Highlights
- The multiple faces of oncogenesis are the result of the inter-relational complexity of genetic, environmental and evolutionary constraints expressing the phenotype, similar to the interrelation of energy, mas and information.
- The kaleidoscopic and multi-level perspective of oncogenesis is a promising area for multiple therapeutic access gates of which only complexity science and trans-disciplinarity are capable.
Highlights
- √
- The multiple faces of oncogenesis are the result of the inter-relational complexity of genetic, environmental and evolutionary constraints expressing the phenotype, similar to the interrelation of energy, mas and information.
- √
- The kaleidoscopic and multi-level perspective of oncogenesis is a promising area for multiple therapeutic access gates of which only complexity science and trans-disciplinarity are capable.
Abstract
Oncogenesis is an extremely complex phenomenon. The mechanisms by which cancer is induced is only partially known. Consequently, therapeutic targets may be uncertain and results are often unsatisfactory. The purpose of this paper is to develop a trans-level and multiple trans- disciplinary perspective describing the kaleidoscopic reality of oncogenesis. This manner of understanding oncogenesis as a complex process characterized by a non-linear dynamic, far from equilibrium and with unpredictable evolution, transcends the classical perspective and requires a paradigm shift. This approach is also facilitated by recent studies that focus on group phenomena, with emerging behaviors in a continuous phase transition. Biological systems, and obviously the human organism, express this type of behavior with critical self-organizing valences in the context of a genome—mesotope (environment)—phenotype interaction. For example, nature has transposed in the ecosystem, among other things, the performance pattern of its mineral history represented by the dynamic energy-matter-information unit (the principle of invariance). And multi-cell biological systems in the phylogenetic tree crown have multiple directed aerobic metabolisms in accordance with specific functions. Cancers, in turn, have a hybrid (anaerobic and aerobic) and unidirectional metabolism whose only and ultimate reason is the survival of the malignant cell. Understanding the transdisciplinary reality of oncogenesis offers novel development paths for new therapeutic strategies compared to current ones which have relatively limited efficiency.
1. Introduction
1.1. Some preliminary notions
Cell division is a complex, programmed, teleological, and controlled cell multiplication process by scissiparity. Consequently, numerical and quantitative cellular growth, type 2n, is arranged according to the constraints imposed by the local conditions. The exponential increase in cell count is regulated by the divisional rate (doubling period, t/2), being controlled locally and perhaps systemically. Normal cells have a limit on the number of divisions, the Hayflick limit being 40-60 divisions. Growth is balanced not only by the rate of cellular apoptosis, but also by a reduction in the rate of cell division with age. In dividing a somatic cell, two similar, not identical, daughter cells, are produced. The division of a cell is carried out “in the mirror”, at least at the genome level, with a polarity consequence of the daughter cells. Cytoskeletal and cytoplasmic organisms segregate relatively randomly.
The most frequent types of cancer (over 90%) arise from the cancerous transformation of normal cells. The bifurcation process is gradually progressive over time, requiring many years of accumulation of a critical number of mutations that lead to a change in the cellular phenotype.
Cancer cell division is an uncontrolled process, gradually occurring over time and progressively accelerating in the frequency of divisions. As a result of this increased rhythm, transcriptional, translational, and consequence folding errors result in an increase in the number of malignant replication errors. The Hayflick limit disappears as a result of telomere restoration by telomerase activation, as well as through other possible mechanisms. Cancer cells have lost memory of apoptosis, the mechanism of cellular loss in tumors being cellular necrosis of ischemic nature. As the number of divisions increase, cancer cells become progressively younger and more aggressive. By canceling local feed-back, cancer cell behavior becomes dictatorial (abolishing the “bootstrap principle” respected by normal cells).
Oncogenesis could be considered a nonlinear dynamic process, an anti-ontogenetic and anti-phylogenetic process which moves back in time to recover the primacy of the unicellular state. The evolution of oncogenesis is far from thermodynamic equilibrium, exhibiting high complexity [1], robustness [2], and adaptability [3].
Ontogeny revises phylogeny (Ernst Haeckel). In ontogeny, information, energy, and matter are used according to an equation of everything, E x m x I = 1, where E is energy, m—mass, I—information. Variable combinations are required for the construction of the future being/entity from the zygote and then through the stages of the embryo, fetus, and body with different growth periods. Biological systems depend on energy, material, and information flows, with humans representing the highest performing in their simultaneous and interactive uses. From a single totipotent cell, there is a complex organism consisting of approximately 200 cell phenotypes corresponding to about 1014 cells or “cell states”. In evolution, the cells, three-dimensional shapes containing energy and information, describe a diagram in the phase space following “continuous phase transitions” [4].
