Abstract
The pancreatic pseudocyst represents the main complication that occurred 3 to 6 weeks after an outbreak of acute or chronic pancreatitis represented by a collection containing pancreatic enzymes without their own epithelial wall. In the present paper, we present a study performed on 7 patients admitted to the Surgical Department of Sibiu County Emergency Clinical Hospital who were diagnosed with pancreatic pseudocyst between 2016 and 2020, and the drainage of the mini-invasive pancreatic pseudocyst by an incision in the right lumbar area, in the case of a 53-year-old patient known to have a history of multiple cardiac defects, the pancreatic pseudocyst being discovered approximately 6 months before, for which the patient underwent conservative treatment, and who had 5 resuscitated cardio-respiratory arrests throughout the evolution.
Introduction
The pancreatic pseudocyst represents the main late complication (3-6 weeks) of severe acute or chronic pancreatitis, with a complication incidence of 10-15% of the cases described in the literature, which increases proportionally with the severity of pancreatitis [1,2,3]. Due to the controversy over the evolution of acute pancreatitis regarding the definition and classification of fluid collections, the Atlanta International Symposium on Acute Pancreatitis (Georgia, 1993) accepted and defined the following anatomical-clinical entities [2,3,4].
Pancreatic and peripancreatic necrosis occur early in the evolution of acute pancreatitis and consist of diffuse or focal areas of non-viable pancreatic tissue, which may be sterile or contaminated [3].
Acute fluid collections (the false pseudocysts) appear early in the evolution of severe acute pancreatitis (within the first 2 weeks) as an inflammatory reaction induced by the necrosis of the pancreas and peripancreatic tissue, having a plasma-like electrolytic composition and a low concentration of pancreatic enzymes, and over 50% of the cases resorb with the resolution of acute pancreatitis [2,4]. Pancreatic abscesses represent circumscribed intra- abdominal collections, usually located in the vicinity of the pancreas, containing purulent fluid and very little necrotic tissue, appeared after the bacterial overpopulation of both necrosis and the false pancreatic pseudocyst [3].
The pancreatic pseudocyst represents a fluid collection rich in pancreatic enzymes, without its own epithelial wall, delimited by the neighboring viscera and/ or by a capsule formed of fibrous and/ or granulation tissue [4,5]. The formation and persistence of the pseudocyst is based on the existence of a permanent communication with the pancreatic canalicular system, and the spontaneous resorption of the pseudocyst is impossible as long as this communication persists [3,4,5].
The aim of the study is to evaluate the usefulness of the percutaneous drainage of the pancreatic pseudocyst in well-selected cases and to determine the type of location of the pseudocyst that allows surgery to be performed.
Materials and Methods
We conducted a retrospective study on 7 patients diagnosed with pancreatic pseudocysts between January 2016 and December 2020 and who were admitted to the Surgical Department of Sibiu County Emergency Clinical Hospital, where the hospitalization charts and their intraoperative protocols were analyzed.
The inclusion criteria were all the patients diagnosed with pancreatic pseudocyst in the period between 2016 and 2020, with an altered general condition, locoregional compression or the occurrence of pseudocyst complications, and the exclusion criteria were the patients with a good general condition and uncomplicated pseudocysts.
The study analyzed the demographic data, the clinical signs upon admission, the postoperative outcomes and their complications. The Excel software was used to process and analyze the statistical data.
Results
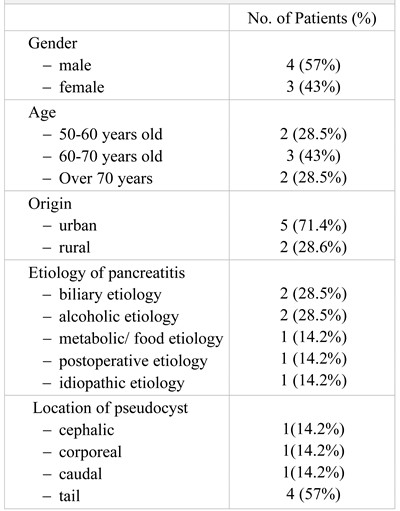
The patients included in the study group were aged between 50 and 70 years, with a mean age of 65.3+/-8.1 years (Table 1). Most patients were from rural areas (5 patients, 71.4%), with a gender ratio M:F of 4:3.

