The Establishment of Expanded Newborn Screening in Rural Areas of a Developing Country: A Model from Health Regions 7 and 8 in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. System Development and Validation
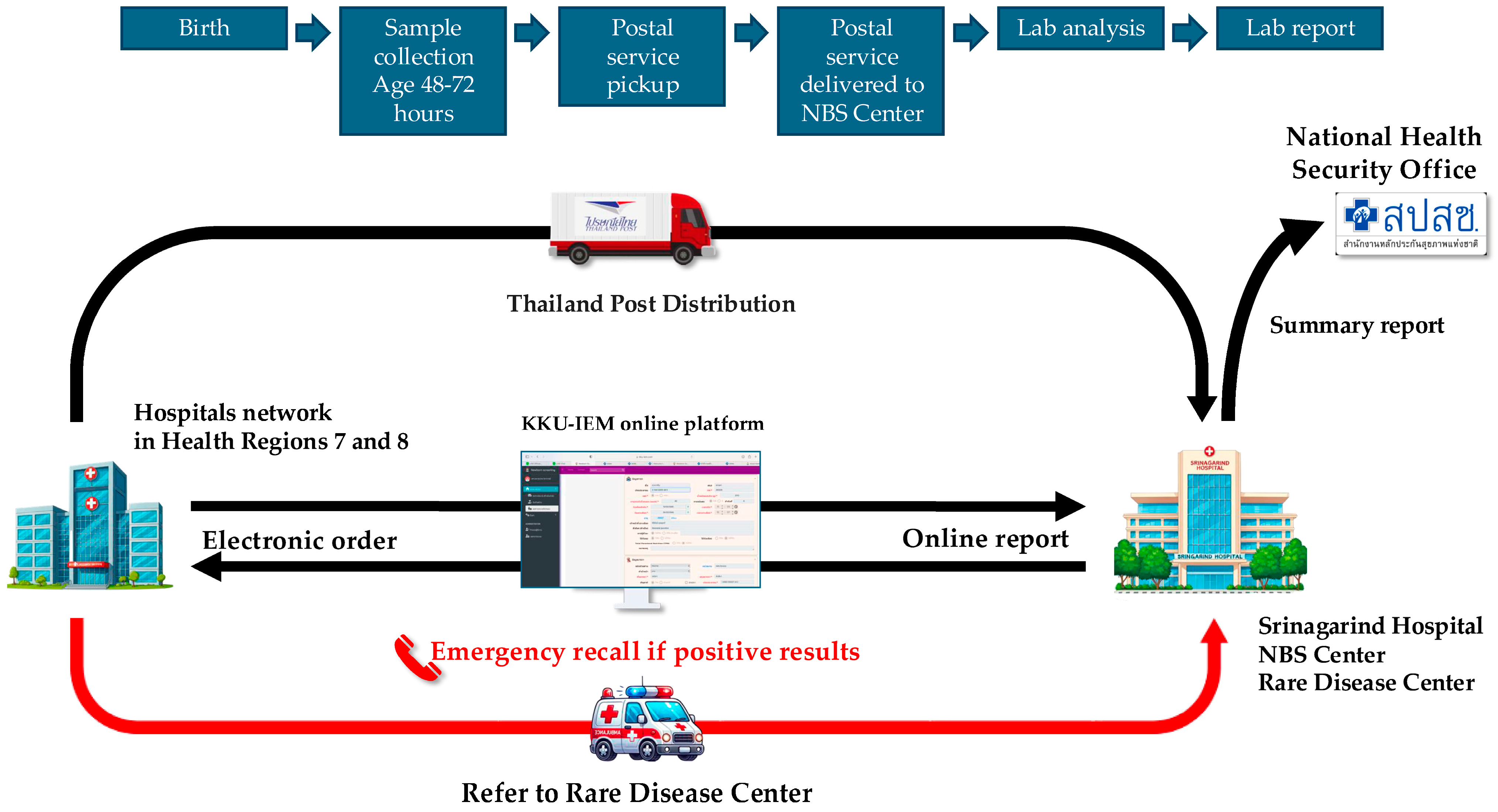
2.2. Software and Logistics Preparation
2.3. Training of Networks
- Very Urgent: Infants must be followed up with for evaluation within 24 h of receiving a positive result.
- Urgent: Infants must be followed up with for evaluation within 48 h of receiving a positive result.