Each phase transition, resulting from the configurations allowed by thermodynamic constraints, corresponds to an enormous number of microscopic states, denoted by W in the Boltzmann entropy formula, S = k log W. All cells in the structural composition of the human organism shared the same initial “quantum” state (holomeria: all in one!). And, from this perspective, they remain “entangled” for the rest of their life. In the intrauterine and post-natal period, there is progressive “sliding”, a “decoherence” by translating the “superposition cellular state packet” and the construction of a tissue, organic, and systemic specificity (an “unsealing” of multi-potentiality with the extraction of a certain specification—eigenstate) (Figure 1). The mesotope* (Mesotope is the immersion environment of terrestrial life with its content in energetic, substantial and informative, interactive elements that condition the behavior of living systems) is responsible for decoherence, for the functional-structural “specification” of tissues and organs in space-time [6]. The organization in a scale-free network of systems is nothing more than a powerful argument to affirm the bottom-up principle of invariance from the genomic, transcriptomic, proteomic, and metabolomics level to cellular, tissue, and organic networks. Obviously, each stage of organization has elements of specificity and each node has its own identity, unlike the Erdὂs-Rényi and Watts-Strogatz models in which there is no difference between the network nodes [7]. Compared to the “traditional” scale-free network, the organ system network is critical and can no longer grow. This situation is compensated by the reconfiguration phenomenon under the conditions of demands, of the environmental energy field fluctuations.
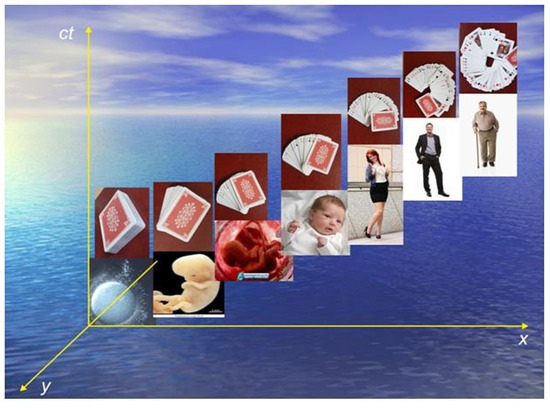
Figure 1.
Metaphor of human ontogenetic decoherence, emergence of antagonistic interactions of genome—mesotope—evolutionary developmental biology. Decoherence or “functional-structural specification” of tissues, organs, systems in space-time Minkowski (time coordinate—complex number) is a diagram of successive chronologically delimited transitions of the human life in time. There is an invariant relativist interval between transitions, Δs. It measures the distance between two events in space-time. Decoherence is of quantum origin and is mainly the result of mesotope intervention on the initial condition of superposition of zygote states (maximal state of genome density) by the intervention of the conscious observer. The exemplification by progressive exposure of playing cards is similar to the Schrödinger cat’s experiment. The mesotope is the “conscious observer” that exposes the game cards and extracts from the coherent potential the specificity of the decoherence of some periods of life.
The mechanisms by which cancer is induced remain obscure as well as the disturbances in the reconfiguration of subsystems following phase transitions. In oncogenesis, there is an initial cell superposition: both the normal cell and the malignant one share the same initial condition of the energy field (similar to the systems that share the same quantum initial state). This means that they still remain in a relationship of correspondence (“spooky action at a distance”—Einstein). Phase transitions describe a “deterministic” pathway from the normal cell to the bifurcation when following an asymmetrical division, a normal cell and a modified, mutant cell, result. Here too, the diagrams are fundamentally different and the biological realities diverge. The phenomenon of cancer transformation involves multiple structural and functional mechanisms and energy and information components embedded in the matrix of a complex spatial network, where the nodes are correlated interactively and at a trans-level state. Less differentiated than the normal cell, the cancer cell exceeds the normal one in terms of the superposition of probabilities, in terms of state density. In other words, from a mathematical point of view, the combinatorial complexity of the cancer cell is superior and it therefore has a functional ascendant on the normal cell. The normal cell is more resilient in terms of functional and structural stability to mesotope fluctuations.
This resilience, understood as a conservative identity of shape in a permanently fluctuating environment. originates in a personalized history, continuously reiterated and stabilized by feedback regulators. From the same perspective, the cancer cell is “fluid” and “obedient”, with no history, easily deformable in a dynamic, fluctuating environment. The instability of the cancer cell is due to the lack of regulatory feedback (an essential feature). The cancer cell is not willing to enter an antagonistic relationship with the factors that aggress upon it. Rather, it conforms, in fact, is reconfigured and thus not destroyed. Cancer easily accepts the change in genomic and phenotypic identity. The only and last reason for cancer is the survival and restoration of cell supremacy through an anti-system riot.
2. Discussions
2.1. Cancer—A Kaleidoscopic Reality
For purely methodological reasons, to make the phenomenology of oncogenesis more accessible, we tried a multiple approach from the perspective of the contribution to knowledge of different sciences, theories, and hypotheses, their invocation being made in an approximately historical order of contribution to understanding this type of pathology.
