The Comparison of Local Tumor Control After Microwave Ablation, Surgical Resection and Combined Treatment for Colorectal Liver Metastases
Abstract
:Introduction
Materials and Methods
Inclusion criteria and data collection
Procedures
Follow-up imaging
Statistical analysis
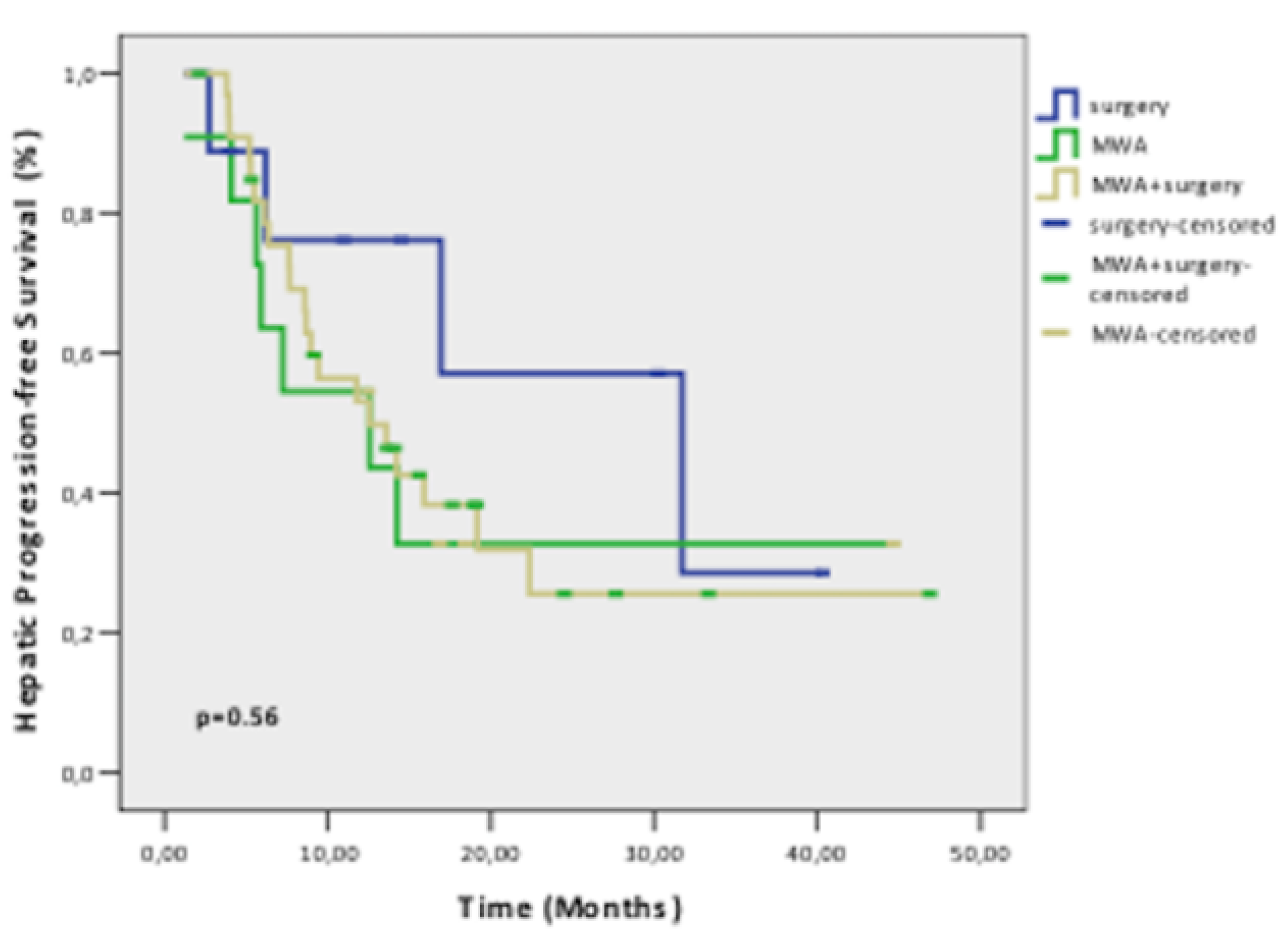
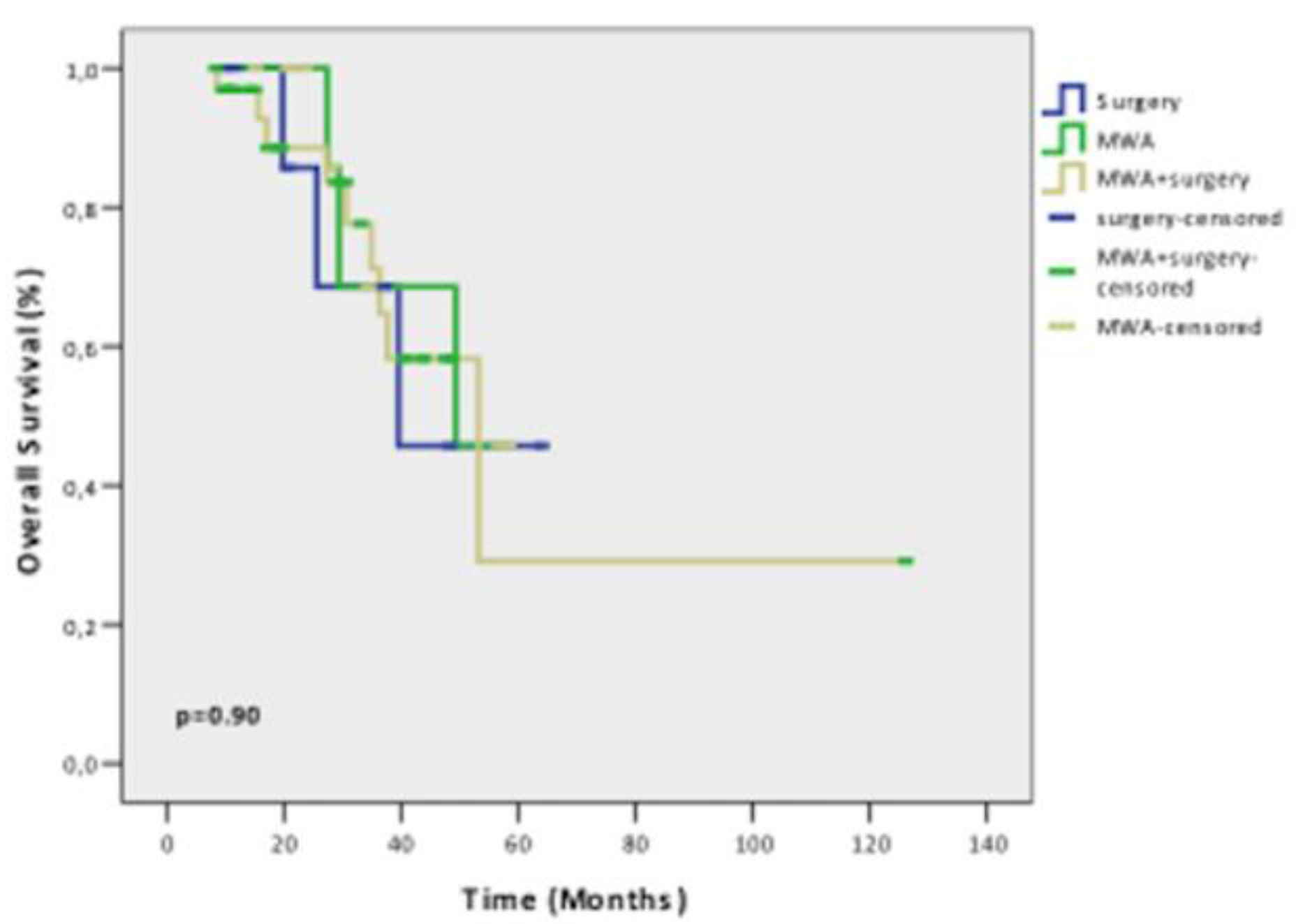
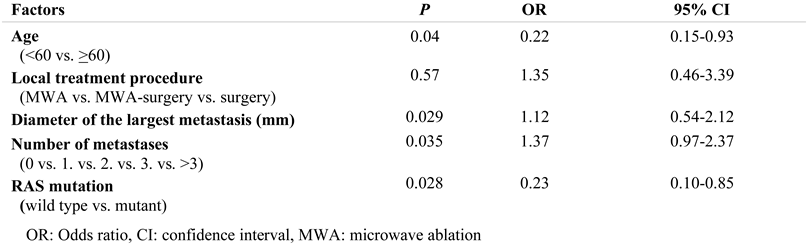
Results
Discussion
Conclusions
Conflicts of Interest disclosure
Compliance with ethical standards
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shimada, H.; Nagano, Y.; Endo, I.; Sekido, H.; Togo, S. Outcome after hepatic resection versus combined resection and microwave ablation for multiple bilobar colorectal metastases to the liver. Surgery. 2006, 139, 263–273. [Google Scholar] [CrossRef]
- Stättner, S.; Primavesi, F.; Yip, V.S.; Jones, R.P.; Öfner, D.; Malik, H.Z.; Fenwick, S.W.; Poston, G.J. Evolution of surgical microwave ablation for the treatment of colorectal cancer liver metastasis: review of the literature and a single centre experience. Surg Today. 2015, 45, 407–415. [Google Scholar] [CrossRef]
- Meijerink, M.R.; Puijk, R.S.; van Tilborg, A.A.J.M.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Heymans, J.; Frankema, J.S.; de Jong, K.P.; Richel, D.J.; Prevoo, W.; Vlayen, J. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2018, 41, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Nieuwenhuizen, S.; Puijk, R.S.; van den Bemd, B.; et al. Resectability and Ablatability Criteria for the Treatment of Liver Only Colorectal Metastases: Multidisciplinary Consensus Document from the COLLISION Trial Group. Cancers (Basel) 2020, 12, 1779. [Google Scholar] [CrossRef]
