Extra Medullar Granulocytic Sarcoma: A Case Report of an Exceptional Localization
Abstract
Introduction
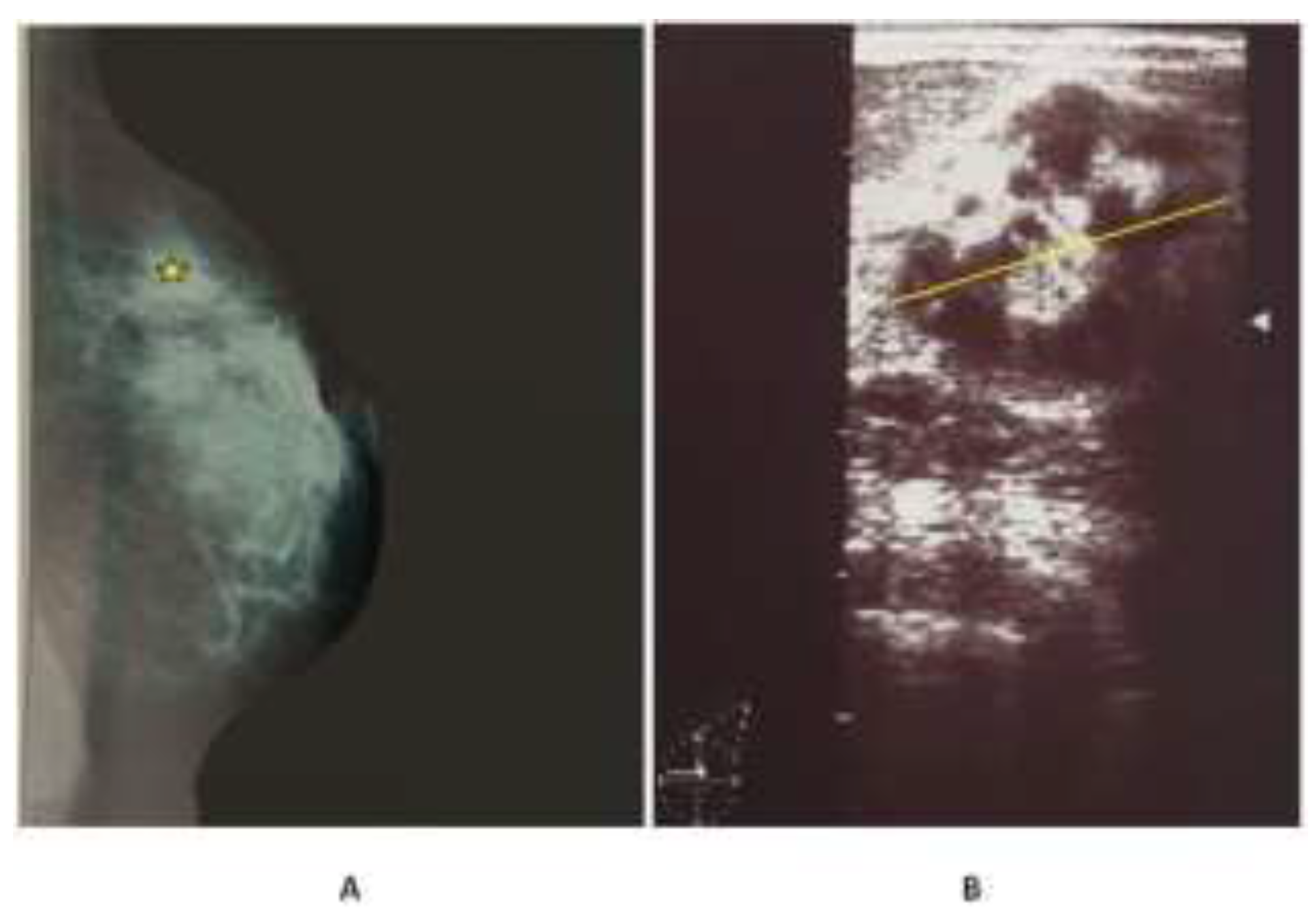
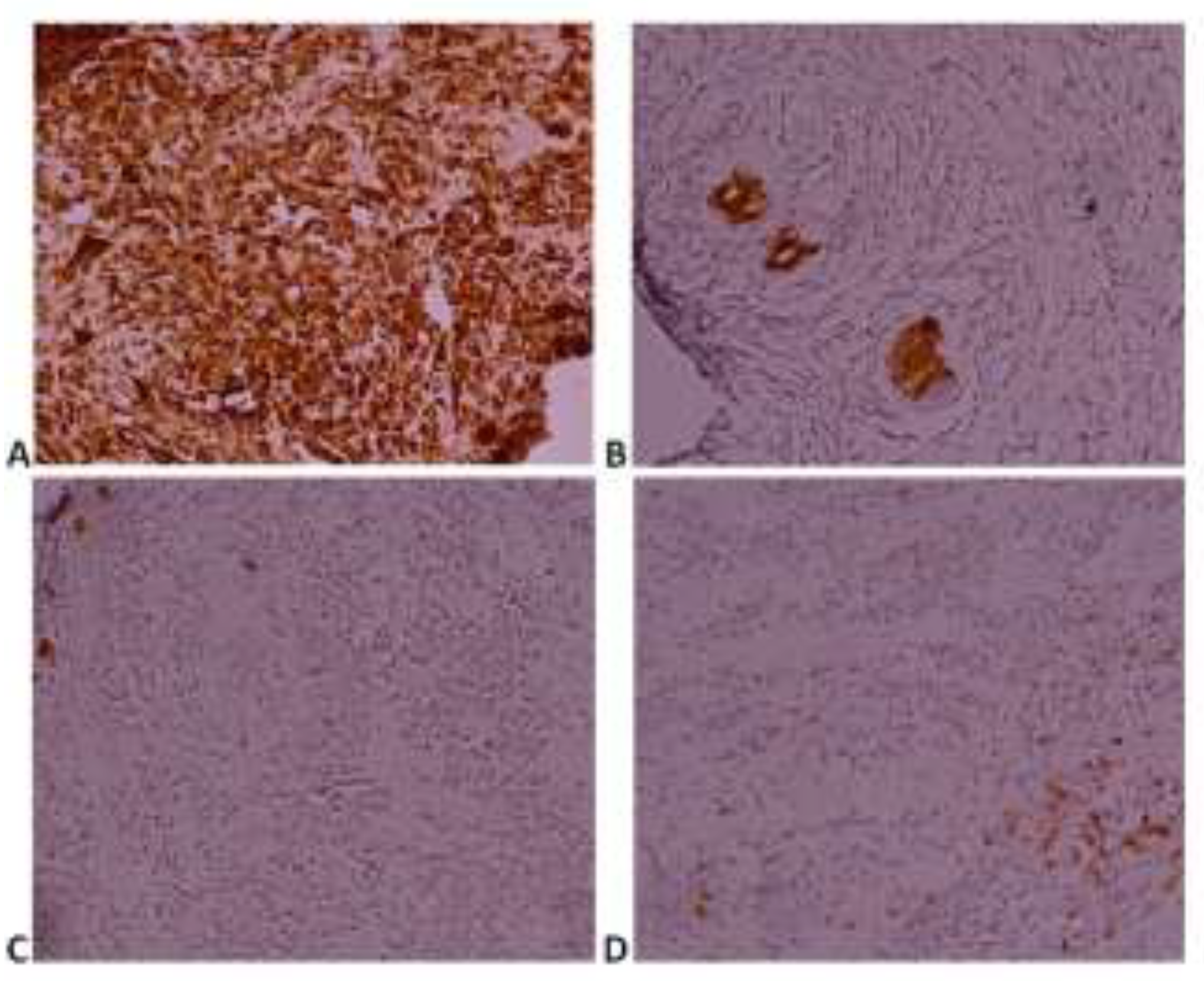
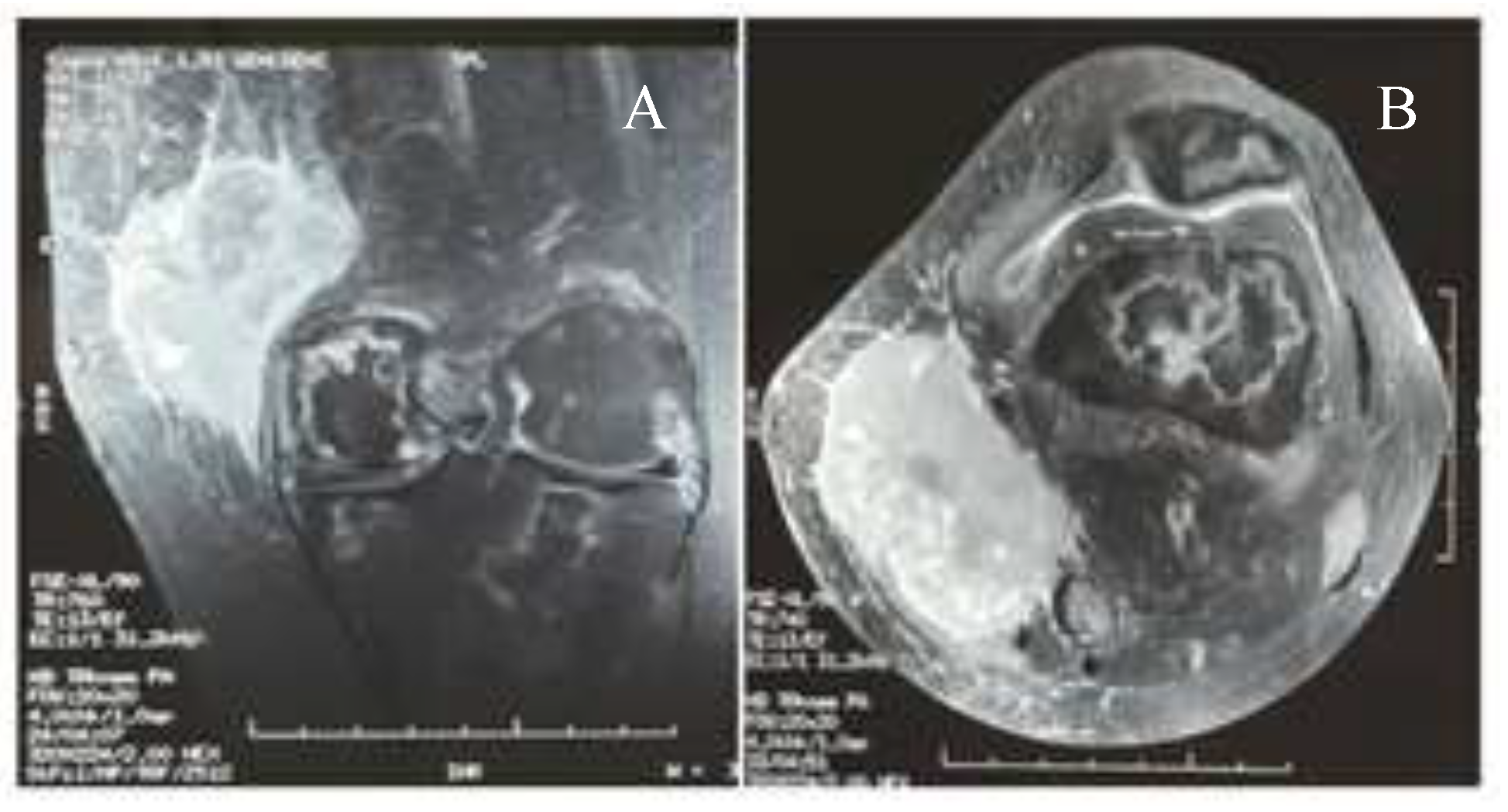
Case presentation
Discussions
Highlights
Conclusions
Compliance with ethical standards
Conflict of interest disclosure
References
- Fernandes Vieira, V.; Vo, Q.D.; Bouquet de la Jolinière, J.; Khomsi, F.; Feki, A.; Hoogewoud, H.-M. Granulocytic Sarcoma Presenting as a Palpable Breast Lump. Front Surg. 2017, 3, 67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Viani, L.; Zannoni, M.; Luzietti, E.; Caramatti, C.; Martella, E.M.; et al. Primitive Myeloid Sarcoma of the Breast: A Case Report. J Clin Case Rep. 2016, 6, 671. [Google Scholar] [CrossRef]
- Kudva, R.; Monappa, V.; Solanke, G.; Valiathan, M.; Rao, A.C.K.; Geetha, V. Myeloid sarcoma: A clinicopathological study with emphasis on diagnostic difficulties. J Cancer Res Ther. 2017, 13, 989–993. [Google Scholar] [PubMed]