Clinical and imaging medicine, whose main tool is observation, considers cancer genesis a local process of uncontrolled proliferation of cellular clones which develops an invasive mass effect. Subsequently, the effects on diathesis, the seeding of new territories by metastasis, and cytotoxicity through cytokines were also understood. Angiogenesis induced by malignant tumors opens up important ways of energy influx at the expense of the host organism. Histopathological studies have identified changes in architecture, cell size and form, structure and geometry of microtubules, nuclear atypia, and chromatin disorganization. Scanning electron microscopy has highlighted impressive changes in plasma membrane relief of cancer cells (the fractal size is around 1.7), similar to lymphocytes, the expression of an in-forma-tional “inflation” (in the sense of Shannon entropy!). Fractal geometry and its defining mechanism, recursion, used only partially by cancer, contribute to understanding the “metastability” of cancers because the repetition of the genetic sequences can effectively detect and correct point mutations of one bit (Hamming code). In cancers, instability (errors) is progressively amplified, simultaneously with the reduction of error correction capability ≥ 2 bits, due to the large accumulation of entropy (non-correlative bits >> 2bits).
Biochemistry has identified ancestral metabolic pathways, aerobic fermentation (Warburg effect), cellular lactic acidosis, telomerase activity, aberrant proteins, and electric field decrease (membrane potential).
2.2. Classical Thermodynamics
Unlike the normal cell characterized by stability (low energy level, i.e., minimal, optimal interaction—Maupertuis, with the mesotope), the cancerous cell has a high energy level, being unstable, similar in this way to an “excited” atomic nucleus. If the excited atomic nucleus disintegrates, the cancer cell, as the “excited” cell, divides. The high energy level is the result of increased in- and out-cellular energy flows. The cancer cell is characterized by the state of energy inflation: enthalpy is increased, Gibbs free energy (used only for system maintenance), and unusable energy defined as entropy and eliminated as heat are also increased. Based on the principle of conserving the total energy of the cell, free energy is consumed at the high and uncontrolled rhythm of the divisions, the result being the stabilization of the oncogenic hub (metastable order) and the practical spraying of nuclear genome stability (disorder). Essential for sustaining life is “access to entropy” (Ludwig Boltzmann), to negentropy (free energy Gibbs) necessary for self-organization that sensitively outweighs the local production of entropy under the imperative of the second law of thermodynamics. In cancer cells, the need for free energy is massive as a result of genomic reconfiguration with each division that is a phase transition. That is why the energy substrate access is wider compared to the rather narrow range of normal, specialized cells. Primary, functional, and behavioral, non-specialized “energy sucker” cancer cells use small molecules (high entropy) from normal neighboring cells, from apoptotic cells or destroyed by proteolysis and lipid dislocation secondary to tumor proximity. The cancer cell has a selective growth advantage due to the excess entropy. Entropy takes into account matter and information, energy, which is the support of information, and information, which is the matrix that drives the organization of matter. From a thermodynamic point of view, entropy is the disorganization, loss of order and structural information of biological systems—the physical aspect of information encoded in matter configurations and in the energy field (Wheeler, 1990, quoted by 8). The death of the body is the maximum degree of entropy, uniformity, paid with the dissipation of heat in the environment. Living systems follow the meaning of “time’s arrow” and, through death, it dissolves into a mineral form when the loss of heat causes entropy to reach zero (S = 0), near the temperature of 0oK (Nernst’s theory reformulated by Max Plank). Practically, the human organism remains alive by the import of entropy [9]. And this is possible only through its evolution away from equilibrium, on the border with disorder, the entropy inflow being balanced by heat dissipation in the mesotope. Imported entropy is partially converted into Gibbs free energy embedded in the order of body structures and partially returns to the environment as degraded energy (heat) or “waste energy”. Thus, the normal cell entropy is lower than that of the cancer cell, in turn, less than the metastatic cell entropy (ΔS normal cell <ΔScancer cell <ΔS metastatic cell).
2.3. Information Theory
From the point of view of information theory, Shannon entropy refers to the loss of structural and functional information. The entropy of a system represents the amount of uncertainty one particular observer has about the state of this system [10]. The breakdown of semantic information blocks (logons—sentences and phrases) in structural information, construction elements, (bits—words, i.e., unrelated information) ultimately leads to increasing the number of bits (Kolmogorov entropy) by “deconstruction” (Jacques Derrida). Consequently, a biological system characterized by order requires by definition a significantly lower number of bits (“deterministic” dynamic system, even in the variant of deterministic chaos) than a “disordered system”, like a cancer cell or other “probabilistic” dynamic systems. In cancer, molecular changes can be quantified in terms of increased entropy or loss of information [11,12,13]. The cancerous cell has lost its memory. The loss of correlative information (the phenomenological, semantic aspect of information) has consequences on the increase of energy and, less significantly, on the mass, according to the equation of everything: E x m x I = 1. The internal adjustment of the equation is found in the compensatory increase of entropy and heat export as metastability effort by deepening aneuploidy. The replication of the malignant cell is followed by increased cellular uncertainty and obviously it has no evolutionary targets. The “infection” with mixed computer viruses, hardware and software, is followed by the progressive destruction of the initial program (increasing the number of bits), with every run of the genetic program (replication). And this increases the risk of new mutations followed by the deepening of aneuploidy. In ontogeny, normal cell replication is progressively more restrictive (environmentally-induced constraints and unitary phenotypic functioning), and because of this, it necessarily has evolutionary targets. If in normal cells, the structural fractal organization of nuclear DNA attenuates gene errors by dissipating and blocking the expression in replication, accelerated cancer cell division amplifies gene errors, an argument for the loss of the fractal pattern of DNA. Life as order emerged from disorder, and the maintenance of order is possible only by exploiting the disorder. There are always real degrees of freedom on the same border with disorder. Cancer, as a pathological form of life, is a disorder unleashed from order, and it remains a disorder only by exploiting the order.
