Highlights
- Thrombocytopenia is a common occurrence in patients with end-stage renal disease undergoing hemodialysis;
- Chronic viral hepatitis B or C in end-stage renal disease patients does not impact platelet counts, suggesting that the natural course of liver disease is slowed in subjects undergoing hemodialysis
Highlights
- Thrombocytopenia is a common occurrence in patients with end-stage renal disease undergoing hemodialysis;
- Chronic viral hepatitis B or C in end-stage renal disease patients does not impact platelet counts, suggesting that the natural course of liver disease is slowed in subjects undergoing hemodialysis
Abstract
Objectives. We evaluated platelet counts in end-stage renal disease and chronic viral hepatitis. Materials and Methods. We studied 70 patients with end-stage renal disease and chronic viral hepatitis and compared them to a control group of 45 patients without hepatitis. Results. The presence of viral hepatitis was associated with a higher prevalence of thrombocytopenia. Correlations between age, C-reactive protein, liver stiffness measurement, and platelet count were observed. C-reactive protein levels > 10 mg/dl were associated with a lower risk of thrombocytopenia in patients with end-stage renal disease and chronic viral hepatitis, yet age > 60 years, dialysis vintage > 10 years, aspartate and alanine aminotransferase levels > 20 IU/L, albumin levels < 3.5 g/dl, and fibrosis stage ≥ 3 were not related. Conclusions. Chronic viral hepatitis leads to a higher prevalence of thrombocytopenia. Platelet counts in these patients begin to decrease significantly once liver fibrosis reaches stage III.
Introduction
Thrombocytopenia (TCP) is defined as a platelet count below 150.000/mm3 and can be caused by a decrease in platelet production, increased destruction of platelets, or spleen sequestration. TCP is considered to be mild if the platelet count is above 70.000/mm3, and severe if below 20.000/mm3. Patients with a count above 50.000/mm3 are generally asymptomatic, but severe cases can present with spontaneous mucosal, intracranial, gastrointestinal, and genitourinary bleeding.
End-stage renal disease (ESRD) is associated with abnormalities in both the number of platelets and their function. TCP etiology in ESRD is multiple and includes uremia, blood loss, sepsis, and heparin treatment. Hemodialysis (HD) in itself has been described as a cause of TCP since dialysis membranes have been shown to trigger platelet adhesion, aggregation, and activation. A thrombocyte count of 50,000 is the guideline threshold value for invasive dialysis access interventions and other surgical procedures [1,2].
Patients on HD also carry a greater risk of presenting with chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, with a much higher prevalence in developing countries [3]. TCP is one of the most widespread complications of chronic viral hepatitis (CVH). It appears secondary to hypersplenism in cirrhosis, immune-mediated mechanisms, shear stress, hyperfibrinolysis, bacterial translocation, sepsis, viral suppression of platelet production in the bone marrow, and decreased thrombopoietin. TCP interferes with interferon during antiviral treatment: therapy is not started if pre-treatment levels are lower than 100.000/mm3; and at a platelet count under 25.000/mm3 after initiation of treatment, therapy is discontinued [4]. Currently recommended interferon-free treatment (grazoprevir and elbasvir) against HCV infection has a more favorable side-effect profile that does not impact platelet count [5]. Although the challenge in the care of HCV patients with thrombocytopenia in initiating or maintaining IFN containing anti-viral therapy can be avoided with the use of Direct Antiviral Agents as the primary treatment modality, baseline thrombocytopenia has the potential of increasing the risk of drug cessation. Also, patients with baseline thrombocytopenia can exhibit compromised sustained virologic response (SVR) rates in comparison with those with acquired thrombocytopenia [6]. Nucleoside analogues such as entecavir are the first line of treatment in chronic HBV infection; TCP is a very rare occurrence in patients undergoing this therapy [7].
