Abstract
Multiple myeloma (MM) is a plasma cell neoplasm, characterized by periods of remission and relapses. The emergence of novel therapies, with multiple mechanisms of action and fewer adverse reactions, brings more and better options and also a higher survival rate. However, MM is still an incurable disease, and patients eventually become refractory to an extensive range of therapies. We present the case of a patient diagnosed with MM standard risk, who was at first refractory to multiple treatment regimens, and then had an unexpected and stable complete response to a newer drug of the same class.
Introduction
Multiple myeloma (MM) is a plasma-cell neoplasm, representing 10-13% of hematologic malignancies [1,2]. Although it is usually a chronic disorder, and many therapies are available, it remains incurable. We present a standard risk MM patient, who first was unexpectedly refractory to multiple treatment regimens, and then had a spectacular and stable response to a newer drug of a previously used class.
Case Presentation
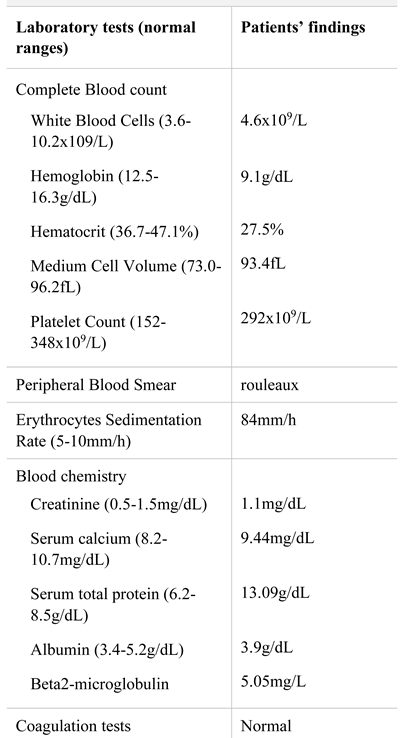
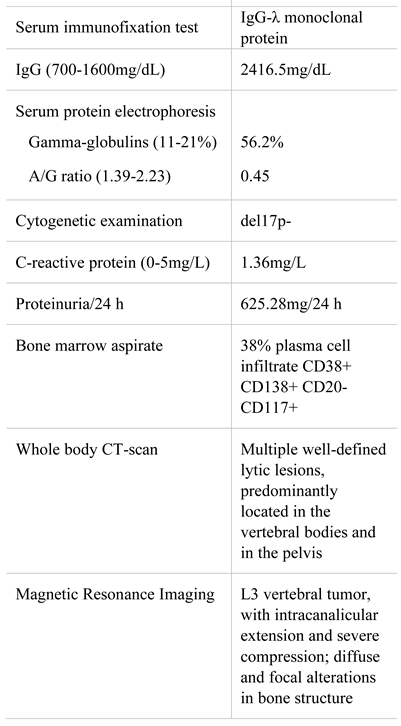
A 63-years-old male was admitted to our department with fatigue and severe low back pain radiating to the lower right extremity, non-related to trauma. His past medical history was unremarkable, except for anemia discovered one-year prior presentation. Physical examination was normal, except for pallor. Laboratory analyses are displayed in Table 1. Diagnosis was multiple myeloma IgG lambda secretory, stage II. Also, he presented an L3- vertebral tumor which was highly suggestive for plasmacytoma.

Table 1.
Laboratory findings at diagnosis.
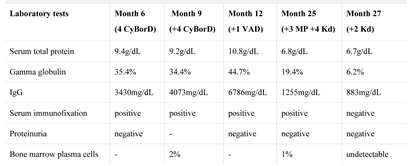
After establishing the diagnosis, chemotherapy was initiated with a melphalan-free regimen with the patient eligible for autologous hematopoietic stem cell transplantation (auto-HCT). We started CyBorD protocol (cyclophosphamide, bortezomib, dexamethasone), without significant complications. The L3 vertebral tumor was excised. Postoperative histopathological exam confirmed the plasmacytoma. Evaluation after four cycles showed only partial response (Table 2).

Table 2.
Evolution of hematological parameters.
We proceeded with hematopoietic stem cell collection (apheresis from peripheral blood)—5.17 × 106 CD34+ cells/kg, enough for two auto-HCTs. During hospitalization, the patient developed femoral-popliteal deep venous thrombosis, complicated with pulmonary thromboembolism. He was started on anticoagulants, with favorable evolution.
We continued with another four cycles of CyBorD, without complications. Evaluation revealed stable disease (Table 2), but far from an appropriate response. The transplant team recommended continuing treatment as before, high-dose melphalan and auto-HCT, but because of the unsatisfactory response, with another regimen.
As the patient did not fit the official reimbursement criteria for thalidomide, and lenalidomide is not available in Romania, the only available regimen was VAD (vincristine, doxorubicin, dexamethasone), which unfortunately was followed by grave complications: a severe infection of the upper left limb, requiring surgical intervention, broad-spectrum antibiotics, and a one-month hospitalization; two weeks after discharge, he developed bronchopneumonia with subsequent septic shock, and paroxysmal atrial fibrillation, all requiring again a prolonged hospitalization and intensive care. Also, reactive depression appeared, necessitating antidepressants.
Meanwhile, the disease rapidly progressed and even the weak response was lost (Table 2). Auto-HCT was delayed due to lack of proper disease control, multiple and severe infectious complications during chemotherapy, and also by patients’ preference. He received three cycles of MP (melphalan, prednisone) protocol.