2.4. Evolutionary Developmental Biology and Deterministic Chaos Theory
The evolution of any normal cell is subject to genetic constraints, evolutionary developmental biology (evo- devo) constraints (similar to the bootstrap theory!) and mesotopic (environmental) constraints. When we reiterate the logistic function x = ax (1—x) in discrete mode we obtain the equation xt+1 = axt (1 -xt), where t is the time that takes the integer values 0, 1, 2, 3... and the co- efficient a ranges between 1 and 4, interval in which behavior can be oscillating or non-oscillating, periodic and chaotic. The co-efficient a is environmentally dependent and reflects field fluctuations. When the co- efficient a takes values between 1 and 3, normal cell replication tends toward a stability phase called attractor (the correlation between the initial conditions and the final state), which geometrically takes the form of a spider web that weaves inwards (Figure 2) and finally stops at a point, point attractor. It is an intriguing similarity with space-time geometry and geodesics. The space bends around every material particle, and the deformation of the space acquires the characteristics of a gravitational attractor. When a > 3.58, the evolution of the logistical function of cellular duplication becomes chaotic. At this value, disordered replication characterizes cancer. It is the maximal energy phase in which there is no other preferential attractor, the cellular proliferation being rapid and uncontrollable, with accumulation of matter capable of accentuating space-time deformation and reproducing a functional phenomenon similar to the singularity of black holes in the cosmic texture, obviously on a very small scale. When a reaches value 4, the division stops abruptly and cancer cell necrosis occurs [14]. The attractor with its basin is illustrative of the behavior of the dynamic system or of the chaotic system. Chaotic systems appear only randomly, but in reality, they are constituted by complex types of stable order elements which, although not regular and repetitive, maintain the dynamic system within the limits of predictable behavior. This is certainly within a probability field where it has a particular geometric configuration. The attractor of cancer expresses a metastability state that parasites the dynamics of the normal surrounding tissue, attractor identifiable in Waddington’s epigenetic landscape [15]. It is well known that malignant tumors stimulate the development of highways by which they preferentially attract energy and information flows detrimental to surrounding normal tissues. The therapeutic removal of malignant tissue determines the attractor’s turn towards a spatial geometry compatible with tissue close to the physiological behavior. In other words, the objectives of cancer therapy become problems of space-time manipulation, of space- time geometry. Constraints of local geometry have been used experimentally in reprogramming specialized mature cells and reversing them into multipotent stem cells [16].
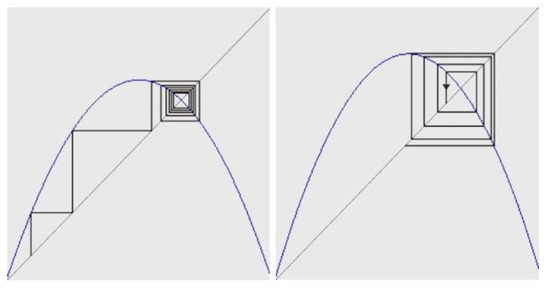
Figure 2.
Attractor geometry (“spider-web”- concentric woven cloth) for logistic function xt+1 = axt(1-xt), when a takes values between 1 and 3, normal cell division tends towards stabilization and then finishes in a point (point attractor). The cancer cell comes out of this range and when a > 3.58, cellular replication becomes chaotic and there is no preferential attractor anymore.
2.5. Quantum mechanics. The cancer cell as an “anti- cell”.
The evolution of the normal cell is forward-looking in time, in the future, and supports the thermodynamic constraints of apoptotic entropy in an interactive relationship with the tissue context (bootstrap theory). The cancer cell has a reverse, anti-ontogenetic and anti- phylogenetic pathway, progressively deeper in time (Davies). Cancer is a reverser to the singularity of coherence, the “sealing” of functional multi-potency from which a single function is extracted: replication. The potentiality is highly energetic, and the higher the energy, the fuzzier the shape, the field of multiple probabilities, realm of multiple histories. The cancer cell becomes solitary and “selfish” (like Dawkins’s selfish gene!) [17]. The similarity in quantum electrodynamics is the antiparticle, one of Feynman’s diagrams being extremely suggestive: the positron has the propagator oriented back in time. In the sudden change of direction of the electron (as a particle), its propagator being oriented in the sense of the “time’s arrow”, an energetic photon is emitted and the electron is metamorphosed in the positron (the antiparticle, which differs only by the inverted charge!), its propagator being overturned back in time (Figure 3). The orientation of the sense of evolution “in the mirror” to the normal cell makes the cancer cell a selfish and aggressive anti-cell compared to the normal, altruistic cell. Thereby, cancer cells develop cellular cannibalism or cellular cytosis, being able to endocyte and digest other tumor cells or lymphocytes [18,19]. Cancer cell metabolism resorts to ancestral pathways (fermentation) under aerobic conditions (Warburg effect). It is a high- speed metabolism that makes the cancer cell an unstable cell like genotype and phenotype; as a result of energy, inflation is an “excited cell”. It dissipates a large amount of heat (Q—degraded energy) in the extracellular environment, but also the total internal energy (U) is significantly higher than the normal cell. These parameters are linked by the formula:
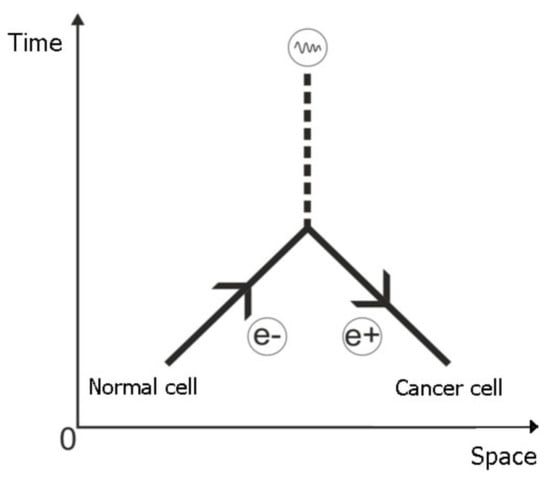
Figure 3.