The natural history of CVH patients with ESRD is milder, usually asymptomatic compared with those affected only by CVH, presumably because of altered immunological response or a lower viral load in subjects on HD. Liver biopsy in ESRD patients, the gold standard for evaluating fibrosis, is generally avoided because of platelet dysfunctions and the important risk of hemorrhage [8,9].
The aim of the current study is to evaluate the platelet counts in ESRD and CVH compared to ESRD only, and determine correlations between TCP and clinical markers in both groups.
Materials and Methods
We performed a multicenter retrospective transversal study, which included 70 patients with ESRD and CVH (20 with HBV, 47 with HCV, and 3 with HBV and HCV co-infection) and a control group of 45 patients with ESRD without CVH. The diagnosis of CVH was confirmed by the presence of the HBs antigen (Ag) or anti-HCV antibody (Ab) for more than 6 months, with a viral load (HBV DNA, HCV RNA) detected by PCR greater than 50 IU/L. Patients in the control group were screened for viral hepatitis by confirming the absence of the HBs Ag and anti-HCV Ab. Patients in both groups had a diagnosis of ESRD and were on HD treatment. High-flux polysulfone dialyzer membranes were used for each patient. Only patients who gave informed consent were included in the study.
We excluded comorbidities that can influence platelet count: aplastic anemia, bone marrow suppression by chemotherapy or irradiation, chronic alcohol abuse, congenital macrothrombocytopenia, human immunodeficiency virus co-infection, or antiviral treatment with interferon. Blood samples for determining alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), C-reactive protein (CRP), hyaluronic acid (HA), and lactate dehydrogenase (LDH) were taken at the start of HD sessions. Fibroscan measurements were made on the day the blood samples were taken, before HD, to determine the Metavir fibrosis stage (F). In HBV patients, the following liver fibrosis thresholds were used: 6 kPa to differentiate between F1/2, 9 kPa for F2/3, and 12 kPa for F3/4. In HCV patients, we used 7 kPa to differentiate between F1/F2, 9.5 kPa for F2/3, and 12 kPa for F3/F4. For patients with HBV/HCV co-infection, the criteria for liver fibrosis in HCV infection were used [10].
We compared the same variable in the two groups with Student’s t test to check for statistical significance between the different values when the distribution was normal, and the Mann-Whitney test when the distribution was not normal. Mean values were presented with standard deviations (SD) and median values with interquartile ranges (IQR). The Shapiro- Wilk test was used to verify whether data were normally distributed. The linear correlation between platelet count and different variables was verified by calculating Pearson’s correlation coefficient r. The Chi-square test and the Odds Ratio (OR) along with its 95% confidence interval (CI) were calculated to identify risk factors associated with TCP. Statistical analysis was carried out with the GraphPad Prism 6 software, version 6.01.
Ethical approval. All procedures involving human participants were in accordance with ethical standards of institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent. Subjects’ participation in the study was voluntary, biological samples were collected after obtaining the written consent for participation.