Table 1.
The general data of the patients included in the study group.
The etiology was established according to the anamnestic data, as well as according to the clinical, laboratory and imaging examinations. The most frequent causes were biliary and alcoholic (28.5%) ones, followed by metabolic, postoperative and idiopathic etiology.
A number of 2 cases were admitted during the Covid- 19 pandemic and followed the specific protocols required by the Sars-Cov-2 regulations for the prevention of the hospital dissemination of Covid-19. People are infected via small droplets from diseased people that are produced by sneezing, coughing, or talking, as well as by touching contaminated surfaces, the virus having the ability to survive for hours or even days [6]. Since the declaration of the COVID-19 pandemic in our country, on March 15th, all the patients presenting to the Emergency Department are treated as potential COVID-19 suspects, and the initial evaluation is made with complete protective personal equipment, consisting of FFP 2 or 3 masks, eye protection, gowns and gloves [7,8]. A RT-PCR test is run 24-48 hours before admission for elective cases and when admission is decided for emergency cases. Special Covid-19 (red zones) and non-Covid-19 (green zone) were designed in our hospital. Before the results of the RT-PCR test are confirmed negative, laparoscopic and endoscopic maneuvers were generally avoided, due to the potential risk of aerosolization and transmission of the Sars-Cov-2 infection [9].
The pancreatic pseudocysts were encountered in every pancreatic segment, but with a higher incidence at the tail level (57%).
The clinical data obtained from the objective clinical examination and the patients' problems and symptoms allowed the suspicion of a pancreatic pathology in most cases, and in the case of patients with a more affected general condition, investigations continued until the diagnosis of certainty was made.
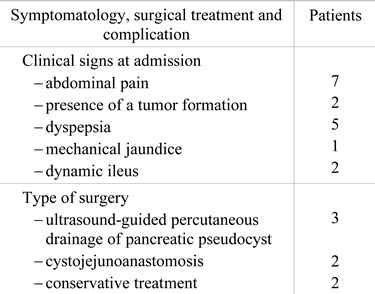
The most encountered complains were abdominal pain, described by all the patients, dyspepsia (71.4%), palpable abdominal tumor (28.5%) and dynamic ileus (28.5%). (Table 2).

Table 2.
Clinical presentation and management.
The surgical treatment was chosen in each case depending on the location of the pseudocyst, and the clinical manifestations present since the time of admission. Ultrasound-guided percutaneous drainage was performed in 43% of the patients, cystojejunoanastomosis was performed in 28.5% of the patients, and the treatment was conservative in 14.2 % of the patients.
The postoperative complications noticed in the study group were the suppuration of the surgical wound (28.5%), bleeding, pancreatic fistula and the incomplete discharge of the pancreatic cyst in 14.28% of the patients. These were managed by conservative approaches in 5 cases and it was necessary to have a surgical re-intervention for the complete evacuation of the pancreatic pseudocyst in one patient (Table 3).

Table 3.
Postoperative complications in the study group.
One particularly challenging case was the one of a 53- year-old patient with personal history of operated duodenal ulcer, chronic alcoholism, hypertension, chronic ischemic cardiac disease, and a 4 cm diameter pancreatic pseudocyst, detected by abdominal ultrasound, for which the patient underwent conservative treatment. The patient was admitted to the Emergency Department for diffuse abdominal pain, fever, loss of appetite, asthenia, weight loss (approximately 37 kilos in the last 10 months). At the time of the examination, the patient was having an altered general condition, was being cachectic, had pale and dry skin and mucous membranes, distended abdomen presenting spontaneous diffuse pain on palpation, present bowel sounds and bowel movements for feces and gas. Biochemically, he had severe anemia (Hb = 7.6g/dl, Ht = 24.8%, amylase = 269UI/ l).
