Introduction
Secondary diabetes is a relatively common entity in chronic pancreatitis, so named pancreatogenic diabetes or types 3c diabetes. It is mostly due to changes in insulin secretion by pancreatic beta cells at islands. As a rule, the insulin secretory deficit is complete, following a long evolution of chronic pancreatitis. Beta cell secretory function decreases relatively parallel to the decline in pancreatic exocrine function [
1]. Most patients with chronic pancreatitis have an exocrine pancreatic insufficiency closely correlated with endocrine function. The prevalence of alteration of glucose tolerance is 40-70%, half of these patients having insulin-susceptible diabetes. Endocrine failure is progressive and occurs on average ten years after the diagnosis of chronic pancreatitis. The severity of endocrine dysfunction is dependent on the degree of pancreatic parenchymal involvement, but is also affected by the level of alcohol consumption. In insulin- dependent patients who develop secondary diabetes mellitus of chronic pancreatitis, beta cell function is somewhat preserved, and glucoregulation is better, compared to patients with type I diabetes [
2,
3].
Data show that T3cDM is commonly associated with chronic pancreatitis, with a prevalence from 25% to 80%. A significant risk factor is the duration of chronic pancreatitis, which also causes the onset of diabetes due to exhaustion of the pancreatic endocrine reserve [
4,
5].
In the case of hereditary pancreatitis, the mean age of secondary pancreatitis diabetes is between 38-53 years, and in the case of chronic pancreatitis for other causes, the onset age is less specific [
6,
7].
Over the past 20 years, significant efforts have been made both in deciphering the genetic mechanisms that can compete with chronic pancreatitis and in the study of risk factors and/or the progression of this disease [
8]. However, the link with T3cDM requires more systematic investigations to determine the genetic potential for differentiation between currently known diabetes, such as type 1 diabetes (T1DM), type 2 diabetes mellitus (T2DM), and latent autoimmune diabetes of adulthood (LADA), etc. [
9].
From an immunological perspective, the association of T1DM with specific HLA alleles has not been demonstrated in secondary diabetes of chronic pancreatitis. This aspect was also able to support the fact that patients with chronic pancreatitis reach late (in the stages of disease evolution) absolute insulin, secondary diabetes being successfully treated with oral antidiabetics [
10].
A small proportion of patients with chronic pancreatitis and secondary diabetes develop a total deficiency of endogenous insulin secretion, but this does not completely alter the secretion of alpha cells, which is still minimally preserved. From a pathophysiological point of view, after an extended period of chronic pancreatitis evolution, the pancreas loses enough beta cells so that insulin secretion decreases to complete deficiency. The small mass of remaining cells no longer responds to glucose level diffusion perisinusoidal. Alterations in the enteroinsular axis also correlate with decreased secretory secretion levels, but diminishing secreted glucagon levels appear as a consequence of exocrine dysfunction of alpha cells. Glycemic contractility is not preserved, hypoglycemia does not stimulate glucagon secretion, and catecholamine secretion is maintained in secondary diabetes compared to T1DM. Hypoglycemia is more severe than in T1DM due to a lack of glucagon secretion stimulation and probably also due to associated liver disease, malnutrition, and alcoholism, all of which are often associated with chronic pancreatitis [
11].
Diagnosis of secondary diabetes by chronic pancreatitis is based on the determination of pancreatic C-peptide and apparently concomitantly with the glycemic profile or the oral glucose tolerance test (OGTT), along with the determination of glycosylated hemoglobin level (HbA1C%). At the onset of decline in endocrine function, HbA1C% or peptide C is not helpful as an assessment criterion.
Determination of post-prandial glycaemia may provide real benefits in assessing the decline in beta cell function. For such situations, diet, alcohol withdrawal, and oral antidiabetics offer the main treatment options. Insulin therapy and basal-bolus enhancement are indicated for patients with good compliance, and a 7% HbA1C target is preferred (compared to 6.0%) to avoid hypoglycemia that may be extremely severe for these patients. In patients with pancreatogenic diabetes, ketoacidoses are rare events [
12,
13].
The secretion of the pancreatic polypeptide is absent in the secondary insulin-dependent pancreatitis due to pancreatic beta cell damage with exocrine damage. The plasma level of somatostatin secretion is increased in patients with insulin-requiring diabetes secondary to chronic pancreatitis [
14,
15].
Long-term complications in patients with diabetes secondary to chronic pancreatitis compared to those in T1DM are highly dependent on the duration of diabetes itself. Life expectancy is generally generally reduced especially if patients do not give up alcohol and tobacco [
16].