Results
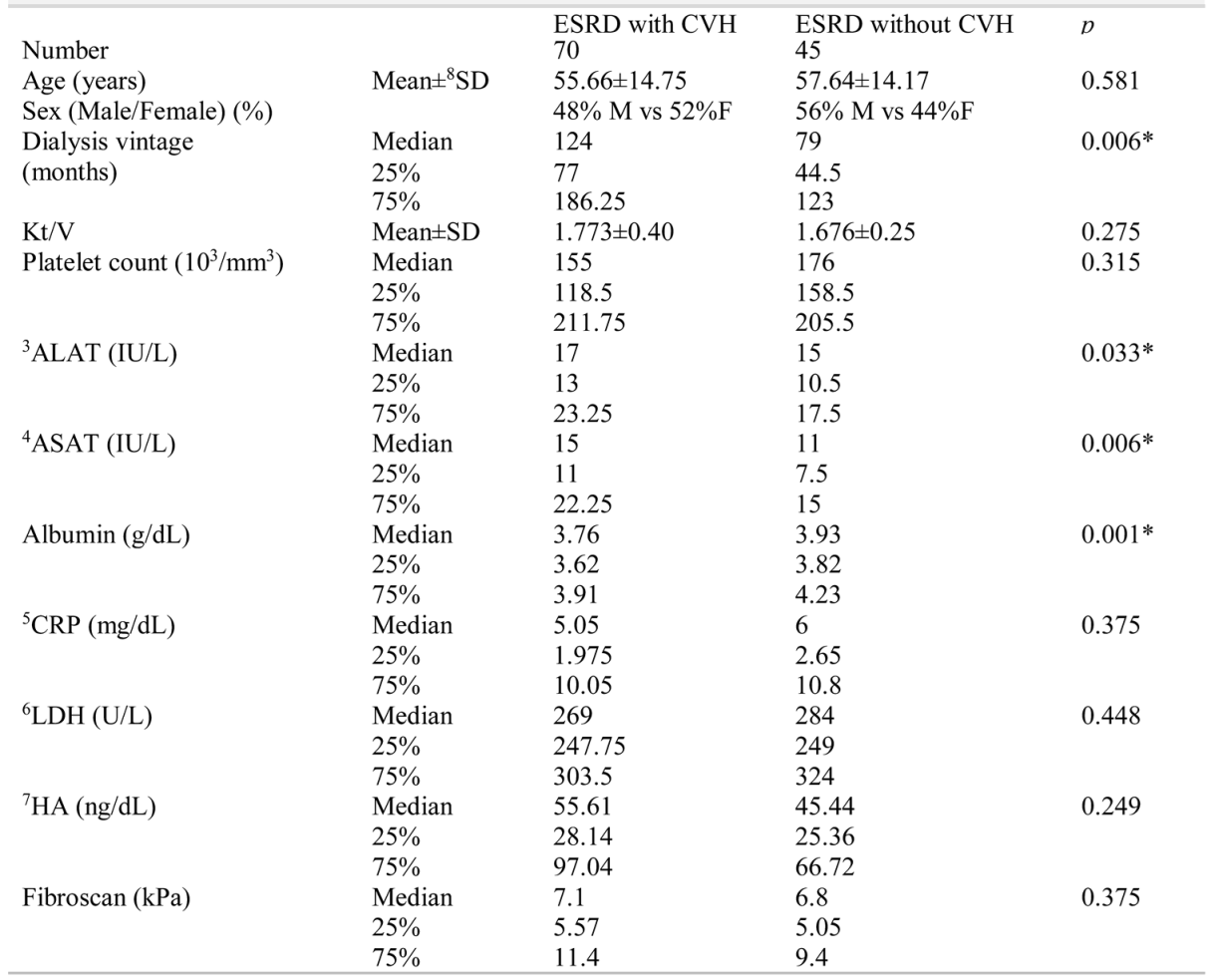
Table 1 presents the clinical characteristics of the two subject groups. The median age was 58.5 years (IQR = 47 - 68) for the group with ESRD and CVH and 56 years (IQR= 48.5 - 67) for the control group (p = 0.87). There were 24 men and 26 women in the CVH group, and 24 men and 21 women in the control group.

Table 1.
Demographical and clinical differences between patients with 1ESRD and 2CVH vs patients with ESRD without CVH.
Platelet count between the two groups was not significantly different (mean of 168.316±65.570/mm3 in the group with CVH vs. a mean of 179.068 ± 46.550/mm3 platelets in the control group, p = 0.46). Platelet count was also not different between patients with HBV and controls (p = 0.13), between patients with HCV and the control group (p = 0.72), and between patients with HCV and patients without HBV (p = 0.31).