Although the many therapeutic options that can be applied in the surgical resolution of the pancreatic pseudocyst pathology by classical, laparoscopic and minimally invasive techniques can be applied, there is a direct contraindication due to the altered general condition of the patient upon admission. Associated hydro- electrolytic rebalancing, correction of anemia by blood administration, administration of anti-secretors, antibiotic therapy, anticoagulants were performed upon admission, but under this treatment the evolution is still unfavorable, for which reason the examination and transfer of the patient to the Intensive Care Unit were requested, where the patient had 5 cardiopulmonary arrests, and thus being resuscitated for 5 times.
An abdominal CT is performed which reveals bilateral pleurisy, left basal pulmonary condensation, pseudocyst in the tail of the pancreas whose dimensions were 9/8 cm, multiple cystic images located in the left renal bed and small hepatic hypodense node (Figure 1).
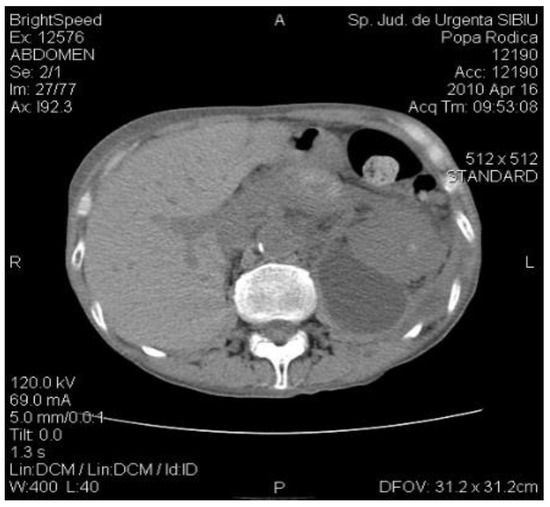
Figure 1.
The CT exam revealed a 4-cm diameter pancreatic pseudocyst in the tail of the pancreas.
The position of the pseudocyst in the tail of the pancreas in direct contact with the space of the left renal bed allowed us to perform an ultrasound-guided incision in the left lumbar area by draining the pseudocyst’s content through drainage tubes with a thick diameter, under intravenously induced anesthesia potentiated locally with Xylin 1% (Figure 2).
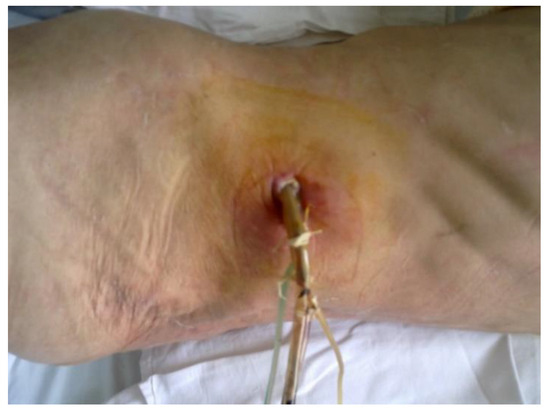
Figure 2.
Drainage tubes inserted into the left renal bed after the drainage of the pseudocyst by ultrasound guided puncture in the left lumbar area, with the evacuation of cloudy fluid.
The efficiency of the external drainage of the pancreatic pseudocyst by inserting drainage tubes in the left lumbar area was favorable, with the persistence of evacuating the contents of the pseudocyst for a longer time of about 3 months, but with the decrease of the flow until its disappearance.
The biochemical tests performed every 3 months did not show the recurrence of a pancreatic reaction whose main role is the formation and maintenance of the pseudocyst.
Postoperatively, the patient was HD and CR stable, with soft, mobile abdomen, painless on palpation, bowel movement present for feces and gas, good digestive tolerance, postoperative wound without reaction, permeable drainage tubes.
The ultrasound examination at 3 postoperative days reveals residual fluid in the cyst, for which reason the surgical re-intervention with local anesthesia by the repositioning of the drainage tubes is decided upon and performed (Figure 3).