2.4. Quality Control
2.5. Re-Evaluation
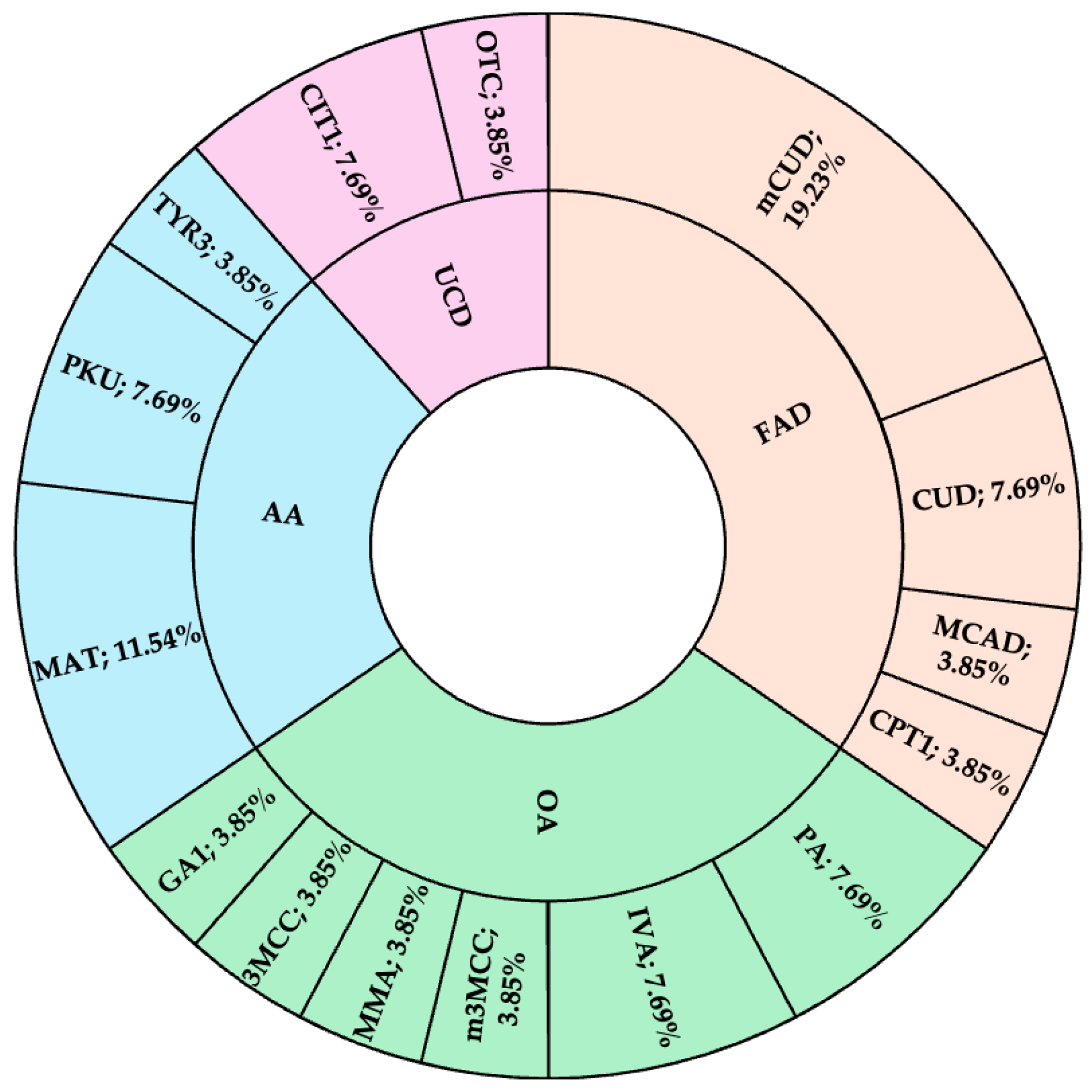
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CH | Congenital hypothyroidism |
| HITAP | Health Intervention and Technology Assessment Program |
| HRs | Health Regions |
| IEMs | Inborn errors of metabolism |
| MS/MS | Tandem mass spectrometry |
| NBS | Newborn screening |
| NHSO | National Health Security Office |
| TPD | Thailand Post Distribution |
| RD | Rare disease |
References
- Guthrie, R. Blood Screening for Phenylketonuria. JAMA 1961, 178, 863. [Google Scholar] [CrossRef]
- Charoensiriwatana, W.; Janejai, N.; Boonwanich, W.; Krasao, P.; Chaisomchit, S.; Waiyasilp, S. Neonatal Screening Program in Thailand. Southeast Asian J. Trop. Med. Public Health 2003, 34 (Suppl. 3), 94–100. [Google Scholar] [PubMed]
- Liammongkolkul, S.; Sanomcham, K.; Vatanavicharn, N.; Sathienkijkanchai, A.; Ranieri, E.; Wasant, P. Expanded newborn screening program in Thailand. Ann. Transl. Med. 2017, 5 (Suppl. 2), AB133. [Google Scholar] [CrossRef]
- Khampang, R.; Angkab, P.; Leelahavarong, P.; Saengsri, W.; Vatanavicharn, N. Feasibility Study of Newborn Screening for Inborn Errors of Metabolism by Tandem Mass Spectrometry. J. Health Syst. Res. 2024, 18, 239–250. [Google Scholar]
- Therrell, B.L.; Padilla, C.D.; Borrajo, G.J.C.; Khneisser, I.; Schielen, P.C.J.I.; Knight-Madden, J.; Malherbe, H.L.; Kase, M. Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020–2023). Int. J. Neonatal Screen. 2024, 10, 38. [Google Scholar] [CrossRef]
- Liu, L.; He, W.; Zhu, J.; Deng, K.; Tan, H.; Xiang, L.; Yuan, X.; Li, Q.; Huang, M.; Guo, Y.; et al. Global Prevalence of Congenital Hypothyroidism among Neonates from 1969 to 2020: A Systematic Review and Meta-Analysis. Eur. J. Pediatr. 2023, 182, 2957–2965. [Google Scholar] [CrossRef]
- Thaisri, H.; Puangtabtim, W.; Krasao, P.; Thongngao, P.; Auttarawanit, S.; Innark, P.; Pankanjanato, R.; Dhepakson, P.; Mahasirimongkol, S. The recall prevalence in congenital hypothyroidism screening due to thyroid stimulating hormone levels among newborns using dried blood spot samples in Thailand from 2015 to 2022. Dis. Control J. 2024, 50, 526–538. [Google Scholar]
- Jaruratanasirikul, S.; Piriyaphan, J.; Saengkaew, T.; Janjindamai, W.; Sriplung, H. The Etiologies and Incidences of Congenital Hypothyroidism before and after Neonatal TSH Screening Program Implementation: A Study in Southern Thailand. J. Pediatr. Endocrinol. Metab. JPEM 2018, 31, 609–617. [Google Scholar] [CrossRef]
- Charoensiriwatana, W.; Srijantr, P.; Janejai, N.; Hasan, S. Application of Geographic Information System in TSH Neonatal Screening for Monitoring of Iodine Deficiency Areas in Thailand. Southeast Asian J. Trop. Med. Public Health 2008, 39, 362–367. [Google Scholar]