Materials and Methods
Two hundred and sixty-one patients, women and men with a chronic history of pancreatitis, who came to the medical visit for re-evaluation, were investigated. Other patients who did not have the diagnosis of pancreatogenic secondary diabetes but who had chronic pancreatitis were also investigated.
Results
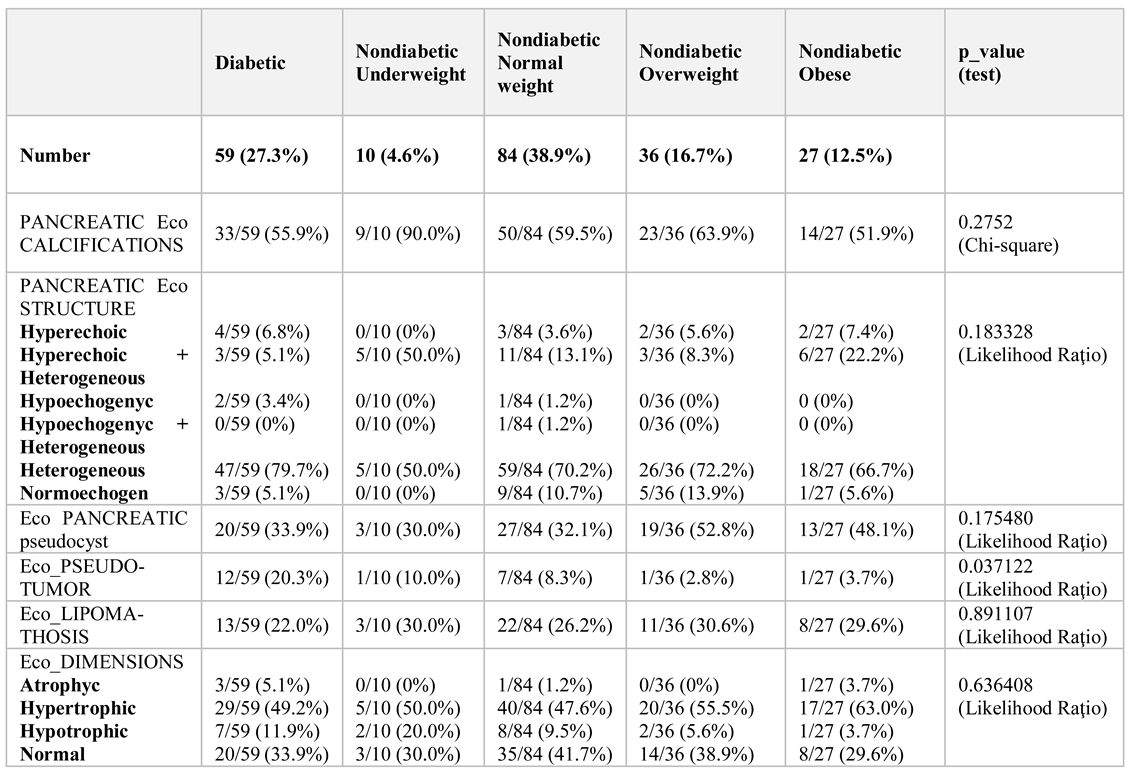
Of the 216 patients diagnosed with chronic pancreatitis, only 59 (27.3%) cases developed secondary diabetes, documented prior to the visit. The patients were 22.2% women and 77.8% men, with average ages of 56.8 years and 53.4 respectively. 63% came from urban areas.