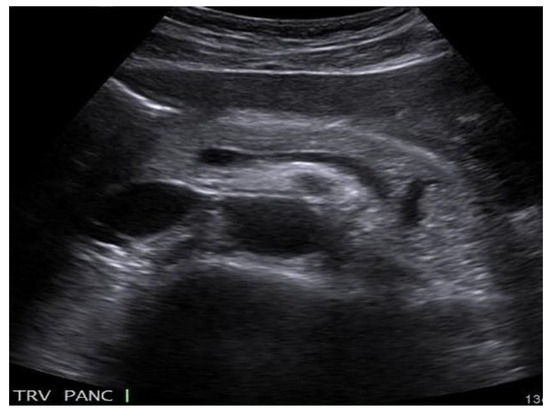
Figure 3.
The ultrasound examination of the pancreatic pseudocyst.
The evolution was favorable, the patient being afebrile, with painless abdomen, good digestive tolerance, drainage gradually decreasing quantitatively, reaching about 80ml/24 h at the time of the hospital discharge, present bowel movement, postoperative wound healing, without reaction. The prolonged hospitalization in intensive care unit led to pressure wounds, which were managed by antibiotics and essential oils, effective against the several microorganisms, such as Bacillus subtilis, Clostridium perfrigens, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans [10].
The abdominal CT at follow-up evidenced basal lung segments without changes, normal aspect of the liver, spleen, and kidneys, gallbladder with fluid and homogeneous content, pancreas with small cystic image in the tail, with an irregular contour, with a diameter of 1.9 cm, drainage tubes visible at this level, with an irregular anterior contour of the pancreas at this level, with the dilation of the Wirsung distal from the described formation (Figure 4).
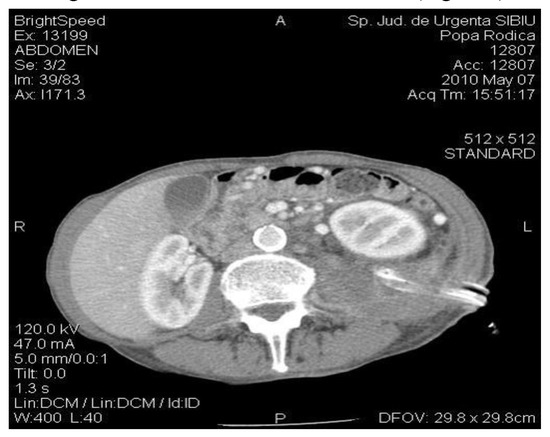
Figure 4.
The CT exam: diminished size of the pancreatic pseudocyst; drainage tubes located in the left renal bed.
Due to the small size of the pancreatic cyst and to the cessation of the drainage on the drainage tubes, it was decided to suppress them.
The patient returned for periodic check-ups every 3 months, when abdominal ultrasounds were performed revealing the total evacuation of the pancreatic pseudocyst and the evaluation of the biological constants without significant changes (Figure 5).
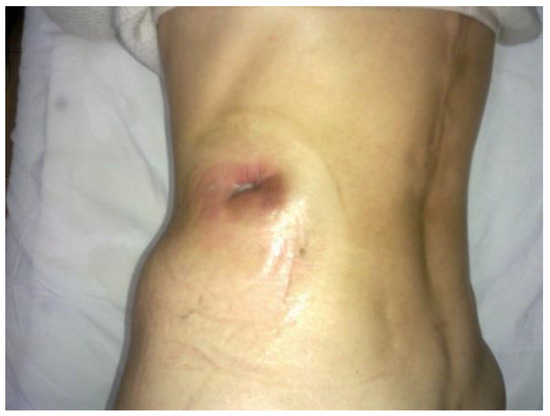
Figure 5.
The postoperative wound after the removal of the drainage tubes.
Discussion
In a recent study by Mofleh et al. [11], the etiology of acute pancreatitis was found to be of a biliary etiology in 35- 49% of the cases and of alcoholic origin in 20% of the cases, results which are similar to the results of our study. Less frequently, acute pancreatitis may have metabolic, posttraumatic or postoperative causes. A rare association was reported by Nasri et al., in a case with neglected diaphragmatic hernia following trauma, which developed into acute pancreatitis by the stretching of the transverse mesocolon due to increased intra-abdominal pressure inducing posterior fascia loosening, thus leading to pancreatic mobility and herniation [12]. In these complicated cases, a preoperative CT exam is extremely valuable for both diagnosis and management [13]. An inadequate diet, rich in fats, alcohol, and spicy food may exacerbate the inflammation of the pancreas [14,15].
