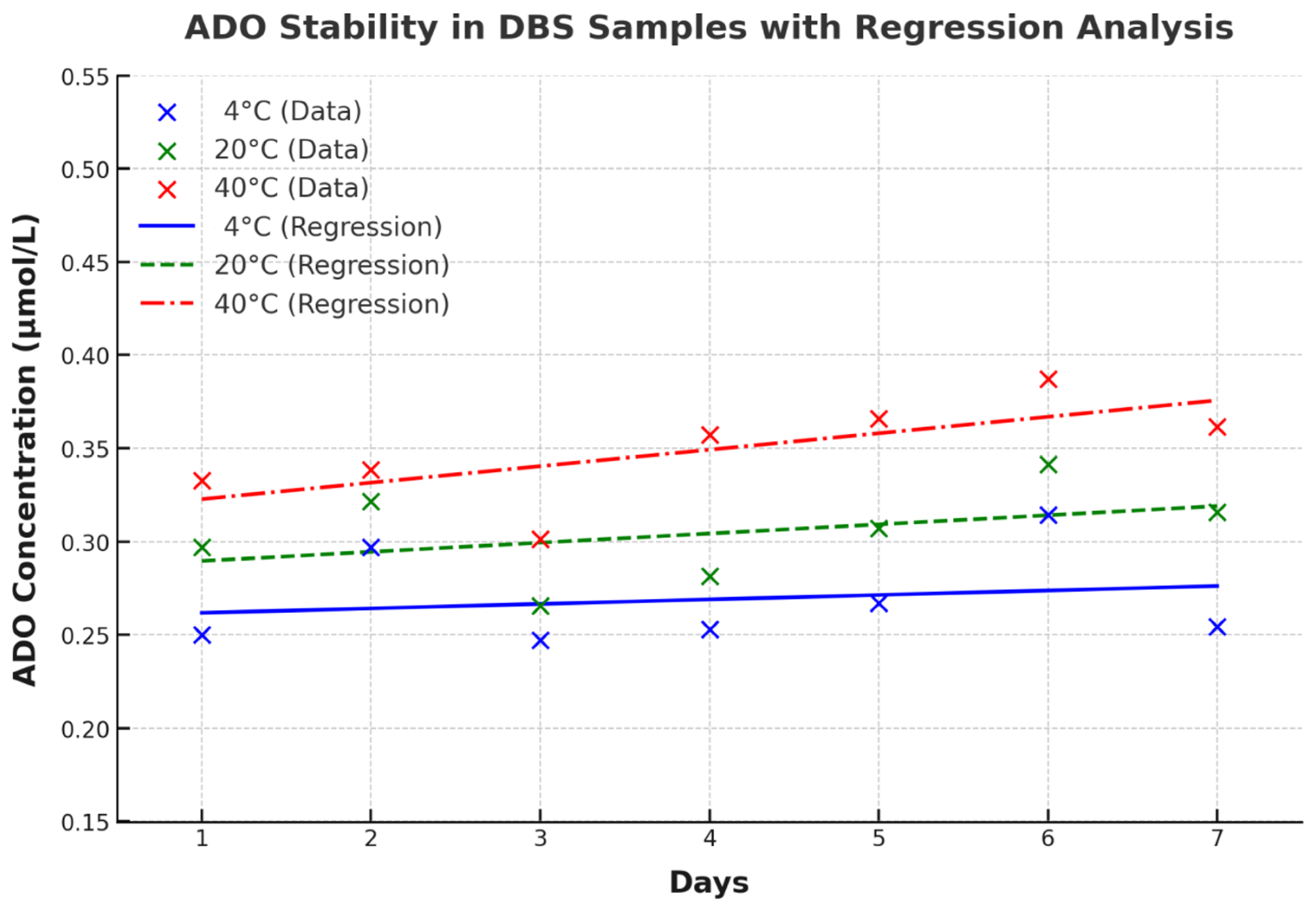
3.4. Short-Term Stability of ADO in DBS with Different Storage Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IMDs | Inherited metabolic disorders |
| ADO | Adenosine |
| DBS | Dried blood spot |
| MS/MS | Tandem mass spectrometry |
| ADA | Adenosine deaminase |
| SCID | Severe combined immunodeficiency |
| TREC | T-cell Receptor Excision Circle |
| QC | Quality control |
| CV | Coefficient of variation |
References
- Tang, C.; Tan, M.; Xie, T.; Tang, F.; Liu, S.; Wei, Q.; Liu, J.; Huang, Y. Screening for neonatal inherited metabolic disorders by tandem mass spectrometry in Guangzhou. Zhejiang Da Xue Xue Bao Yi Xue Ban 2021, 50, 463–471. [Google Scholar] [CrossRef]
- Ruoppolo, M.; Malvagia, S.; Boenzi, S.; Carducci, C.; Dionisi-Vici, C.; Teofoli, F.; Burlina, A.; Angeloni, A.; Aronica, T.; Bordugo, A.; et al. Expanded Newborn Screening in Italy Using Tandem Mass Spectrometry: Two Years of National Experience. Int. J. Neonatal Screen. 2022, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Kononets, V.; Zharmakhanova, G.; Balmagambetova, S.; Syrlybayeva, L.; Berdesheva, G.; Zhussupova, Z.; Tautanova, A.; Kurmambayev, Y. Tandem mass spectrometry in screening for inborn errors of metabolism: Comprehensive bibliometric analysis. Front. Pediatr. 2025, 13, 1463294. [Google Scholar] [CrossRef]
- Ma, S.; Guo, Q.; Zhang, Z.; He, Z.; Yue, A.; Song, Z.; Zhao, Q.; Wang, X.; Sun, R. Expanded newborn screening for inborn errors of metabolism by tandem mass spectrometry in newborns from Xinxiang city in China. J. Clin. Lab. Anal. 2020, 34, e23159. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Qiu, Y.; Zhang, C. Expanded newborn screening for inherited metabolic disorders by tandem mass spectrometry in a northern Chinese population. Front. Genet. 2022, 13, 801447. [Google Scholar] [CrossRef]
- Lee, B.; Heo, W.Y.; Kim, J.A.; Lee, H.S.; Hwang, N.; Park, H.D.; Sung, S.I.; Chang, Y.S.; Park, W.S.; Lee, S.Y. Comprehensive Evaluation of the NeoBase 2 Non-derivatized MSMS Assay and Exploration of Analytes with Significantly Different Concentrations Between Term and Preterm Neonates. Ann. Lab. Med. 2023, 43, 153–166. [Google Scholar] [CrossRef]
- Wan, Z.; Liu, W.; Zhai, Y.; Ma, Z.; Cao, Z. Performance Validation of the NeoBase 2 Non-Derivatized MSMS Assay Kit and Cutoff Values Establishment of Term and Preterm Neonates. Fetal Pediatr. Pathol. 2024, 43, 366–375. [Google Scholar] [CrossRef]
- Aranda, C.S.; Gouveia-Pereira, M.P.; da Silva, C.J.M.; Rizzo, M.; Ishizuka, E.; de Oliveira, E.B.; Condino-Neto, A. Severe combined immunodeficiency diagnosis and genetic defects. Immunol. Rev. 2024, 322, 138–147. [Google Scholar] [CrossRef]
- Flinn, A.M.; Gennery, A.R. Adenosine deaminase deficiency: A review. Orphanet J. Rare Dis. 2018, 13, 65. [Google Scholar] [CrossRef]