- Liammongkolkul, S.; Boonyawat, B.; Vijarnsorn, C.; Tim-Aroon, T.; Wasant, P.; Vatanavicharn, N. Phenotypic and Molecular Features of Thai Patients with Primary Carnitine Deficiency. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2023, 65, e15404. [Google Scholar] [CrossRef]
- Chau, J.F.T.; Yu, M.H.C.; Chui, M.M.C.; Yeung, C.C.W.; Kwok, A.W.C.; Zhuang, X.; Lee, R.; Fung, J.L.F.; Lee, M.; Mak, C.C.Y.; et al. Comprehensive Analysis of Recessive Carrier Status Using Exome and Genome Sequencing Data in 1543 Southern Chinese. NPJ Genom. Med. 2022, 7, 23. [Google Scholar] [CrossRef]
- Engel, K.; Höhne, W.; Häberle, J. Mutations and Polymorphisms in the Human Argininosuccinate Synthetase (ASS1) Gene. Hum. Mutat. 2009, 30, 300–307. [Google Scholar] [CrossRef] [PubMed]
- McCullough, B.A.; Yudkoff, M.; Batshaw, M.L.; Wilson, J.M.; Raper, S.E.; Tuchman, M. Genotype Spectrum of Ornithine Transcarbamylase Deficiency: Correlation with the Clinical and Biochemical Phenotype. Am. J. Med. Genet. 2000, 93, 313–319. [Google Scholar] [CrossRef]
- Caldovic, L.; Abdikarim, I.; Narain, S.; Tuchman, M.; Morizono, H. Genotype–Phenotype Correlations in Ornithine Transcarbamylase Deficiency: A Mutation Update. J. Genet. Genomics Yi Chuan Xue Bao 2015, 42, 181–194. [Google Scholar] [CrossRef]
- Wichajarn, K.; Liammongkolkul, S.; Vatanavicharn, N.; Wattanasirichaigoon, D. Clinical and Laboratory Findings and Outcomes of Classic Organic Acidurias in Children from North-Eastern Thailand: A 5-Year Retrospective Study. Asian Biomed. 2017, 11, 41–47. [Google Scholar]
- Beyzaei, Z.; Nabavizadeh, S.; Karimzadeh, S.; Geramizadeh, B. The Mutation Spectrum and Ethnic Distribution of Non-Hepatorenal Tyrosinemia (Types II, III). Orphanet J. Rare Dis. 2022, 17, 424. [Google Scholar] [CrossRef]
- Heylen, E.; Scherer, G.; Vincent, M.-F.; Marie, S.; Fischer, J.; Nassogne, M.-C. Tyrosinemia Type III Detected via Neonatal Screening: Management and Outcome. Mol. Genet. Metab. 2012, 107, 605–607. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Chiang, S.-C.; Huang, A.; Hwu, W.-L. Spectrum of Hypermethioninemia in Neonatal Screening. Early Hum. Dev. 2005, 81, 529–533. [Google Scholar] [CrossRef]
- Barić, I.; Staufner, C.; Augoustides-Savvopoulou, P.; Chien, Y.-H.; Dobbelaere, D.; Grünert, S.C.; Opladen, T.; Petković Ramadža, D.; Rakić, B.; Wedell, A.; et al. Consensus Recommendations for the Diagnosis, Treatment and Follow-up of Inherited Methylation Disorders. J. Inherit. Metab. Dis. 2017, 40, 5–20. [Google Scholar] [CrossRef]