- Leung, U.; Kuk, D.; D'Angelica, M.I.; Kingham, T.P.; Allen, P.J.; DeMatteo, R.P.; Jarnagin, W.R.; Fong, Y. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015, 102, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Groeschl, R.T.; Pilgrim, C.H.; Hanna, E.M.; Simo, K.A.; Swan, R.Z.; Sindram, D.; Martinie, J.B.; Iannitti, D.A.; Bloomston, M.; Schmidt, C.; Khabiri, H.; Shirley, L.A.; Martin, R.C.; Tsai, S.; Turaga, K.K.; Christians, K.K.; Rilling, W.S.; Gamblin, T.C. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014, 259, 1195–1200. [Google Scholar] [CrossRef]
- Alexander, E.S.; Wolf, F.J.; Machan, J.T.; Charpentier, K.P.; Beland, M.D.; Iannuccilli, J.D.; Haas, R.H.; Dupuy, D.E. Microwave ablation of focal hepatic malignancies regardless of size: A 9-year retrospective study of 64 patients. Eur J Radiol. 2015, 84, 1083–1090. [Google Scholar] [CrossRef]
- Takahashi, H.; Kahramangil, B.; Berber, E. Local recurrence after microwave thermosphere ablation of malignant liver tumors: results of a surgical series. Surgery. 2018, 163, 709–713. [Google Scholar] [CrossRef]
- Pathak, S.; Jones, R.; Tang, J.M.; Parmar, C.; Fenwick, S.; Malik, H.; Poston, G. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis. 2011, 13, e252–e265. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; de Baére, T.; Pereira, P.L.; Tarantino, F.P. CIRSE Standards of Practice on Thermal Ablation of Liver Tumours. Cardiovasc Intervent Radiol. 2020, 43, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; et al.; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014, 273, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Sangha, V.K.; Morris-Stiff, G.; Malik, H.Z.; Guthrie, A.J.; Toogood, G.J.; Lodge, J.P.; Prasad, K.R. Outcomes of intensive surveillance after resection of hepatic colorectal metastases. Br J Surg. 2010, 97, 1552–1560. [Google Scholar] [CrossRef]
- Camacho, J.C.; Petre, E.N.; Sofocleous, C.T. Thermal Ablation of Metastatic Colon Cancer to the Liver. Semin Intervent Radiol. 2019, 36, 310–318. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave ablation: principles and applications. Radiographics. 2005, 25 (Suppl. 1), S69–S83. [Google Scholar] [CrossRef]