Feynman diagram. The electron propagator evolves in time. When the electron radiates a photon (the bifurcation) the propagator trajectory returns in time as a propagator of the positron. There is similarity between the evolution of normal cell time and “the return in time” of cancer cell.
ΔU = Q−L
where L is the transfer of energy through coherent motion in the mechanical, chemical, osmotic, electric work.
Metabolism, in the lactic acid atmosphere, reanimates telomerase activity (ancestral enzyme) that contributes, together with other phylogenetic archaic factors (c-myc oncogene present in fresh-water hydra) to the immortality of the cancer cell. It is, at the level of current knowledge, an ontological paradox: youth without old age and life without death are contained in the cancer cell [6]! The cancer cell is a “primitivized” cell by losing memory and immerging in archaic time (Figure 4). It is an “anti-cell” with “antimatter inclusions” that make it thermodynamically immune!
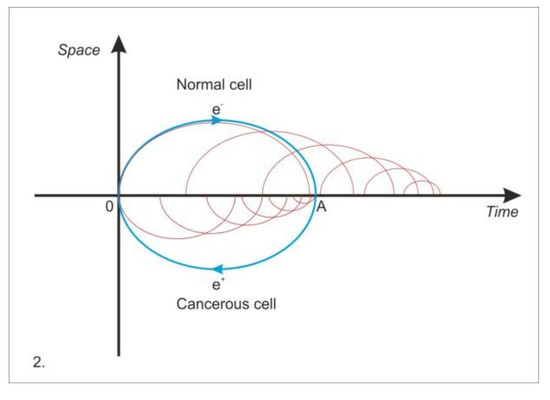
Figure 4.
Initial common destiny of the normal and cancer cell—trajectory 0A. After the coexistence in “superposition”, the normal cell follows the thermodynamic trajectory (the “time arrow”) and the cancer cell enters an “anti- thermodynamic” trajectory specific to the antimatter. If in quantum mechanics, the transition from one state to another is instantaneous, without intermediate stages, in biology, macroscopic domain (mesoscopically, in the background), we must admit as a peculiarity of perceptual continuity, intermediate states in the phase transition. The phase space diagram is made by “clinging”, by the synthesis of many phase transitions. In the normal cell, the thermodynamic orientation progressively reduces the coherence and magnitude of the metabolic processes, resulting their deceleration to the entropic extinction. The cancer cell, initially an occult presence, is reversed by anti-thermodynamics. Its consistency of metabolic processes increases and manifests itself through the superposition of all functions. It results in a maximization of replicative potential and the loss of specific memory functions.
Based on the principle of Cusanus-Bohr’s complementarity (Bohr said the opposite elements are not contradictory), antimatter is a necessary reality in phase opposition with matter. In fact, anti-matter is not an anti- structure, it is the “same” matter with “mirror” image, the same particle that at successive moments turns, behaviorally, to the contrary, similar to the fermions intuited by Ettore Majorana in 1937 (Majorana fermions), particles which are their own antiparticles as has been recently demonstrated (e.g.,: neutrino, K0 kaon). The antimatter intervenes in a lightning manner, like a switch, capable of causing a bifurcation, a quiet phase transition or a brutal discontinuity similar to macroscopic catastrophes (René Thom) with impressive energy release. On a microscopic scale, a phase transition is also the passage of an atom or elementary particle from one state to another with the emission of a photon, without passing through intermediate states.