- Hershfield, M.; Tarrant, T. Adenosine Deaminase Deficiency. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1483/ (accessed on 9 May 2025).
- Sauer, A.V.; Brigida, I.; Carriglio, N.; Aiuti, A. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front. Immunol. 2012, 3, 265. [Google Scholar] [CrossRef]
- Yan, L.; Sun, X.; Lou, B.; Zhang, Y.; Zhuang, D.; Jia, J.; Zhang, L.; He, Y.; Xu, L.; Wu, S.; et al. Carrier frequency and incidence estimation of deficiency of adenosine deaminase 2 in the Chinese population based on massive exome sequencing data. Clin. Immunol. 2024, 269, 110394. [Google Scholar] [CrossRef]
- van der Spek, J.; Groenwold, R.H.; van der Burg, M.; van Montfrans, J.M. TREC Based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review. J. Clin. Immunol. 2015, 35, 416–430. [Google Scholar] [CrossRef]
- Shinwari, K.; Bolkov, M.; Tuzankina, I.A.; Chereshnev, V.A. Newborn Screening through TREC, TREC/KREC System for Primary Immunodeficiency with limitation of TREC/KREC. Comprehensive Review. Antiinflamm Antiallergy Agents Med. Chem. 2021, 20, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Hartog, N.; Hershfield, M.; Michniacki, T.; Moloney, S.; Holsworth, A.; Hurden, I.; Fredrickson, M.; Kleyn, M.; Walkovich, K.; Secord, E. Newborn tandem mass spectroscopy screening for adenosine deaminase deficiency. Ann. Allergy Asthma Immunol. 2022, 129, 776–783.e772. [Google Scholar] [CrossRef] [PubMed]
- la Marca, G.; Giocaliere, E.; Malvagia, S.; Funghini, S.; Ombrone, D.; Della Bona, M.L.; Canessa, C.; Lippi, F.; Romano, F.; Guerrini, R.; et al. The inclusion of ADA-SCID in expanded newborn screening by tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 88, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hu, Z.; Yang, J.; Zhang, Y.; Shi, Y.; Zhu, S.; Yang, R.; Huang, X. Effects of delivery and storage conditions on concentrations of amino acids and carnitines in neonatal dried blood spots. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020, 49, 565–573. [Google Scholar] [CrossRef]
- Shimada, Y.; Kawano, N.; Goto, M.; Watanabe, H.; Ihara, K. Stability of amino acids, free and acyl-carnitine in stored dried blood spots. Pediatr. Int. 2022, 64, e15072. [Google Scholar] [CrossRef]
- Dijkstra, A.M.; de Blaauw, P.; van Rijt, W.J.; Renting, H.; Maatman, R.; van Spronsen, F.J.; Maase, R.E.; Schielen, P.; Derks, T.G.J.; Heiner-Fokkema, M.R. Important Lessons on Long-Term Stability of Amino Acids in Stored Dried Blood Spots. Int. J. Neonatal Screen. 2023, 9, 34. [Google Scholar] [CrossRef]
- van Rijt, W.J.; Schielen, P.; Özer, Y.; Bijsterveld, K.; van der Sluijs, F.H.; Derks, T.G.J.; Heiner-Fokkema, M.R. Instability of Acylcarnitines in Stored Dried Blood Spots: The Impact on Retrospective Analysis of Biomarkers for Inborn Errors of Metabolism. Int. J. Neonatal Screen. 2020, 6, 83. [Google Scholar] [CrossRef]