- Feng, J.; Yang, C.; Zhu, L.; Zhang, Y.; Zhao, X.; Chen, C.; Chen, Q.; Shu, Q.; Jiang, P.; Tong, F. Phenotype, Genotype and Long-Term Prognosis of 40 Chinese Patients with Isobutyryl-CoA Dehydrogenase Deficiency and a Review of Variant Spectra in ACAD8. Orphanet J. Rare Dis. 2021, 16, 392. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Wang, Y.Y.; Ma, D.Y.; Wang, X.; Cheng, W.; Jiang, T. Retrospective Analysis of Isobutyryl CoA Dehydrogenase Deficiency. Minerva Pediatr. 2024, 76, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, A.; Selvanathan, A.; Pandithan, D.; Kim, W.; Kava, M.P.; Boneh, A.; Coman, D.; Tolun, A.A.; Bhattacharya, K. 3-Methylglutaconyl-CoA Hydratase Deficiency: When Ascertainment Bias Confounds a Biochemical Diagnosis. JIMD Rep. 2022, 63, 568–574. [Google Scholar] [CrossRef] [PubMed]
- van Calcar, S.C.; Gleason, L.A.; Lindh, H.; Hoffman, G.; Rhead, W.; Vockley, G.; Wolff, J.A.; Durkin, M.S. 2-Methylbutyryl-CoA Dehydrogenase Deficiency in Hmong Infants Identified by Expanded Newborn Screen. WMJ Off. Publ. State Med. Soc. Wis. 2007, 106, 12–15. [Google Scholar]
- van Maldegem, B.T.; Duran, M.; Wanders, R.J.; Niezen-Koning, K.E.; Hogeveen, M.; Ijlst, L.; Waterham, H.R.; Wijburg, F.A. Clinical, Biochemical, and Genetic Heterogeneity in Short-Chain Acyl-Coenzyme A Dehydrogenase Deficiency. JAMA 2006, 296, 943–952. [Google Scholar] [CrossRef]
- Grünert, S.C.; Stucki, M.; Morscher, R.J.; Suormala, T.; Bürer, C.; Burda, P.; Christensen, E.; Ficicioglu, C.; Herwig, J.; Kölker, S.; et al. 3-Methylcrotonyl-CoA Carboxylase Deficiency: Clinical, Biochemical, Enzymatic and Molecular Studies in 88 Individuals. Orphanet J. Rare Dis. 2012, 7, 31. [Google Scholar] [CrossRef]
- Chuenwattana, S.; Imtawil, K.; Sornkayasit, K.; Rattanathongkom, A.; Charoenwat, B.; Wichajarn, K. Neonatal Intrahepatic Cholestasis Caused by Citrin Deficiency (NICCD) in Thai Infants: Case Reports on Clinical Presentation, Genotype Analysis, and Considerations for Negative Newborn Screening. Med. Rep. 2024, 4, 100051. [Google Scholar] [CrossRef]
- Chen, H.A.; Hsu, R.H.; Chen, Y.H.; Hsu, L.W.; Chiang, S.C.; Lee, N.C.; Hwu, W.L.; Chiu, P.C.; Chien, Y.H. Improved Diagnosis of Citrin Deficiency by Newborn Screening Using a Molecular Second-Tier Test. Mol. Genet. Metab. 2022, 136, 330–336. [Google Scholar] [CrossRef] [PubMed]
- de Sain-van der Velden, M.G.; Rinaldo, P.; Elvers, B.; Henderson, M.; Walter, J.H.; Prinsen, B.H.; Verhoeven-Duif, N.M.; de Koning, T.J.; van Hasselt, P. The Proline/Citrulline Ratio as a Biomarker for OAT Deficiency in Early Infancy. JIMD Rep. 2012, 6, 95–99. [Google Scholar]