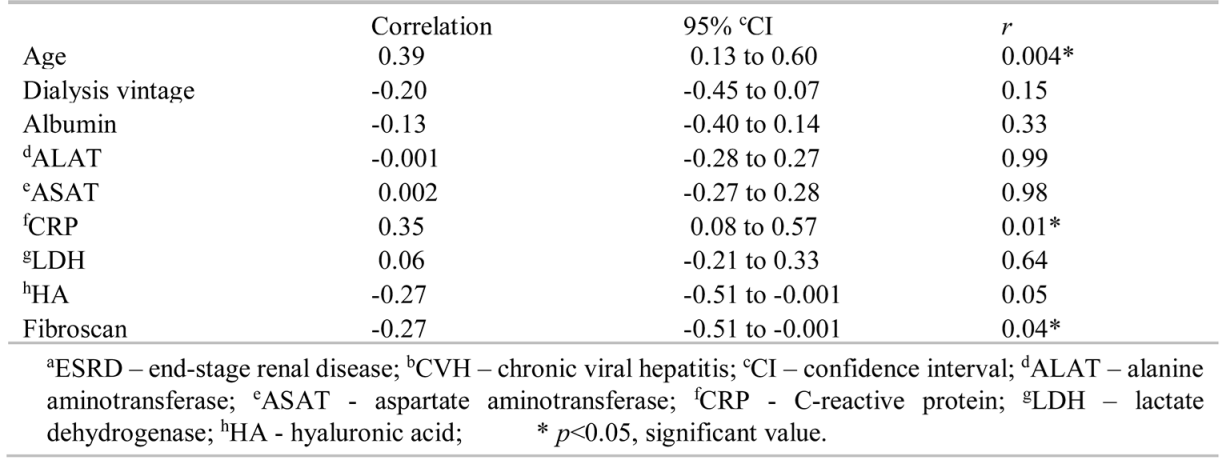
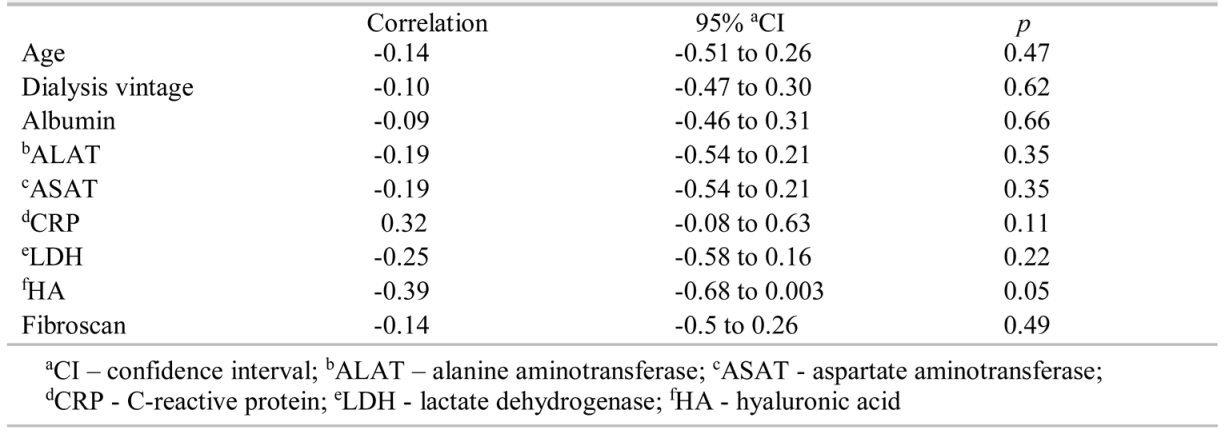
The dialysis vintage of patients with ESRD and CVH (mean of 137.6±83.95 months) was significantly higher (p = 0.004) than that of the control group (mean of 83.12±54.66 months). Patients in the group with ESRD and CVH had higher ALAT (p = 0.03), ASAT (p = 0.006) values, and lower serum albumin concentration (p = 0.001) levels, although median values were in the normal range. No statistically significant difference was found in CRP, LDH, HA, and Fibroscan values between the two groups. Positive correlations were observed between platelet counts and age (r = 0.39, 95% CI = 0.13 to 0.60, p = 0.004) and CRP (r = 0.35, 95% CI = 0.08 to 0.57, p = 0.01), and negative correlations between Fibroscan values (r = -0.27, 95% CI = -0.51 to -0.001, p =0.04) and platelet count were observed in patients with ESRD and CVH (Table 2). In the control group, none of the variables correlated with platelet count (Table 3).