Patients with acute pancreatitis should be followed-up on a long term for local and general complications. Diabetes mellitus represents a heavy burden for society in the form of a long-term disability, healthcare use and costs [16,17]. In a systematic review by Das et al., diabetes mellitus was reported to be much more frequent in the following 5 years after an episode of acute pancreatitis compared to the normal population [17].
The imaging follow-up by CT examinations of pancreatic pseudocysts showed that 40% of them with a diameter of less than 6 cm spontaneously regress if there is no communication with the pancreatic duct [18].
However, previously published studies found that symptomatic pseudocysts and those with a diameter over 6 cm must be drained surgically or by nonsurgical and minimally invasive methods (echo-CT-guided puncture, laparoscopic internal or external drainage, drainage by digestive endoscopy), because they may develop evolutionary complications represented by the relapse of acute pancreatitis, cyst abscess, rupture of the cyst in the peritoneum or fistula formation in adjacent viscera. Radiologically guided percutaneous drainage allows the insertion of a catheter that will drain the pancreatic secretions on the outside, having as disadvantage the case in which the pseudocyst communicates with the main pancreatic duct, turning the pseudocyst into an external pancreatic fistula.
The indications of this method still remain limited (immature or infected pseudocysts located or fused in the immediate vicinity of the abdominal wall), due to the large number of failures (54% described in the literature) and relapses (63% described in the literature), as well as the incidences of complications that can be generated by lesions of the adjacent viscera, digestive bleeding, cellulitis at the site of implantation of the catheter [19,20].
Other modern methods of drainage of the pseudocyst included laparoscopic and endoscopic drainage techniques, by different approaches. Laparoscopic transgastric pseudocystogastrostomy is the most common method of internal drainage, which involves the occurrence of two conditions: the adherence of the pseudocyst to the gastric posterior wall and their optimal ratio for a declive drainage.
Posterior laparoscopic pseudocystogastrostomy has been described as a technical alternative to transgastric pseudocystogastrostomy. Unlike the anterior approach, which involves the adherence of the pseudocyst to the gastric posterior wall, the indication for the posterior approach is represented by the pseudocysts that come in contact with the greater gastric curvature.
Laparoscopic pseudocystojejunostomy is indicated in pseudocysts that bulge in the submesocolic space and do not adhere to the gastric posterior wall, with the anastomosis of the cystic formation with an excluded jejunal loop (Roux) or in continuity (with or without anastomosis at the foot of the loop) [21,22,23].
Laparoscopic transgastric cystogastrostomy involves the creation of a transgastric cystogastrostomy, without the need of creating pneumoperitoneum. It is performed with the help of laparoscopic instruments inserted directly into the stomach through the abdominal wall during its maximum distension after insufflation with the help of endoscopic instruments.
Transpapillary drainage is recommended in pseudocysts that communicate with the ductal system and it involves performing an endoscopic sphincterotomy with its risks and inserting a drainage catheter [24]. Direct transmural drainage through the gastric wall consists in creating an internal derivative between the pseudocyst and the stomach performed endoscopically.
The choice of the type of surgery depends on the size, location and relationship that the pseudocyst has with the neighboring structures, but also on the general condition of the patient [25].
The fact that the pseudocyst in the presented case bulges at the level of the left renal lodge, being in direct contact with the posterior wall of the lodge, allowed us to perform an echo-guided puncture and to insert a large caliber drainage tube through a mini-incision, without damaging the neighboring parenchymal structures and with an efficient drainage of the pseudocyst contents [26,27,28].
The widening of the puncture with a minimal incision offers the advantage of digital and instrumental control of the pseudocyst cavity, the fragmentation of the contents, the debridement and the lavage of the remaining cavity [29].
Carefully perioperative care is extremely important, with fluid and metabolic rebalance, especially in elderly patients, with multiple comorbidities, to ensure a favorable outcome [30,31,32]. Regular patient monitoring is a key element and it allows: monitoring the efficiency of drainage and the position of drainage tubes by repeated ultrasound examinations, monitoring the biological constants, and imaging tracking of the size of the remaining cavity by injecting radiological contrast medium into the drainage tube [33,34,35].