Experimentally, reversal of the time arrow has been found in the case of two spins. Numerical simulations show that reversing the time arrow can also occur for a spin that interacts with larger spin environments [20]. “There is order in the form of correlations” (David Jennings—Oxford University) and “the order is like fuel”, consumed to reverse the flow of heat and the meaning of the energy arrow. That means that the cold gets colder and the warm gets warmer. The normal “warm” cell heats up and becomes “warmer” as a malignant cell. Quantum thermodynamics comes to support and clarify phenomena that the classic paradigms find bizarre. In the last 50 years, quantum mechanics has become a more prominent presence in biology, although the “substance of life” is warm (300-310 K), wet and noisy, conditions considered to have been restrictive until then. The foundation of our cosmic nature is quantum mechanics through specific mechanisms at the DNA level (proton tunneling, genes superposition, interference), proteins, enzymes, metabolism, in the process of vision, smell, consciousness, etc. Cancer cell entropy is estimated to be twice as high as normal cell entropy. The in-“forma”- tional expression of malignant cell entropy is made “in its form”, in its shape, the membrane relief approaching that of lymphocytes being also conditioned by cytoskeletal plasticity. In the generalized form of the entropy law (quantum thermodynamics), which takes into account the interactive correlations between components, reversibility is firm, energy dissipation being the exchange currency to reduce entropy as a local, orderly, symmetrical and stable pattern. The dissipation of energy in the body’s “micromedium tissue” also increases its entropy (extracellular matrix disorganization and stiffening, disruption of M2 macrophages). Chromatin studies in the MRI have revealed that its disorganization obviously has also perturbed the memory function. And, just like in the brain, the recent genetic memory related to the “inhibitor factors” of the genetic behavior of “inscriptions” on social, community life of cells, group cell altruism (apoptosis). Storing new information involves deleting the content of the original genetic memory to create a place in the “genetic software” with the heat payment of kTln2 (Boltzmann’s statistical entropy) as established by the Landauer principle, also recently verified in quantum mechanics. In the malignant cell, current information is about molecules and micro-molecules taken from healthy cells or from normal apoptotic cells or destroyed by proteolysis and adipose dislocation by tumor cells in order to be used for their own growth. Nuclear DNA entropy, equivalent to a progressive excess of unrelated information (huge number of bits) generates electrochemical disorder in synthesis and metabolism, in polarization and resting membrane potential, in cytosolic pH reduction, etc. This involves complex processes of reconfiguration of the cellular genome in the context of genetic instability propagated in molecular instability and cellular reorganization. Electron transfer blocking, the occurrence of dipole-dipole interactions, membrane dielectric layers, disrupt static cellular electromagnetism and electrochemical, molecular gradient levels, etc. The cancer cell is an “energy parasite” that compensates for the growth of entropy by extracting free energy from healthy cells. The cancer cell holds high potency, but the clutter of the nuclear genome prevents upgrading towards distinct functions except survival and replication. The higher the number of cellular divisions, the sooner the malignant cell accesses pathways and metabolic mechanisms specific to the archaic time of the primitive phylogeny of cell phenotype, profoundly altered, making it impossible to establish the parental, oncogenic origin cell lineage. Therapeutic insufficiency in cancers is the result of a hiatus between the aerobic metabolism of the body and primitive metabolism, acidophilic, of undifferentiated cancers. The cancer cell’s “hybrid metabolism” has other priorities in the entropy partition as compared to healthy cell metabolism. “Hybrid metabolism” is difficult to intercept and correct as the “hybrid war” built and developed based on parameters other than the classical ones. Cancer therapy is applied on the basis of eminent classical schemes. In the metabolomics network, the targets (nodes) whose role is that of a switch can be identified and can reorient time’s arrow. Then, the cancer cell, instead of being destroyed, can be “reeducated”, “re-entwined” on the trajectory of classical thermodynamics, of entropy, under whose auspices the destiny of any organism is carried out.
The malignant cell is in a continuous and alert phase transition, resulting in the immanent cellular uncertainty [21,22] and hence the probability field of the directions of use of the energy potential. Deleting qubits with each phase transition involves very high energy costs across the chain from transcription to translation. The effort of genomic “stabilization” of maintaining an informational software, although ultimately failing as anaplasia, has proportional energy costs. Heat, as degraded energy, gets through convection into the cytosol, then extracellularly, the organism representing the entropy uptake system. Initially, it has a physical bath behavior, but in time, the disorder becomes significant and the import of negentropy from the environment can no longer compensate for it. Electromagnetic field fluctuations generate an increased state density with a field of probability extended as the phase space. The evolution of macroscopic biological systems, however, is characterized by determinism, even if, frequently, it is unpredictable according to the theory of deterministic chaos. The causal, epigenetic phenomena triggered by the macroscopic environment propagate at the quantum level where they are “absorbed” and “dissipated” into a probability field, the effects being not linear and immediate. The correlation phenomena in quantum thermodynamics generate paradoxical, bizarre, unexplained events for the conceptual framework of the macroscopic world. That is why uncertainty, superposition, interference, entanglement, as quantum phenomena, have diffused into the biological, cellular reality and are difficult to be intercepted therapeutically, at least for the time being, with classical means. From this perspective, it seems that our therapeutic targets are wrong, and therefore the results are unsatisfactory.