Table 2.
Correlation between platelet count and other variables in patients with aESRD and bCVH.

Table 3.
Correlation between platelet count and other variables in the control group.
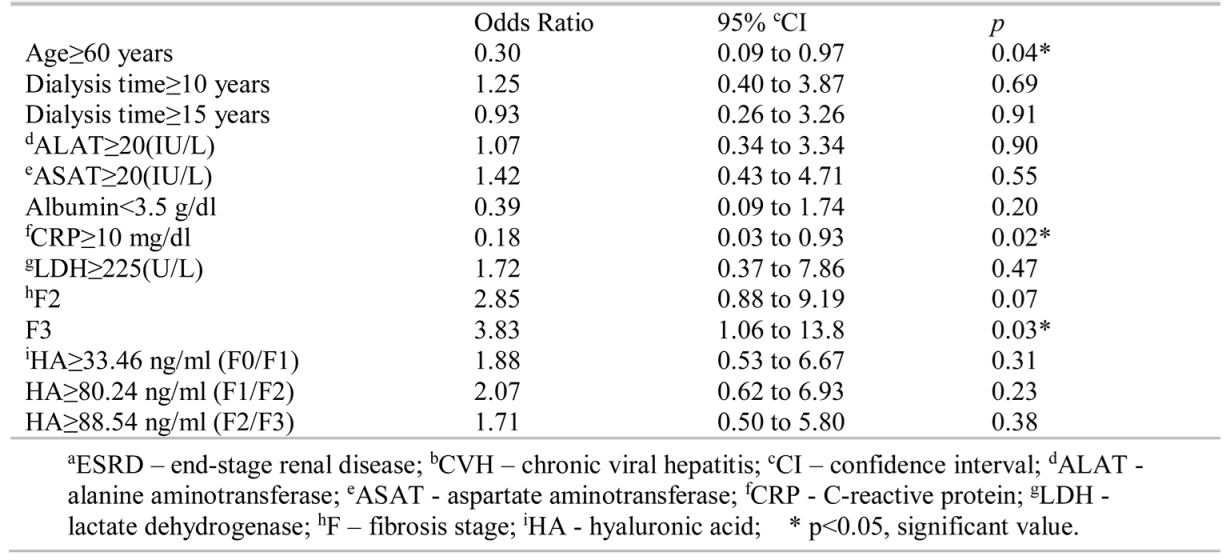
The prevalence of TCP in our study was 44% (31 subjects) in the group with ESRD and CVH (Figure 1), significantly higher (OR=3.14, 95% CI=1.01 to 9.7, p=0.04) than the 20% (9 subjects) in the control group (Figure 1). Univariate analysis showed that among the risk factors presented in Table 4, a CRP value higher than 10 mg/dl was associated with fewer TCP cases in the group of subjects with CVH.
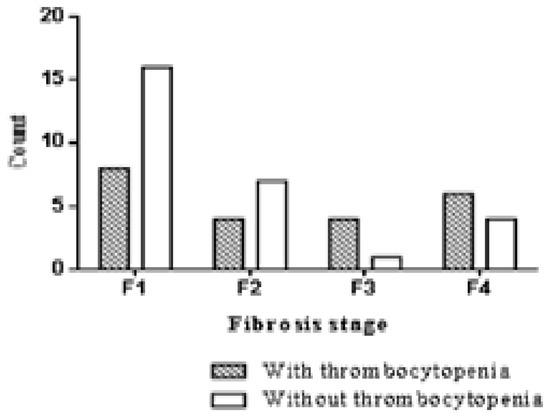
Figure 1.
Thrombocytopenia prevalence in patients with chronic viral hepatitis according to fibrosis stage.