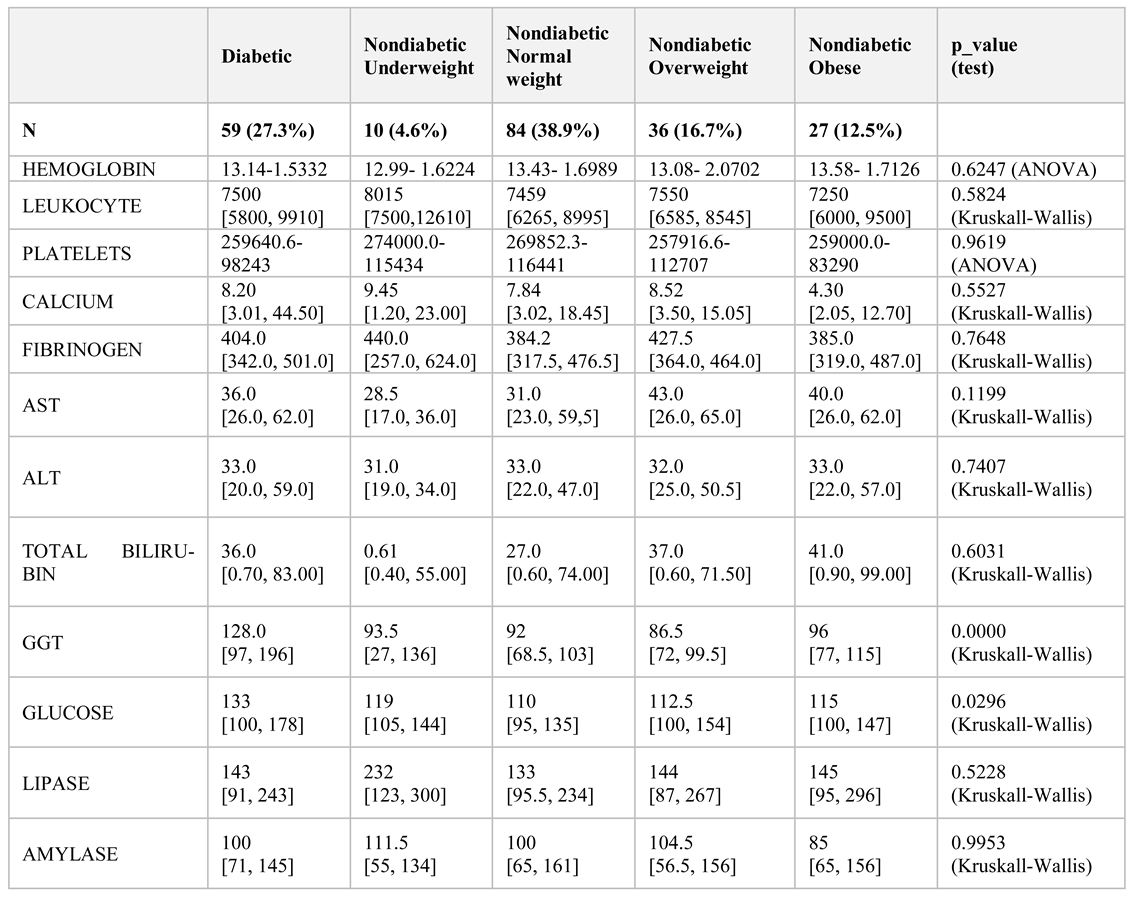
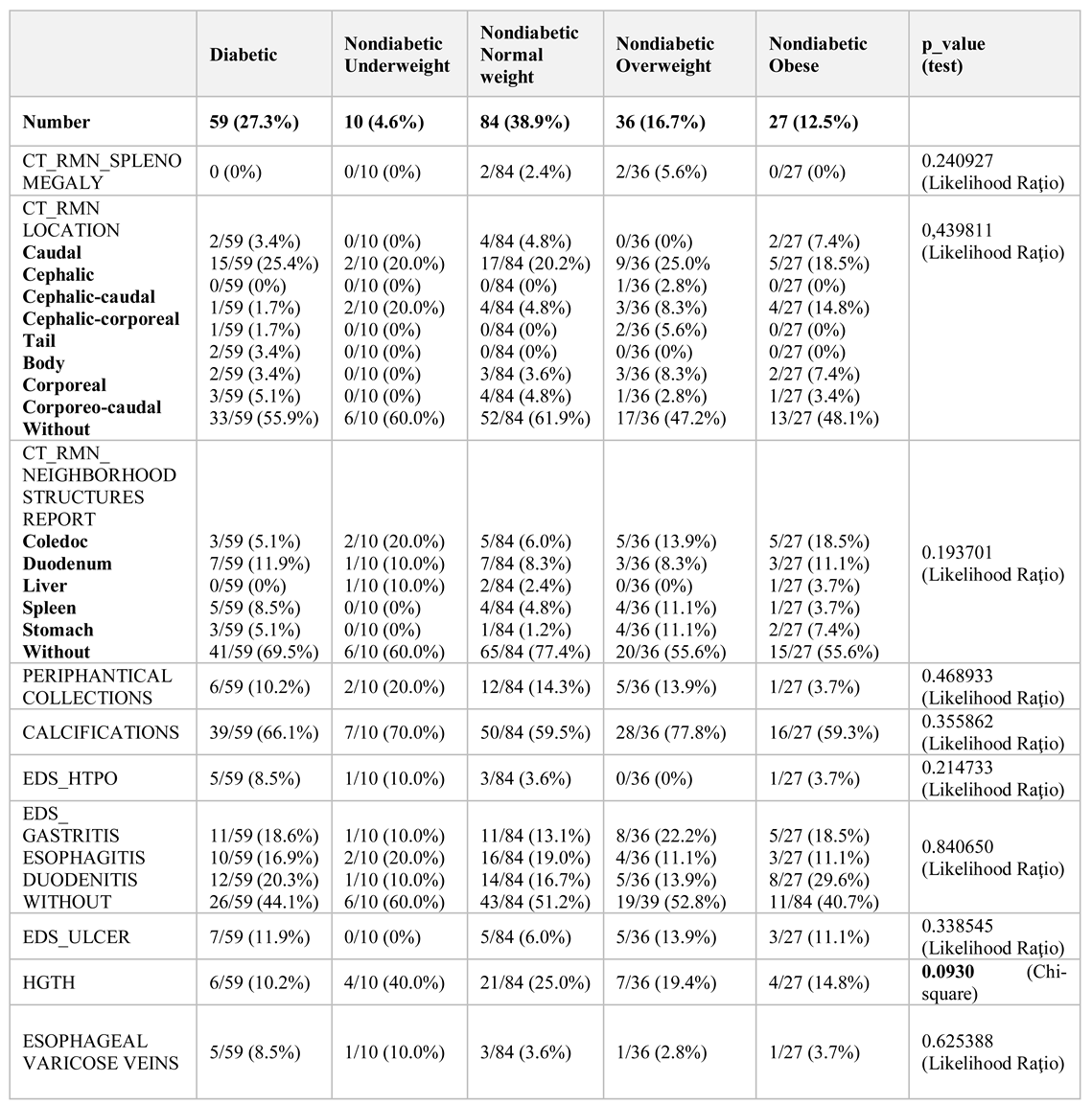
The mean duration of chronic pancreatitis was six years. Non-diabetic patients were compared with patients who had previously been diagnosed with diabetes and who were analysed for body mass index (BMI). Biochemical data and imaging investigations were performed: abdominal ultrasound, computed tomography, and nuclear magnetic resonance (
Table 1 and
Table 2). We used IBM SPSS 20.0 for statistical analysis Excel and Epi Info.
Following the analysis of the batches,
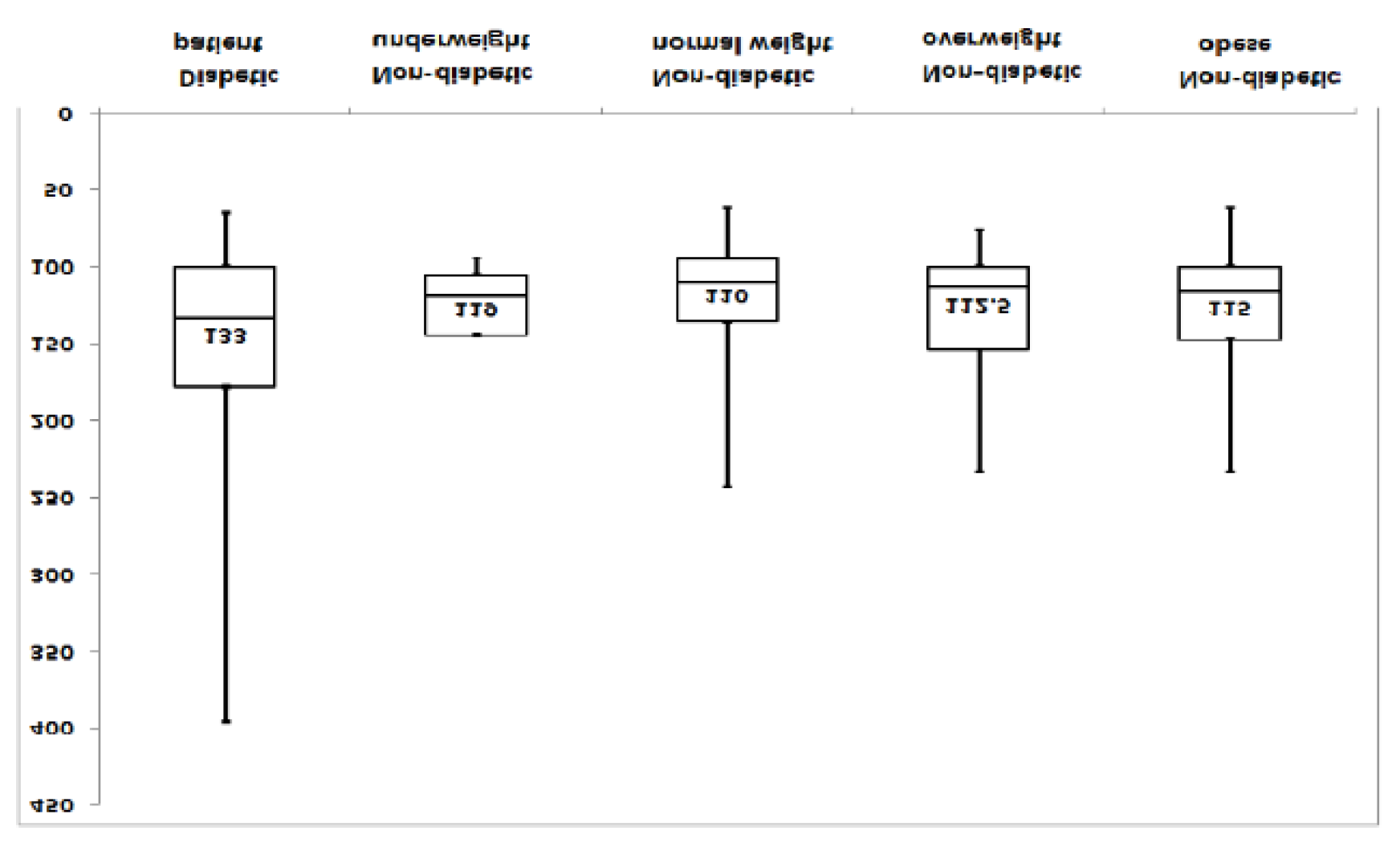
diabetes patients had an average basal blood sugar of 133 mg / dL [25%, 75%] = [100, 178], the minimum value was 65 mg / dL, maximum: 396 mg / dL,
non-diabetic patients showed the following: minimum value is 94 mg / dL, maximum: 145 mg / dL, median: 119 mg / dL, 25% 75% = [105, 144],
normoponderal nondiabetics: the minimum value is 62 mg / dl, maximum: 243 mg / dl, median: 110 mg / dl, [25%, 75%] = [95, 135.5] the lowest is 62 mg / dl, the maximum: 234 mg / dl, the median: 112.5 mg / dl, [25%, 75%] = 100, 154,
obese nondiabetics: / dl, median: 115 mg / dl, [25%, 75%] = [100, 147]).