The advantages of percutaneous drainage are represented by the simplicity of the technique in the case of an experienced surgical team, the possibility of performing it at the patient's bedside under some conditions and the less expensive cost. The disadvantages are long hospital stay, the risk of infection, the risk of external pancreatic fistula, repeated hospitalization, and the recurrence of pseudocysts. Our study has some limitations: the limited number of patients included in the study, the retrospective character and the incomplete radiological follow-up.
However, the present research provides useful information on a topic that is still under debate: when and how it is appropriate to treat pancreatic pseudocysts. We considered the symptoms of persisting pain, weight loss, jaundice or obstruction as main indicators for the surgical intervention, and our clinical management was less influenced by the size and the duration of the pancreatic pseudocyst.
On a long term follow-up, we consider that along with the imaging and the surgical evaluation of the pancreas, the patients with chronic pancreatic suffering should be monitored by a specialist in diabetes and nutrition, taking into consideration the high incidence of type 3C diabetes in these situations [36].
Highlights
- ✓ The pancreatic pseudocyst represents the main late complication (3-6 weeks) of severe acute or chronic pancreatitis, with an incidence of complications of 10-15%.
- ✓ The modern therapeutic attitude pays more attention to the surgical procedures that preserve the pancreas, and one of these is the percutaneous drainage guided by imaging.
Conclusions
The identification of a pancreatic cystic lesion is a challenging diagnosis, and the abdominal ultrasound is usually sufficient, but specifying the nature of the cyst is much more difficult. The type of pancreatic cystic lesion decisively determines the strategy and the operative tactics, and the errors of the therapeutic conduct can have severe effects on the prognosis, including the vital one. The modern therapeutic attitude pays more attention to the surgical procedures that preserve the pancreas, and one of these is the imaging-guided percutaneous drainage for critical patients who cannot undergo surgery.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Hao, W.M.; Chen, Y.; Jiang, Y.; Yang, A. Endoscopic Versus Laparoscopic Treatment for Pancreatic Pseudocysts. Pancreas 2021, 50, 788–795. [Google Scholar] [CrossRef]
- Nemeş, R.; Curcă, T.; Paraliov, T.; Munteanu, M.; Paşalega, M.; Dincă, N.; Mesina, C.; Martin, L.; Cheie, M.; Talpoşi, L.; et al. Cystic tumors of the pancreas. Considerations upon 34 operated cases. Rom J Gastroenterol. 2002, 11, 303–8. [Google Scholar] [PubMed]
- Ferrucci, J.T.; Mueller, P.R. Interventional approach to pancreatic fluid collections. Radiol. Clin. N. Am. 2003, 41, 1217–1226. [Google Scholar] [CrossRef]
- Baillie, J. Pancreatic pseudocysts (Part I). Gastrointest Endosc. 2004, 59, 873–879. [Google Scholar] [CrossRef]
- Klöppel, G. Pseudocysts and other non-neoplastic cysts of the pancreas. Semin Diagn Pathol. 2000, 17, 7–15. [Google Scholar] [PubMed]
- Suceveanu, A.I.; Dragoi, C.M. ASSERTION FOR MONTELUKAST IN THE COVID-19 PANDEMICS? Farmacia 2020, 68, 579–585. [Google Scholar] [CrossRef]