- Song, P.; Sheng, L.; Sun, Y.; An, Y.; Guo, Y.; Zhang, Y. The clinical utility and outcomes of microwave ablation for colorectal cancer liver metastases. Oncotarget. 2017, 8, 51792–51799. [Google Scholar] [CrossRef]
- Urbonas, T.; Anderson, E.M.; Gordon-Weeks, A.N.; Kabir, S.I.; Soonawalla, Z.; Silva, M.A.; Gleeson, F.V.; Reddy, S. Factors predicting ablation site recurrence following percutaneous microwave ablation of colorectal hepatic metastases. HPB (Oxford). 2019, 21, 1175–1184. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ruarus, A.H.; Vroomen, L.G.P.H.; et al.; COLLISION Trial Group Colorectal liver metastases: surgery versus thermal ablation (COLLISION)—A phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018, 18, 821. [Google Scholar] [CrossRef]
- Liang, P.; Wang, Y.; Yu, X.; Dong, B. Malignant liver tumors: treatment with percutaneous microwave ablation-- complications among cohort of 1136 patients. Radiology. 2009, 251, 933–940. [Google Scholar] [CrossRef]
- Shibata, T.; Niinobu, T.; Ogata, N.; Takami, M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 2000, 89, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, X.; Liang, P.; Han, Z.; Cheng, Z.; Yu, J.; Liu, F.; Hu, Y. Does primary tumor location impact the prognosis of colorectal liver metastases patients after microwave ablation? - Lessons from 10 years' experience. Oncotarget. 2017, 8, 100791–100800. [Google Scholar] [CrossRef] [PubMed]
- Philips, P.; Groeschl, R.T.; Hanna, E.M.; Swan, R.Z.; Turaga, K.K.; Martinie, J.B.; Iannitti, D.A.; Schmidt, C.; Gamblin, T.C.; Martin, R.C. Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br J Surg. 2016, 103, 1048–1054. [Google Scholar] [CrossRef]