- Millington, D.S. How mass spectrometry revolutionized newborn screening. J. Mass. Spectrom. Adv. Clin. Lab. 2024, 32, 1–10. [Google Scholar] [CrossRef]
- Mak, C.M.; Lee, H.C.; Chan, A.Y.; Lam, C.W. Inborn errors of metabolism and expanded newborn screening: Review and update. Crit. Rev. Clin. Lab. Sci. 2013, 50, 142–162. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, R.; Huang, X.; Tian, Y.; Pei, X.; Bohn, M.K.; Zou, L.; Wang, Y.; Li, H.; Wang, T.; et al. Reference Standards for Newborn Screening of Metabolic Disorders by Tandem Mass Spectrometry: A Nationwide Study on Millions of Chinese Neonatal Populations. Front. Mol. Biosci. 2021, 8, 719866. [Google Scholar] [CrossRef] [PubMed]
- Supriya, M.; De, T.; Christopher, R. Effect of temperature on lysosomal enzyme activity during preparation and storage of dried blood spots. J. Clin. Lab. Anal. 2018, 32, e22220. [Google Scholar] [CrossRef] [PubMed]
- Kloosterboer, M.; Hoffman, G.; Rock, M.; Gershan, W.; Laxova, A.; Li, Z.; Farrell, P.M. Clarification of laboratory and clinical variables that influence cystic fibrosis newborn screening with initial analysis of immunoreactive trypsinogen. Pediatrics 2009, 123, e338–e346. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Johansson, S.; Wang, Y.Q. Adenosine and the regulation of metabolism and body temperature. Adv. Pharmacol. 2011, 61, 77–94. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T.; Kalinchuk, A.V. Adenosine, energy metabolism and sleep homeostasis. Sleep. Med. Rev. 2011, 15, 123–135. [Google Scholar] [CrossRef]
- Lowy, B.A.; Williams, M.K. Studies on the metabolism of adenosine and adenine in stored and fresh human erythrocytes. Blood 1966, 27, 623–628. [Google Scholar] [CrossRef]
- Jimmerson, L.C.; Bushman, L.R.; Ray, M.L.; Anderson, P.L.; Kiser, J.J. A LC-MS/MS Method for Quantifying Adenosine, Guanosine and Inosine Nucleotides in Human Cells. Pharm. Res. 2017, 34, 73–83. [Google Scholar] [CrossRef]
- Townsend, M.K.; Bao, Y.; Poole, E.M.; Bertrand, K.A.; Kraft, P.; Wolpin, B.M.; Clish, C.B.; Tworoger, S.S. Impact of Pre-analytic Blood Sample Collection Factors on Metabolomics. Cancer Epidemiol. Biomark. Prev. 2016, 25, 823–829. [Google Scholar] [CrossRef]

| Month | LC Average (µmol/L) | LC SD (µmol/L) | LC CV (%) | HC Average (µmol/L) | HC SD (µmol/L) | HC CV (%) | QC Lot No. |
|---|---|---|---|---|---|---|---|
| April | 0.740 | 0.071 | 9.64 | 4.56 | 0.236 | 5.18 | 744302 |
| May | 0.815 | 0.104 | 12.8 | 5.05 | 0.354 | 7.01 | 746505 |
| June | 0.833 | 0.094 | 11.3 | 5.16 | 0.327 | 6.34 | 746505 |
| July | 0.783 | 0.092 | 11.8 | 4.83 | 0.337 | 6.97 | 746505 |
| August | 2.69 | 0.186 | 6.91 | 7.31 | 0.451 | 6.17 | 749559 |