Figure 1.
Basal blood glucose assessment.
Figure 1.
Basal blood glucose assessment.
Basal blood glucose (a jeun) was 133 mg/ dl (normal <100 mg / dl) in the diabetic and non-diabetic groups with an average mean value between 110 and 119 mg/ dl, which suggests that basal glycemia is already being altered in patients who have not been diagnosed with diabetes secondary to chronic pancreatitis. Patients diagnosed with secondary diabetes mellitus with chronic pancreatitis had a mean HbA1c% of 7.9 and peptide C of 0.7 ng / mL. Reference values for peptide C were 0.81-3.85 ng / mL.
Non-diabetic patients also had altered glycemic values, requiring additional HbA1C% and C-peptide tests, possibly performing a glucose tolerance test (OGTT). The glycemic difference between the two groups, diabetic vs. non-diabetic, is explained by the fact that diabetic patients were already undergoing specific metformin and basal insulin therapy, and those in the second batch were found with glycemic values altered during the re-evaluation visit without having previously received extensive diabetic investigations or specific treatment. Differences between batches were significant (p = 0.0296 Kruskal-Wallis): Diabetics-Nondiabetic- normoponderal: p value = 0.010725.
Determination of gamma-glutamyl transpeptidase (GGT) in:
diabetic patients show a minimum value is 19, maximum, 2513, median: 128, [25%, 75%] = [97, 196];
underweight nondiabetics: 308, median: 93.5, [25%, 75%] = [27, 136],
normoponderal nondiabetics: minimum value is 15, maximum: 973, median: 92, [25%, 75%] = [68.5,
overweight: the minimum is 23, maximum: 932, median: 96, [25%, 75%] = [27, 99.5] and obese nondiabetic patients: = [77, 115].
Comparing the values between batches, significant differences are obtained (0.0000 Kruskall-Wallis): Diabetic-Nondiabetic normoponderal: p value = 0.000072 and Diabetic-Nondiabetic overweight: p value = 0.000469 (
Figure 2).
GGT catalysis the transfer of the γ-glutamyl group from peptides such as glutathione (GSH) to other amino acids. The increase in serum GGT is also due to cell membrane damage by toxins (including alcohol), ischemia, infections, or cell membrane disruption of the enzyme as a result of bile acid action. GGT is an enzyme specific to the liver and bile ducts. Normal levels in women are <36 U / L and in men <61 U / L.