- Odisio, B.C.; Yamashita, S.; Huang, S.Y.; Harmoush, S.; Kopetz, S.E.; Ahrar, K.; Shin Chun, Y.; Conrad, C.; Aloia, T.A.; Gupta, S.; Hicks, M.E.; Vauthey, J.N. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017, 104, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Calandri, M.; Yamashita, S.; Gazzera, C.; Fonio, P.; Veltri, A.; Bustreo, S.; et al. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018, 28, 2727–2734. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Solbiati, L.; Zanus, G.; Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012, 35, 868–874. [Google Scholar] [CrossRef]


© 2022 by the author. 2022 Fatma Zeynep Güngören, Cengiz Erol, Ahmet Bilici, Murat Dayangaç, Mehmet Şeker, Ömer Fatih Ölmez, Onur Yaprak, Özcan Yıldız, Mustafa Öncel.
Share and Cite
Güngören, F.Z.; Erol, C.; Bilici, A.; Dayangaç, M.; Şeker, M.; Ölmez, Ö.F.; Yaprak, O.; Yıldız, Ö.; Öncel, M. The Comparison of Local Tumor Control After Microwave Ablation, Surgical Resection and Combined Treatment for Colorectal Liver Metastases. J. Mind Med. Sci. 2022, 9, 125-132. https://doi.org/10.22543/7674.91.P125132
Güngören FZ, Erol C, Bilici A, Dayangaç M, Şeker M, Ölmez ÖF, Yaprak O, Yıldız Ö, Öncel M. The Comparison of Local Tumor Control After Microwave Ablation, Surgical Resection and Combined Treatment for Colorectal Liver Metastases. Journal of Mind and Medical Sciences. 2022; 9(1):125-132. https://doi.org/10.22543/7674.91.P125132
Chicago/Turabian StyleGüngören, Fatma Zeynep, Cengiz Erol, Ahmet Bilici, Murat Dayangaç, Mehmet Şeker, Ömer Fatih Ölmez, Onur Yaprak, Özcan Yıldız, and Mustafa Öncel. 2022. "The Comparison of Local Tumor Control After Microwave Ablation, Surgical Resection and Combined Treatment for Colorectal Liver Metastases" Journal of Mind and Medical Sciences 9, no. 1: 125-132. https://doi.org/10.22543/7674.91.P125132
APA StyleGüngören, F. Z., Erol, C., Bilici, A., Dayangaç, M., Şeker, M., Ölmez, Ö. F., Yaprak, O., Yıldız, Ö., & Öncel, M. (2022). The Comparison of Local Tumor Control After Microwave Ablation, Surgical Resection and Combined Treatment for Colorectal Liver Metastases. Journal of Mind and Medical Sciences, 9(1), 125-132. https://doi.org/10.22543/7674.91.P125132