| September | 2.67 | 0.179 | 6.68 | 7.21 | 0.425 | 5.90 | 749559 |
| October | 2.69 | 0.179 | 6.65 | 7.53 | 0.450 | 5.98 | 749559 |
| November | 2.75 | 0.187 | 6.81 | 7.48 | 0.402 | 5.38 | 749559 |
| December | 0.571 | 0.083 | 14.6 | 4.79 | 0.282 | 5.89 | 750938 |
| January | 0.565 | 0.073 | 12.9 | 4.69 | 0.306 | 6.51 | 750938 |
| February | 0.587 | 0.088 | 15.0 | 4.73 | 0.285 | 6.02 | 750938 |
| ADO Reference Interval | 0.19–2.02 µmol/L | 0.10–1.14 µmol/L | ||
|---|---|---|---|---|
| Number of Positives (n) | Positive Rate (%) | Number of Positives (n) | Positive Rate (%) | |
| April | 2 | 0.08 | 13 | 0.51 |
| May | 19 | 0.4 | 94 | 1.95 |
| June | 7 | 0.15 | 197 | 4.21 |
| July | 8 | 0.17 | 337 | 6.96 |
| August | 5 | 0.1 | 169 | 3.3 |
| September | 10 | 0.2 | 182 | 3.63 |
| October | 6 | 0.11 | 132 | 2.33 |
| November | 2 | 0.04 | 66 | 1.21 |
| December | 4 | 0.07 | 17 | 0.3 |
| January | 5 | 0.11 | 16 | 0.34 |
| February | 1 | 0.11 | 2 | 0.21 |
| Total | 72 | 0.15 | 1225 | 2.48 |
| 4 °C | 20 °C | 40 °C | 4 °C vs. 20 °C (p-Value) | 4 °C vs. 40 °C (p-Value) | 20 °C vs. 40 °C (p-Value) | |
|---|---|---|---|---|---|---|
| Day 1 | 0.25 ± 0.03 | 0.30 ± 0.04 | 0.33 ± 0.05 | 0.0704 | 0.0022 * | 0.2657 |
| Day 2 | 0.30 ± 0.03 | 0.32 ± 0.04 | 0.34 ± 0.04 | 0.1661 | 0.0395 * | 0.2695 |
| Day 3 | 0.25 ± 0.03 | 0.27 ± 0.03 | 0.30 ± 0.07 | 0.3119 | 0.1297 | 0.2128 |
| Day 4 | 0.25 ± 0.02 | 0.28 ± 0.04 | 0.36 ± 0.08 | 0.0971 | 0.0152 * | 0.0374 * |
| Day 5 | 0.27 ± 0.03 | 0.31 ± 0.05 | 0.37 ± 0.08 | 0.2047 | 0.0290 * | 0.0353 * |
| Day 6 | 0.31 ± 0.04 | 0.34 ± 0.00 | 0.39 ± 0.04 | 0.2719 | 0.0149 * | 0.0738 |
| Day 7 | 0.25 ± 0.03 | 0.32 ± 0.04 | 0.36 ± 0.08 | 0.0084 * | 0.0310 * | 0.1908 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Liu, J.; Li, X.; Hong, D.; Wu, S.; Chen, C.; Li, H. Seasonal Fluctuations and Stability of Adenosine in Dried Blood Spots for Neonatal Screening. Int. J. Neonatal Screen. 2025, 11, 63. https://doi.org/10.3390/ijns11030063
Yang X, Liu J, Li X, Hong D, Wu S, Chen C, Li H. Seasonal Fluctuations and Stability of Adenosine in Dried Blood Spots for Neonatal Screening. International Journal of Neonatal Screening. 2025; 11(3):63. https://doi.org/10.3390/ijns11030063
Chicago/Turabian StyleYang, Xiangchun, Jing Liu, Xia Li, Dongyang Hong, Shanshan Wu, Changshui Chen, and Haibo Li. 2025. "Seasonal Fluctuations and Stability of Adenosine in Dried Blood Spots for Neonatal Screening" International Journal of Neonatal Screening 11, no. 3: 63. https://doi.org/10.3390/ijns11030063
APA StyleYang, X., Liu, J., Li, X., Hong, D., Wu, S., Chen, C., & Li, H. (2025). Seasonal Fluctuations and Stability of Adenosine in Dried Blood Spots for Neonatal Screening. International Journal of Neonatal Screening, 11(3), 63. https://doi.org/10.3390/ijns11030063