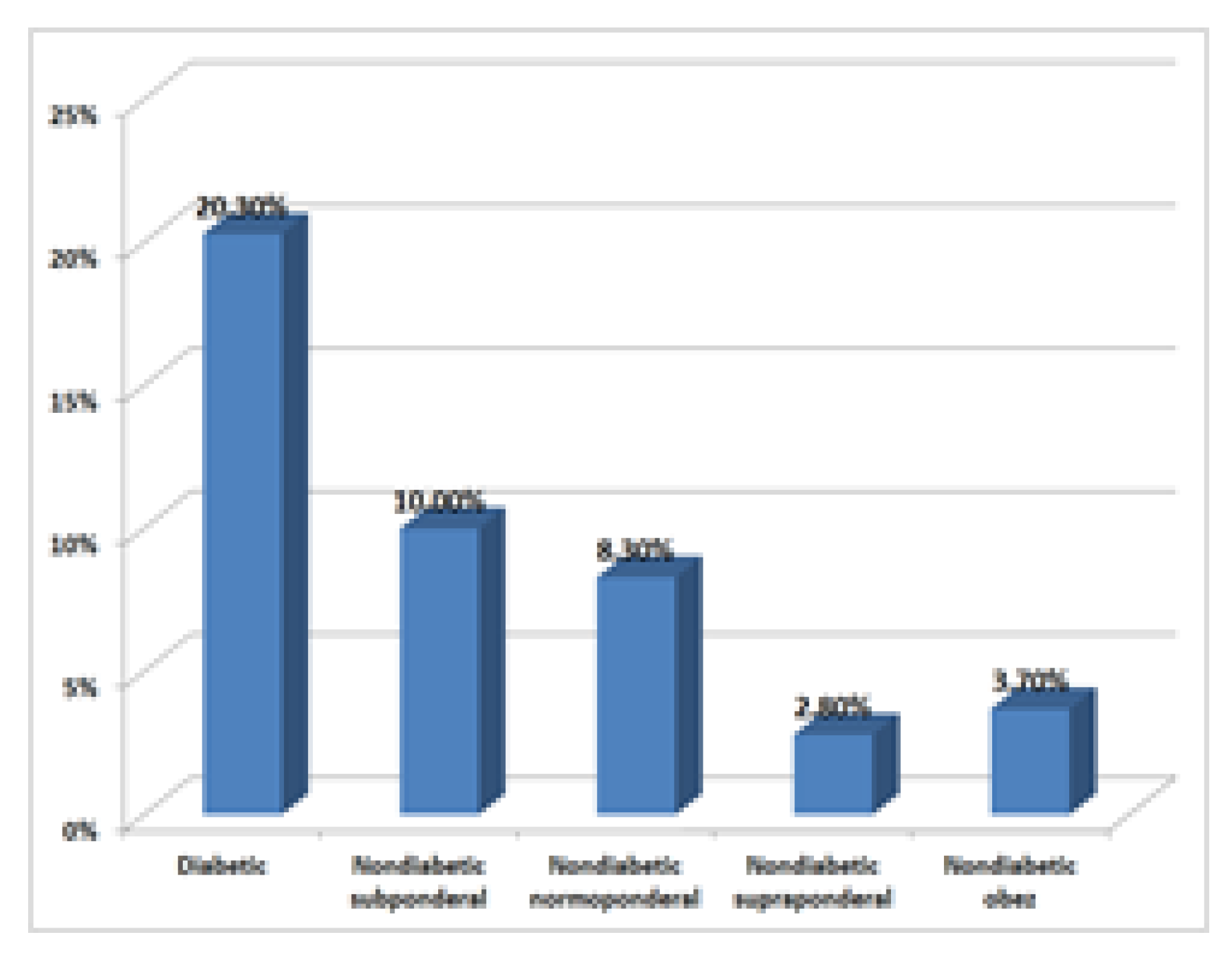
Imaging investigations, especially routine abdominal ultrasound, revealed an incidence of tumours and pseudotumors in diabetic patients 12/59 (20.3%), underweight nondiabetics 1/10 (10.00%), normal nondiabetic 7/84 (8.3%), overweight overdose 1/36 (2.8%), and obese nondiabetics 1/27 (3.7%).
CT scan confirmed these data. Tumour density at the pancreatic level was the highest in the diabetic group, 20.3%, reiterating the hypothesis that the incidence is higher compared to non-diabetic patients, as previous research has shown
By comparing the percentages between diabetics (20.3%) and overweight nondiabetics (2.8%), p = 0.06354, marginally statistically significant (
Figure 3).