- Serban, D.; Socea, B.; Badiu, C.D.; Tudor, C.; Balasescu, S.A.; Dumitrescu, D.; Trotea, A.M.; Spataru, R.I.; Vancea, G.; Dascalu, A.M.; et al. Acute surgical abdomen during the COVID-19 pandemic: Clinical and therapeutic challenges. Exp. Ther. Med. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Costea, D.-O.; Enache, F.-D.; Baz, R.; Suceveanu, A.P.; Suceveanu, A.I.; Ardeleanu, V.; Mazilu, L.; Costea, A.C.; Botea, F.; Voinea, F. Confirmed child patient with covid-19 infection, opperated for associated surgical pathology – first pediatric case in Romania. Romanian Biotechnol. Lett. 2020, 25, 2107–2110. [Google Scholar] [CrossRef]
- Serban, D.; Smarandache, C.G.; Tudor, C.; Duta, L.N.; Dascalu, A.M.; Aliuș, C. Laparoscopic Surgery in COVID-19 Era—Safety and Ethical Issues. Diagnostics 2020, 10, 673. [Google Scholar] [CrossRef]
- Radu, N.; Voicescu, M.; Radu, E.; Tanasescu, C. Biomaterial with antioxidant and antifungal activities, obtained from romanian indigenous plants. Mol. Cryst. Liq. Cryst. 2017, 655, 243–249. [Google Scholar] [CrossRef]
- Al Mofleh, I.-A. Severe acute pancreatitis: Pathogenetic aspects and prognostic factors. World J. Gastroenterol. 2008, 14, 675–84. [Google Scholar] [CrossRef]
- Nasri, S.; Guerrouj, I.; Abbou, W.; Aichouni, N.; Kamaoui, I.; Skiker, I. Diaphragmatic hernia a rare cause of acute pancreatitis: Case report. Radiol. Case Rep. 2021, 17, 572–576. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Savlovschi, C.; Borcan, R.; Pantu, H.; Serban, D.; Gradinaru, S.; Smarandache, G.; Trotea, T.; Branescu, C.; Musat, L.; et al. [Clinical case--voluminous diaphragmatic hernia--surgically acute abdomen: diagnostic and therapeutical challenges]. Chirurgia (Bucur) 2011, 106, 657–60. [Google Scholar] [PubMed]
- Barreto, S.G.; Thomas, T.; Mah, L. Systematic review of diet in the pathogenesis of acute pancreatitis: A tale of too much or too little? Saudi J. Gastroenterol. 2012, 18, 310–5. [Google Scholar] [CrossRef]
- Radu, N.; Roman, V.; Bostan, M.; Radu, E.; Tanasescu, C. Influence of some spice food based bioproducts on human monocytic cells line type THP-1. Mol. Cryst. Liq. Cryst. 2017, 655, 114–123. [Google Scholar] [CrossRef]
- Serban, D.; Papanas, N.; Dascalu, A.M.; Stana, D.; Nicolae, V.A.; Vancea, G.; Badiu, C.D.; Tanasescu, D.; Tudor, C.; Balasescu, S.A.; et al. Diabetic Retinopathy in Patients With Diabetic Foot Ulcer: A Systematic Review. Int. J. Low. Extremity Wounds 2020, 20, 98–103. [Google Scholar] [CrossRef]
- Das, S.L.M.; Singh, P.P.; Phillips, A.R.J.; Murphy, R.; Windsor, J.A.; Petrov, M.S. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut 2014, 63, 818–831. [Google Scholar] [CrossRef]
- Alonso, V.; Guéye, D.; Monnin, V.; Kalfa, N. Radiological and surgical management of bleeding pancreatic pseudocyst in a pediatric patient. Cir Pediatr. 2021, 34, 147–150. [Google Scholar]
- Warshaw, A.L.; Rattner, D.W. Timing of Surgical Drainage for Pancreatic Pseudocyst. Ann. Surg. 1985, 202, 720–724. [Google Scholar] [CrossRef]
- Nealon, W.H.; Walser, E. Surgical Management of Complications Associated with Percutaneous and/or Endoscopic Management of Pseudocyst of the Pancreas. Ann. Surg. 2005, 241, 948–960. [Google Scholar] [CrossRef]
- Kaban, G.K.; A Perugini, R.; Czerniach, D.R.; Litwin, D.E. Pancreatic pseudocyst drainage. Oper. Tech. Gen. Surg. 2004, 6, 55–62. [Google Scholar] [CrossRef]