- Marsden, D.; Bedrosian, C.L.; Vockley, J. Impact of Newborn Screening on the Reported Incidence and Clinical Outcomes Associated with Medium- and Long-Chain Fatty Acid Oxidation Disorders. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 816–829. [Google Scholar] [CrossRef]
- Grünert, S.C. Clinical and Genetical Heterogeneity of Late-Onset Multiple Acyl-Coenzyme A Dehydrogenase Deficiency. Orphanet J. Rare Dis. 2014, 9, 117. [Google Scholar] [CrossRef]
| Groups | Target Diseases | Abbreviation | Urgency Levels * |
|---|---|---|---|
| Disorders of amino acid metabolism | 1. Phenylketonuria | PKU | Urgent |
| 2. Tetrahydrobiopterin defects | BH4 | Urgent | |
| 3. Maple syrup urine disease | MSUD | Very Urgent | |
| 4. Tyrosinemia type 1 | TYR1 | Very Urgent | |
| 5. Tyrosinemia type 2 | TYR2 | Urgent | |
| 6. Tyrosinemia type 3 | TYR3 | Urgent | |
| 7. Homocystinuria | HCY | Urgent | |
| 8. Hypermethioninemia | MAT | Urgent | |
| 9. Hyperornithinemia with gyrate atrophy | HOGA | Urgent | |
| Disorders of organic acid metabolism | 1. Glutaric acidemia type 1 | GA1 | Urgent |
| 2. Isovaleric acidemia | IVA | Very Urgent | |
| 3. Methylmalonic acidemia | MMA | Very Urgent | |
| 4. Propionic acidemia | PA | Very Urgent | |
| 5. Multiple carboxylase deficiency | MCD | Very Urgent | |
| 6. Adenosylcobalamin synthesis defects | Cbl A/B | Very Urgent | |
| 7. Beta-Ketothiolase deficiency | BKT | Very Urgent | |
| 8. 3-Hydroxy-3-Methylglutaryl-CoA lyase deficiency | HMG | Very Urgent | |
| 9. Isobutyryl-CoA dehydrogenase deficiency | IBD | Urgent | |
| 10. 2-Methylbutyryl-CoA dehydrogenase deficiency | MBD | Very Urgent | |
| 11. 3-Methylcrotonyl-CoA carboxylase deficiency | MCC | Very Urgent | |
| 12. 3-Methylglutaconyl-CoA hydratase deficiency | MGA | Very Urgent | |
| 13. Malonic aciduria | MA | Urgent | |
| 14. Combined methylmalonic acidemia and homocystinuria | Cbl C/D | Very Urgent | |
| Urea cycle disorders | 1. Citrullinemia type 1 | CIT1 | Very Urgent |
| 2. Citrullinemia type 2 or Citrin deficiency | CIT2 | Very Urgent | |
| 3. Argininosuccinic aciduria | ASA | Very Urgent | |
| 4. Argininemia | ARG | Urgent | |
| 5. Hyperammonemia-Hyperornithinemia-Homocitrullinuria syndrome | HHH | Urgent | |
| 6. Ornithine transcarbamylase deficiency | OTC | Very Urgent | |
| Disorders of fatty acid oxidation | 1. Medium-chain acyl-CoA dehydrogenase deficiency | MCAD | Urgent |
| 2. Long-chain hydroxyacyl-CoA dehydrogenase deficiency | LCHAD | Very Urgent | |
| 3. Very-long-chain acyl-CoA dehydrogenase deficiency | VLCAD | Very Urgent | |
| 4. Short-chain acyl-CoA dehydrogenase deficiency | SCAD | Urgent | |