2.6. Entropy and Network Science
Cancer cell entropy is determined by the quantitative analysis of gene expression or of various modified, expressed protein molecules. Network differential entropy between normal and cancer samples is determined by comparing the mRNA network and the interactive protein network expressed in normal (mRNA-IPn) and cancer cells (mRNA-IPc). Differential entropy can identify important genetic modules involved in cancer that are otherwise undetectable [23,24]. Real network events, obviously in biological ones, “are far from being random” [6]. They are second order phase transitions that can be reversible, that is, “we can distinguish polynomial laws regardless of the direction from which we approach the critical point, from order to disorder or vice versa” [6]. Near the critical point, “the distance that spins know each other is progressively higher” [6] and the imminence of the bifurcation (the theory of deterministic chaos!) becomes reality. In this situation, the cellular system is either reconfigured or lost. “The transition from disorder to order has begun to present an astonishing degree of mathematical consistency [26]. But the other way around! West confirms the increase in network entropy in cancers and brings new arguments for the greater significance of local network entrances compared to systemic, non-local ones [25]. The theory of dynamic systems asserts that the “elasticity”, resilience, and robustness of a macroscopic system, R, correlates with the level of uncertainty or entropy (disorder), S, of the dynamic, microscopic processes [26], atomic-molecular, which underpin the structural-functional organization of the macroscopic system. More specifically, ΔR·ΔS > 0, where ΔR and ΔS represent changes in the robustness and entropy of the system [27]. Hence, robustness, R, correlates with the level of uncertainty, entropy, S. In Boltzmann’s relation, S = k ln W, and S is the maximum for the maximum value of W –the number of microstates, the number of possible system particle conformations.
Stimulation of an oncogene (network node) by inhibiting the suppressor factors leads to the opening of new pathways in the regulatory gene network. In terms of local network entropy, there is a reduction in uncertainty in those paths that open up to the information flow. Thus, within a physiological network module, the management of information flows is reconfigured by deflecting them on the new open paths by lifting the interdiction (blocking) suppressors. The novelty of the newly opened path, made much more attractive by the new epigenetic landscape, is preferentially accessed and proposed, for this reason, for selection, by favoring flows as stated by the constructional theory launched by Andrei Bejan [28]. This theory, though not explicitly stated, has the principle of Maupertuis’s minimal action as its engine. However, any biological system develops reconfigurations and passive constraints of the hierarchy of flow networks. As a result, there is often an alternation with the principle of maximum action capable of increasing the organization and efficiency of the system [29]. The necessary energy and time costs, significant when phase transitions are bifurcated, can be minimized by the evolution of the system near the edge of chaos. Here opens up the opportunity to extract energy for the construction of the new order capable of promoting the increase of the body’s efficiency standards facing environmental dynamics. The informational and energetic configuration of the epigenetic landscape due to field fluctuations is the one that decides the direction of system evolution.
3. Conclusions
As a stage conclusion, we consider that the multiple faces of oncogenesis are the result of the inter-relational complexity of genetic, environmental (mesotope), and evolutionary constraints expressing the phenotype, similar to the inter-relation of energy, mass, and information (Euler’s three-body problem, unsolved, no general analytical solution as Poincaré showed). If in the last relationship, information is the mathematical and physical matrix, in the first, it seems that the evolutionary developmental biology (the phenotype) fulfills this role, which it strives to play! So far, cancer could not be defeated in direct confrontation! The future will demonstrate if we can prove the ability and the wisdom to accede to the holistic clairvoyance of the human body’s behavior immersed in the mesotope (environment) that contains and restrains it. The kaleidoscopic and multi- levelled perspective of oncogenesis is also a promising area for multiple therapeutic access gates which only complexity science and transdisciplinarity may be able to reach. Reversing the evolution of the cancer cell and re- educating it (repairing and recovering cellular memory) can be an appealing alternative to the extremely aggressive classical therapeutic war resulting in serious collateral effects.
Conflicts of Interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Deisboeck, T.S.; Berens, M.E.; Kansal, A.R.; Torquato, S. Stemmer-Rachamimov AO, Chiocca EA. Pattern of self-organization in tumour systems: Complex growth dynamics in a novel brain tumour spheroid model. Cell Prolif. 2001, 34, 115–34. [Google Scholar] [PubMed]
- Kitano, H. Towards a theory of biological robustness. Mol Syst Biol. 2007, 3, 137 PMID: 17882156. [Google Scholar] [CrossRef] [PubMed]
- Rockmore, R. Cancer complex nature. Santa Fe Institute Bulletin. 2005, 20, 20. Available online: https://sfi- edu.s3.amazonaws.com/sfi-edu/production/.../10/.../spring2005v20n1.pdf.