- Roth, J. Minimally invasive approaches to pancreatic pseudocysts. Curr. Surg. 2003, 60, 591–592. [Google Scholar] [CrossRef]
- Park, A.E.M.; Heniford, B.T.M. Therapeutic Laparoscopy of the Pancreas. Ann. Surg. 2002, 236, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, A.; Lewis, M.P.N.; Rhodes, M. Stapled laparoscopic cystgastrostomy. Surg. Endosc. 2004, 19, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ekremoğlu, M.; Severcan, Ç.; Pasaoğlu, Ö.; Şen, B.; Pasaoğlu, H. An investigation of acute effects at various doses of malathion on glucose homeostasis and insulin resistance in rat liver, pancreas and serum. J. Mind Med Sci. 2020, 7, 85–93. [Google Scholar] [CrossRef]
- Naveena, R. Clinical spectrum of obstructive jaundice: a descriptive crosssectional study. J. Clin Invest Surg. 2021, 6, 53–57. [Google Scholar] [CrossRef]
- Clark, H.L.; Illipparambil, L.C.; Khurana, S. Mediastinal pancreatic pseudocyst masquerading as diffuse alveolar haemorrhage. BMJ Case Rep. 2021, 14, e240677. [Google Scholar] [CrossRef]
- Stoian, A.; Hainarosie, R.; Pietrosanu, C.; Rusescu, A.; Andronache, L.; Paunica, S.; Balalau, C.; Pituru, T. Modern concepts in non-surgical esthetics; a review. J. Mind Med Sci. 2019, 6, 190–195. [Google Scholar] [CrossRef]
- Szakó, L.; Gede, N.; Váradi, A.; Tinusz, B.; Vörhendi, N.; Mosztbacher, D.; Vincze, Á.; Takács, T.; Czakó, L.; Izbéki, F.; et al. Early occurrence of pseudocysts in acute pancreatitis—A multicenter international cohort analysis of 2275 cases. Pancreatology 2021, 21, 1161–1172. [Google Scholar] [CrossRef]
- Serban, D.; Socea, B.; Balasescu, S.A.; Badiu, C.D.; Tudor, C.; Dascalu, A.M.; Vancea, G.; Spataru, R.I.; Sabau, A.D.; Sabau, D.; et al. Safety of Laparoscopic Cholecystectomy for Acute Cholecystitis in the Elderly: A Multivariate Analysis of Risk Factors for Intra and Postoperative Complications. Medicina 2021, 57, 230. [Google Scholar] [CrossRef]
- Savlovschi, C.; Brănescu, C.; Serban, D.; Tudor, C.; Găvan, C.; Shanabli, A.; Comandaşu, M.; Vasilescu, L.; Borcan, R.; Dumitrescu, D.; et al. Amyand's hernia--a clinical case. Chirurgia (Bucur) 2010, 105, 409–414. [Google Scholar] [PubMed]
- Keskin, A.; Karslioglu, B. Did Covid-19 pandemic narrow the spectrum of surgical indications? J. Clin. Investig. Surg. 2021, 6, 58–63. [Google Scholar] [CrossRef]
- Berbece, S.; Ardeleanu, V.; Constantin, V.; Paunica, I.; Toma, A. Therapeutic approach for Amyand’s hernia; a case report. J. Mind Med Sci. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Chen, B.-B.; Mu, P.-Y.; Lu, J.-T.; Wang, G.; Zhang, R.; Huang, D.-D.; Shen, D.-H.; Jiang, T.-T. Sinistral portal hypertension associated with pancreatic pseudocysts - ultrasonography findings: A case report. World J. Clin. Cases 2021, 9, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Ditu, G.; Diculescu, M.; Manuc, M.; Manuc, D.; Diaconu, C.; Suceveanu, A.; Nitipir, C.; Hainarosie, R.; Poiana, C.; et al. Pancreatogenic type 3C diabetes. J. Mind Med Sci. 2018, 5, 270–277. [Google Scholar] [CrossRef]
- Igwe, P.; Ray-Offor, E.; Karibi, E.; Okeke, U.; Ugwa, O.; Jebbin, N. Giant pseudocyst of the pancreas: A report of three cases. Int. J. Surg. Case Rep. 2020, 77, 284–297. [Google Scholar] [CrossRef]
© 2022 by the author. 2022 Mihai Faur, Andrei Moisin, Alexandru Dan Sabau, Dan Sabau.