| 5. Short-chain hydroxyacyl-CoA dehydrogenase deficiency | SCHAD | Urgent | |
| 6. Trifunctional protein deficiency | TFP | Very Urgent | |
| 7. Multiple acyl-CoA dehydrogenase deficiency | MAD | Very Urgent | |
| 8. Carnitine-acylcarnitine translocase deficiency | CACT | Very Urgent | |
| 9. Carnitine palmitoyltransferase type 1 deficiency | CPT1 | Urgent | |
| 10. Carnitine palmitoyltransferase type 2 deficiency | CPT2 | Very Urgent | |
| 11. Primary systemic carnitine deficiency (carnitine uptake defect) | CUD | Urgent |
| Tests | Newborn Screening (Neonates) | Positive Results (Cases) | Recall Rate (%) | Number of Infants Undergoing Confirmatory Tests (%) | Confirmed Cases | Incidence Rate | Positive Predictive Value (%) |
|---|---|---|---|---|---|---|---|
| CH | 122,004 | 287 | 1:425.1 (0.24) | 284 (99.0) | 101 | 1:1208 (0.08) | 35.2 |
| IEMs | 122,004 | 529 | 1:230.6 (0.43) | 508 (96.0) | 20 | 1:6100 (0.02) | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichajarn, K.; Sawatjui, N.; Prasongdee, P.; Panklin, A.; Sornkayasit, K.; Chungkanchana, N.; Tessiri, S.; Wintachai, P.; Dechyotin, S.; Pasomboon, C.; et al. The Establishment of Expanded Newborn Screening in Rural Areas of a Developing Country: A Model from Health Regions 7 and 8 in Thailand. Int. J. Neonatal Screen. 2025, 11, 26. https://doi.org/10.3390/ijns11020026
Wichajarn K, Sawatjui N, Prasongdee P, Panklin A, Sornkayasit K, Chungkanchana N, Tessiri S, Wintachai P, Dechyotin S, Pasomboon C, et al. The Establishment of Expanded Newborn Screening in Rural Areas of a Developing Country: A Model from Health Regions 7 and 8 in Thailand. International Journal of Neonatal Screening. 2025; 11(2):26. https://doi.org/10.3390/ijns11020026
Chicago/Turabian StyleWichajarn, Khunton, Nopporn Sawatjui, Prinya Prasongdee, Amrin Panklin, Kanda Sornkayasit, Natchita Chungkanchana, Supharada Tessiri, Preawwalee Wintachai, Sumalai Dechyotin, Chalanda Pasomboon, and et al. 2025. "The Establishment of Expanded Newborn Screening in Rural Areas of a Developing Country: A Model from Health Regions 7 and 8 in Thailand" International Journal of Neonatal Screening 11, no. 2: 26. https://doi.org/10.3390/ijns11020026
APA StyleWichajarn, K., Sawatjui, N., Prasongdee, P., Panklin, A., Sornkayasit, K., Chungkanchana, N., Tessiri, S., Wintachai, P., Dechyotin, S., Pasomboon, C., Ratanapontee, J., Thanakitsuwan, S., & Rattanathongkom, A. (2025). The Establishment of Expanded Newborn Screening in Rural Areas of a Developing Country: A Model from Health Regions 7 and 8 in Thailand. International Journal of Neonatal Screening, 11(2), 26. https://doi.org/10.3390/ijns11020026