- Longo, G.; Montévil, M. From physics to biology by extending criticality and symmetry breakings. Prog Biophys Mol Biol, 2011, 106, 340–7. [Google Scholar] [CrossRef] [PubMed]
- Calotă, F. Defaying the hazard (In Romanian). Editura Academiei Române: București, 2017; pp. 39–461. ISBN 978-973-27-2823-9. [Google Scholar]
- Albert-László, Barabási. Linked. Noua ştiinţă a reţelelor (In Romanian). Editura Brumar: Timişoara, 2017; p. 111. ISBN 978067260885. [Google Scholar]
- Meijer, D.K.F. Information: What Do You Mean? Syntropy Journal. 2013, 3, 1–49 http://wwwlife energyscienceit/english/2013. [Google Scholar]
- Boltzmann, L. McGuinness, B., Ed.; The Second Law of Thermodynamics; Theoretical Physics and Philosophical Problems. Vienna Circle Collection; Springer: Dordrecht, The Netherlands, 1974; Volume 5, pp. 24–27. ISBN 978-94-010-2091-6. [Google Scholar] [CrossRef]
- Adami, C. Information theory in molecular biology. Physics of Life Reviews. 2004, 1, 3–22. Available online: www.elsevier.com/locate/plrev.
- Davies, P.C.; Demetrius, L.; Tuszynski, J.A. Implications of quantum metabolism and natural selection for the origin of cancer cells and tumor progression. AIP Adv. 2012, 2, 11101. [Google Scholar] [CrossRef]
- Frieden, B.R.; Gatenby, R. Information dynamics in living systems: Prokaryotes, eukaryotes, and cancer. PLoS ONE. 2011, 6, e22085. [Google Scholar] [CrossRef]
- Davies, P.C.; Rieper, E.; Tuszynski, J.A. Self-organization and entropy reduction in a living cell. Biosystems. 2013, 111, 1–10. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mambo, E.; Sidransky, D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006, 25, 4663–74. [Google Scholar] [CrossRef]
- Motofei, I.G. Biology of Cancer; From Cellular Cancerogenesis to Supracellular Evolution of Malignant Phenotype. Cancer Invest. 2018, 36, 309–317. [Google Scholar] [CrossRef]
- Waddington, C.H. The Strategy of the Genes; George Allen & Unwin Ltd.: London, UK, 1957; p. 16. [Google Scholar]
- Roy, B.; Venkatachalapathy, S.; Ratna, P.; Wang, Y.; Jokhun, D.S.; Nagarajan, M.; Shivashankar, G.V. Laterally confined growth of cells induces nuclear reprogramming in the absence of exogenous biochemical factors. Proc Natl Acad Sci USA 2018, 115, E4741–E4750. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, R. The Selfish Gene: 30th Anniversary Edition-with a New Introduction by the Author; Oxford Landmark: Science, 2006; pp. 12–65. ISBN 13:978-0198788607, ISBN 10: 0198788606. [Google Scholar]
- Lugini, L.; Matarrese, P.; Tinari, A.; Lozupone, F.; Federici, C.; Iessi, E.; Gentile, M.; Luciani, F.; Parmiani, G.; Rivoltini, L.; Malorni, W.; Fais, S. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006, 66, 3629–38. [Google Scholar] [CrossRef] [PubMed]
- Overholtzer, M.; Mailleux, A.A.; Mouneimne, G.; Normand, G.; Schnitt, S.J.; King, R.W.; Cibas, E.S.; Brugge, J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007, 131, 966–79. [Google Scholar] [CrossRef]
- Micadei, K.; Peterson, J.P.S.; Souza, A.M.; Sarthour, R.S.; Oliveira, S.; Landi GBatalhão, T.; Serra, R.; Lutz, E. Reversing the thermodynamic arrow of time using quantum correlations. arXiv 2017. [Google Scholar]
- Potten, C.S.; Loeffler, M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990, 110, 1001–20 PMID: 2100251. [Google Scholar] [PubMed]
- Theise, N.D.; Krause, D.S. Toward a new paradigm of cell plasticity. Leukemia. 2002, 16, 542–8. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E.; Severini, S. Increased entropy of signal transduction in the cancer metastasis phenotype. BMC Syst Biol. 2010, 4, 104. [Google Scholar] [CrossRef]
- Hudson, N.J.; Reverter, A.; Dalrymple, B.P. A differential wiring analysis of expression data correctly identifies the gene containing the causal mutation. PLoS Comput Biol. 2009, 5, e1000382. [Google Scholar] [CrossRef]
- West, J.; Bianconi, G.; Severini, S.; Teschendorff, A.E. Differential network entropy reveals cancer system hallmarks. Sci Rep. 2012, 2, 802. [Google Scholar] [CrossRef]
- Demetrius, L.; Manke, T. Robustness and network evolution-an entropic principle. Physica. 2005, A 346, 688. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell. 2011, 144, 646–74. [Google Scholar] [CrossRef] [PubMed]
- Bejan, A. Constructal theory of pattern formation. Hydrol Earth Syst Sci 2007, 11, 753–768. Available online: www.hydrol-earth-syst-sci.net/11/753/2007/.
- Mesina, C.; Stoean, L.C.; Stoean, R.; Sandita, V.A.; Gruia, C.L.; Foarfa, M.C.; Ciobanu, D. Immunohistochemical expression of CD8, CDX2, P53, D2-40 and KI 67 in colorectal adenocarcinoma, conventional and malignant colo-rectal polyps. Revista de Chimie. 2018, 69, 419–428. [Google Scholar]
© 2019 by the author. 2019 Firmilian Calotă, Cristian Meșină, Stelian Ștefăniță Mogoantă, Dragoș Calotă