A few months later, carfilzomib became available. We decided to start Kd (carfilzomib, dexamethasone) protocol, 20 months after diagnosis. Evaluation after four cycles showed very good partial response, and after six cycles, a complete response (Table 2). Evolution under treatment with Kd protocol was favorable. Currently, the patient is at cycle 24, maintaining CR, and with no other complications except for mild hematological toxicity.
Discussions
Life expectancy in MM depends on the response to therapy. It has increased in parallel with the emergence of novel therapies with various mechanisms of action [1,3,4]. The clinical evolution of MM, as with other lymphoproliferative disorders [5,6,7] is marked by periods of remission and relapse, the latter becoming more frequent and more aggressive with each regimen [1,4]. Despite the multiple options available, MM is still incurable, and patients ultimately become refractory to a broad spectrum of drugs [1].
Proteasome inhibitors are an effective treatment of MM [8]. Here, we used a bortezomib-based regimen as first- line, approved for transplant eligible patients. Our patient first had only an insufficient partial response, and later became refractory—aggressive MM although the initial prognosis markers were not unfavorable (standard risk, stage II). Since no other options were available at the time, we chose VAD regimen. We administered only one cycle when severe, life-threatening infectious complications appeared (after a less intensive protocol than the previous one). We decided to use a regimen with lower toxicity, melphalan-based, as stem cells were already collected. No response was observed after any of those regimens. When carfilzomib became available, though the patient had a refractory disease with a high and progressive burden and multiple previous complications, we decided to continue treatment with Kd regimen. The response was extremely favorable and mostly unexpected: stable CR and no adverse reactions whatsoever.
It is known that carfilzomib has greater selectivity and irreversibly inhibits the proteasome, and thus responses may appear even in bortezomib-refractory patients [8]. However, median progression free survival (PFS) for Kd regimen in bortezomib-exposed patients with 2-3 previous treatment lines, is 13.1months [9]. Our patient already has a double PFS and maintains response. Also, CR rates reported for Kd combination even in standard risk patients are actually quite low (13.0%) [10].
So, after the unexpected inadequate first response, our patient surprised us again with such a favorable outcome: CR and prolonged PFS at Kd, without notable adverse reactions, in a patient treated with three lines and previous severe complications.
Highlights
- ✓
- Unexpected favorable and durable response to a newer drug of the same class that was inefficient as first line, in a patient who had become refractory provides renewed hope for the patient, as newer and more effective drugs are constantly being developed.
Conclusions
We aim to illustrate hereby the case of a patient who did not have an unfavorable prognosis at diagnosis by using the known markers, and yet responded poorly and progressed under standard therapy. But, he had the chance to benefit from a newer drug, although from the same class as the one to which he had already showed resistance. Nevertheless, he showed an unexpected complete and stable response, beyond what statistics might have predicted.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. The patient has signed the informed consent accepting that his data can be used for academic purposes (articles, presentations, teaching etc.).
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Rajkumar, S.V. Multiple myeloma: 2018 update on diagnosis, risk-stratification and management. Am J Hematol 2018, 93, 981–1114. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Anderson, K. Multiple myeloma. N Engl J Med 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Pawlyn, C.; Davies, F.E. Towards personalized treatment in multiple myeloma based on molecular characteristics. Blood 2019, 133, 660–675. [Google Scholar] [CrossRef] [PubMed]
- Naymagon, L.; Abdul-Hay, M. Novel agents in the treatment of multiple myeloma: A review about the future. J Hematol Oncol 2016, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Bontoux, C.; Bruneau, J.; Molina, T.J. Histopathological classification of chronic B-lymphoproliferative disorders. Presse Med. 2019, 48, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Damlaj, M.; El Fakih, R.; Hashmi, S.K. Evolution of survivorship in lymphoma, myeloma and leukemia: Metamorphosis of the field into long term follow-up care. Blood Rev. 2019, 33, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Găman, M.; Vlădăreanu, A.M.; Dobrea, C.; et al. A Challenging Case of Kikuchi-Fujimoto Disease Associated with Systemic Lupus Erythematosus and Review of the Literature. Case Rep Hematol. 2018, 2018, 1791627. [Google Scholar] [CrossRef] [PubMed]
- Groen, K.; van de Donk, N.W.C.J.; Stege, C.A.M.; Zweegman, S.; Nijhof, I.S. Carfilzomib for relapse and refractory multiple myeloma. Cancer Manag Res. 2019, 11, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Joshua, D.; Chng, W.-J.; Palumbo, A.; Goldschmidt, H.; Hájek, R.; et al. Impact of Prior Treatment on Patients with Relapsed Multiple Myeloma Treated with Carfilzomib and Dexamethasone vs Bortezomib and Dexamethasone in the Phase 3 ENDEAVOR Study. Leukemia 2017, 31, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Chng, W.J.; Goldschmidt, H.; Dimopoulos, M.A.; Moreau, P.; Joshua, D.; Palumbo, A.; et al. Carfilzomib- dexamethasone vs bortezomib-dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia 2017, 31, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. 2020 Minodora Cezarina Onisâi1, Iuliana Iordan1, Mihaela Gaman, Horia Bumbea, Ana-Maria Vlădăreanu1