- Huang, X.E.; Li, Y.J.; Zhou, X.D. Granulocytic sarcoma of the breast: A case report. Oncol Lett. 2015, 10, 2447–2449. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, A.; Dolek, B.A.; Barca, N.; Aktas, H.; Araz, L.; Kulacoglu, S. Ultrasound Findings in a Case of Myeloid Sarcoma of the Breast. J Belg Soc Radiol. 2016, 100, 15. [Google Scholar] [CrossRef] [PubMed]
- Girshova, L.; Romanova, E.; Kholopova, I.; Nikulina, T.; Mirolubova, J.; Zaritskey, A. Isolated Myeloid Sarcoma Involving the Breast. Blood. 2012, 120, 4345. [Google Scholar] [CrossRef]
- Zhai, J.; Kong, X.; Yang, X.; Gao, J.; Xuan, L.; Wang, X.; Wang, J.; et al. An uncommon granulocytic sarcoma of the breast: a case report and literature review. Onco Targets Ther. 2018, 11, 3685–3690. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, J.R.; Admirand, J.H.; Gualco, G.; Medeiros, L.J. Myeloid Sarcoma Involving the Breast. Arch Pathol Lab Med. 2005, 129, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Pileri, S.A.; Ascani, S.; Cox, M.C.; Campidelli, C.; Bacci, F.; Piccioli, M.; et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007, 21, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.F.; Saydam, G.; Sahin, F.; Baran, Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013, 3, 265–270. [Google Scholar] [PubMed]
- Bakst, R.L.; Tallman, M.S.; Douer, D.; Yahalom, J. How I treat extramedullary acute myeloid leukemia. Blood. 2011, 118, 3785–3793. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, I. A clinical review of breast involvement in acute leukemia. Leuk Lymphoma. 2006, 47, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Avni, B.; Koren-Michowitz, M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011, 2, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Kinoshita, T.; Yashiro, N.; Ikeda, Y.; O’Uchi, T. MR findings of granulocytic sarcoma of the breasts. Br J Radiol. 2006, 79, e112–e115. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the author. 2020 Bouhani Malek, Sakhri Saida, Jaidane Olfa, Adouni Olfa, Chargui Riadh, Rahal Khaled
Share and Cite
Malek, B.; Saida, S.; Olfa, J.; Olfa, A.; Riadh, C.; Khaled, R. Extra Medullar Granulocytic Sarcoma: A Case Report of an Exceptional Localization. J. Mind Med. Sci. 2020, 7, 110-113. https://doi.org/10.22543/7674.71.P110113
Malek B, Saida S, Olfa J, Olfa A, Riadh C, Khaled R. Extra Medullar Granulocytic Sarcoma: A Case Report of an Exceptional Localization. Journal of Mind and Medical Sciences. 2020; 7(1):110-113. https://doi.org/10.22543/7674.71.P110113
Chicago/Turabian StyleMalek, Bouhani, Sakhri Saida, Jaidane Olfa, Adouni Olfa, Chargui Riadh, and Rahal Khaled. 2020. "Extra Medullar Granulocytic Sarcoma: A Case Report of an Exceptional Localization" Journal of Mind and Medical Sciences 7, no. 1: 110-113. https://doi.org/10.22543/7674.71.P110113
APA StyleMalek, B., Saida, S., Olfa, J., Olfa, A., Riadh, C., & Khaled, R. (2020). Extra Medullar Granulocytic Sarcoma: A Case Report of an Exceptional Localization. Journal of Mind and Medical Sciences, 7(1), 110-113. https://doi.org/10.22543/7674.71.P110113