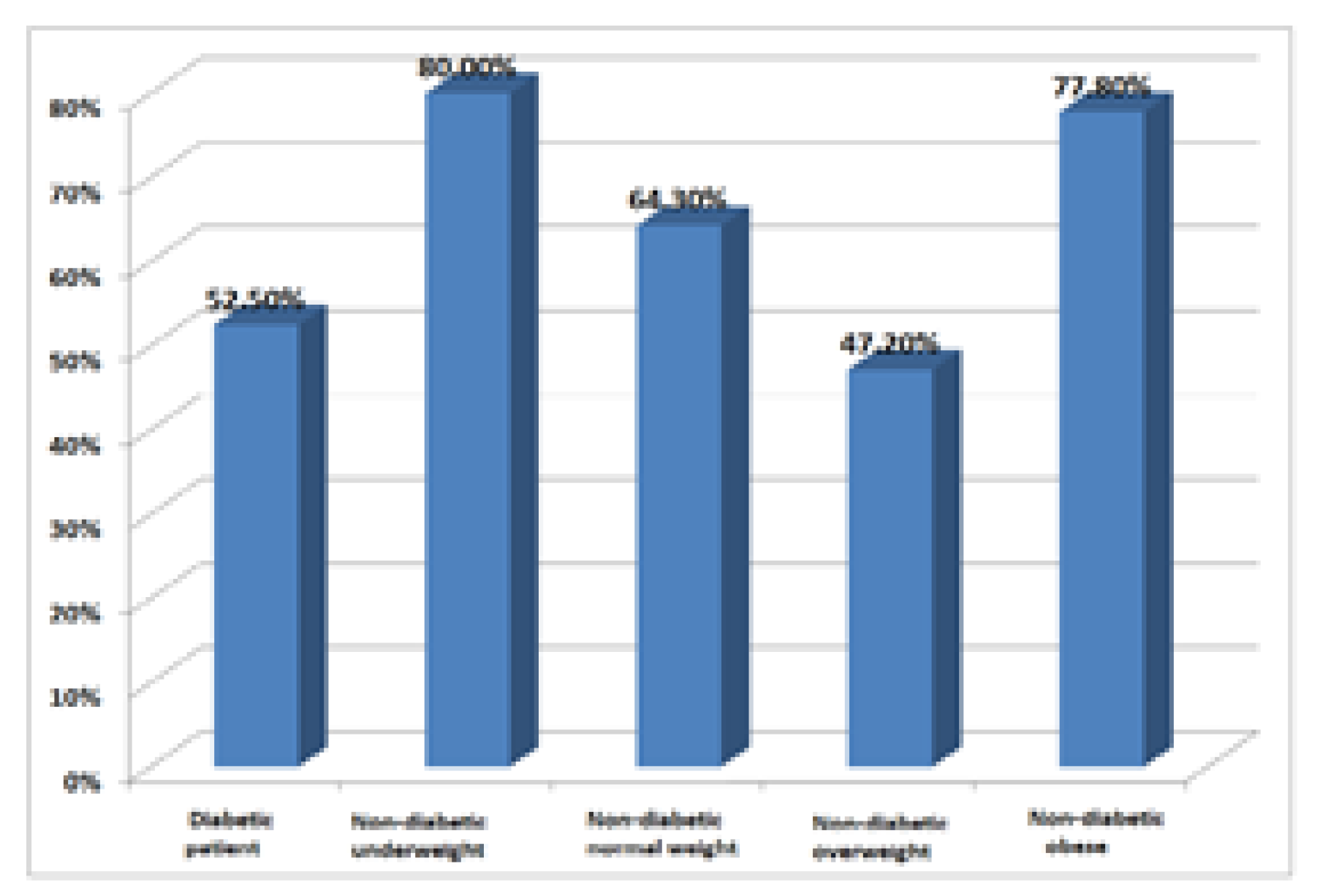
Consumption of ethanol for diabetic patients was 52.5% compared to non-diabetic patients 41,4%. Chronic ethanol consumption is not only a risk factor that worsens chronic pancreatitis but is also a known risk factor for chronic liver disease. Chronic pancreatic and hepatic injury can lead to the installation of caloric protein malnutrition. Underweight patients with chronic pancreatitis should therefore also be investigated from a nutritional perspective, malnutrition further negatively affects the prognosis.
The association between alcohol consumption and sex in the whole sample showed that the proportion of alcohol-consuming women (27.1%) differed significantly from the percentage of men consuming alcohol (70.2%) (p = 0.000001 Chi-square). Smoker status was described in 18.8% of women and 62.5% of men (p = 0.000001 Chi-square) (
Figure 4).
Discussions
Compared to type I DM, T3cDM shows many different properties: glycemic lability, more frequent hypoglycemic episodes, and minimum incidence of ketoacidosis. The need for insulin administration to achieve satisfying diabetes mellitus compensation is significantly lower and response of peripheral tissues to endogenous and exogenous insulin substantially higher compared to type I diabetics. These clinical differences result from decreased but always preserved insulin secretion, decreased glucagon production, impaired external pancreatic secretion, excessive alcohol or tobacco use, or insufficient or irregular food intake. Secondary DM in chronic pancreatitis is accompanied by chronic, microangiopathic, and neuropathic complications analogous to other DM types [
16].
Diabetes secondary to pancreatic involvement was initially described and referred to as pancreatogenic diabetes. The type IIIC diabetes nomenclature was first proposed in 2014 by the American Diabetes Association, with the name type 3c diabetes now commonly accepted [
17,
18,
19]. The most widely reported conditions are the presence of chronic pancreatitis (79%), pancreatic ductal adenocarcinomas (8%), haemochromatosis (7%), cystic fibrosis (4%), and previous pancreatic surgery (2%) [
20,
21,
22].
As diagnostic criteria, Ewald and Brezel proposed 3 major criteria: documentation of exocrine pancreatic insufficiency (abnormalities of the pancreas), documented imagistic (ultrasound, CT or MRI), and absence of markers for T1DM 1 [
23]. Minor criteria involve documenting cellular dysfunction (pancreatic peptide C assay, glycemic profile), insulin resistance, alteration of secretory secretion (glucagon-like peptide- 1 [GLP-1] and/ or pancreatic polypeptides), and evaluation of liposoluble vitamin deficiency (A, D, E, K). Although the duration of chronic pancreatitis somewhat influences these criteria, the clear differentiation between T1DM, T2DM and pancreatogenic type 3C diabetes shows an imprecise standardization of β-cell dysfunction and insulin resistance. The lack of the concrete data on glucose homeostasis in T3cDM makes some interpretations difficult, so there are still many limitations in this regard, namely there are still no precise data that can standardize both diagnosis and therapeutic options (and that is why more studies would be necessary. However, with the little information available thus far, we can conclude that T3cDM is an important pathological entity, most of the time underestimated and clinically ignored, initially treated as a T2DM that evolves into insulin-deficiency, specific to T1DM. Consistent with other studies, it is essential to create management strategies for T3cDM, which must include therapeutic options for improving intestinal absorption, assessing nutritional status, and minimizing malnutrition. As our study has shown, a small percentage of patients with chronic pancreatitis (27.3%) developed T3cDM, the rest requiring investigations to confirm or nullify the diagnosis. The non-diabetic patients, who had changed basal blood glucose values, were mostly overweight and obese, and therefore cannot be ruled out in this context of insulin resistance. This situation should be investigated by laboratory tests (peptide C determination, OGTT test) which for objective reasons could not be achieved in this study. Patients predisposed to T3cDM are mostly men with a history of chronic alcohol and tobacco use, again, consistent with other research studies. Imaging evaluations have detected some abnormalities of the pancreas, from various degrees of a tumour or pseudotumor to more complicated clinical situations requiring surgery and even pancreatectomy [
24]. While any patient with chronic pancreatitis should be monitored for the development of diabetes, those with long-standing duration of the disease, prior partial pancreatectomy, and early onset of calcific disease may be at higher risk. Those patients developing DM are likely to have co-existing pancreatic exocrine insufficiency. The treatment administered on one hand, and the enzymatic insufficiency instituted following chronic pancreatitis on the other hand, contribute to the disease expression and evolution [
21]. The major risks in all these circumstances are those of severe hypoglycemia characteristic of T3cDM. Optimization of lifestyle, nutritional intervention, and education of the patient with T3cDM did not differ from that of patients with type 2 diabetes [
22,
23].
The major drawback of our study is that we initially intended to assess the evolution of chronic pancreatitis and glycemic values in patients previously diagnosed with pancreatogenic diabetes mellitus. However, we were unable to continue observations on non-diabetic patients, although they were detected in the course of the hospital visit through basal blood glucose changes. A second limitation stemmed from lack of availability of patients, access to more complex determinations in the diagnosis of pancreatic T3cDM diabetes, and the assessment of glycemic variability utilizing modern monitoring devices (continuous blood glucose monitoring systems).
Conclusions
In light of prior research, together with our results, we can conclude that T3cDM is a new pathological entity that needs to be explored more deeply to lead to improved diagnosis and therapeutic stability. More studies are needed in this regard, from both epidemiological and pathophysiological perspectives, as no conclusive data exist in present regarding the population and pathophysiological incidence (due to the fact that the exact mechanisms of onset of T3cDM are not fully known).