Table 4.
Different variables and their association with thrombocytopenia in patients with aESRD and bCVH.
Liver fibrosis stage 3 was a risk factor for TCP. We found no significant difference in platelet count between F≥2 and F0-1 patients (p=0.25) or between F≥3 and F0-2 patients (p=0.37) when F was evaluated by HA. We used 80.24 ng/ml as the serum HA concentration for the F1/2 threshold, and 88.54 ng/ml for F2/F3, values that we validated for the detection of liver fibrosis in a previous study [11].
Discussions
Although TCP prevalence was higher in patients with ESRD and CVH, we did not observe a statistically significant difference in platelet count between the two groups, because most patients had mild TCP with values above 100,000/mm3.
This result is different from results reported by Nishida et al., which evidenced a significantly lower platelet count in patients on HD with CVH compared to subjects on HD alone (159.000 ±53.000/mm3vs 193.000±73.000/mm3, p<0.001) [12].
A significantly lower platelet count (p<0.01) in patients on HD with HCV (145.300±38.300/mm3) compared to patients on HD without HCV (174.900± 50.600/mm3) was also reported by Ando et al. Their study also found that megakaryocytopoiesis showed a time-dependent reduction in patients on HD, and peripheral destruction and sequestration in patients with HCV infection contributed to the etiology of TCP [13].
The dialysis process by itself could influence platelet count: Ahmed et al. found a lower platelet count in the blood samples taken 15 minutes after HD started compared to the ones taken before HD, both in the ESRD group and in the ESRD and CVH group. This change in platelet count, although statistically significant, was not considered clinically important [14]. Bat et al. observed an increase in absolute immature platelet number, immature platelet fraction, and increased thrombopoietin levels three hours after the HD session. Complement activation during HD leads to immediate platelet consumption and release of immature platelets from the bone marrow [15].
A longer dialysis vintage is a risk factor for infection with HBV and HCV [3]. A dialysis vintage longer than 10 years is associated with higher cardiovascular disease as well as infection-related and all-cause mortality [16]. In our study, patients with CVH had a longer dialysis vintage than control patients, but a dialysis vintage longer than 10 years was not a TCP risk factor.
We found ALAT and ASAT to be higher in the ESRD and chronic hepatitis group. Nishida et al. also found this significant difference in ALAT and ASAT levels and concluded that ALAT over 20 IU/L was significantly associated with TCP in patients with HCV infection and ESRD [12]. In contrast, Ahmed et al. found no significant difference regarding ALAT and ASAT values [14]. Aminotransferases have been observed to be lower in patients undergoing HD and peritoneal dialysis. Increases of 15-35% in their levels after HD have been attributed to hemoconcentration. Patients with ESRD and CVH have higher aminotransferase levels than those with ESRD alone, but lower values than patients with CVH without ESRD. Factors hypothesized to be responsible for this decrease in aminotransferases are hemodilution, the reduction of viremia after the dialysis procedure, increased production of hepatocyte growth factor (and accelerated liver regeneration), an increase in α-interferon production after HD, and activation of CD69+ lymphocytes [17].
Fluid retention, especially before HD, is a cause for hemodilution-related hypoalbuminemia [18]. Kubrusly et al. found a significant increase in albumin levels post- HD compared to pre-HD [19]. In patients with CVH, albumin is a marker of liver function and its protein synthesis, and is significantly decreased in cirrhosis compared to lower liver fibrosis stages. A positive correlation was observed between platelet count and albumin by Osada et al. (r=0.59, p<0.001) [20] and Zucker et al. (p=0.38, p<0.01) [21]. In patients with HCV infection alone, a 0.5 g/dl decrease in albumin concentration was associated with a 33% increase in the relative risk of TCP (p<0.005). In our study, albumin levels approached the lower part of the reference range for both groups and as expected, patients with CVH and ESRD had lower albumin concentration compared with the control group. We observed no correlation between albumin and platelet count. A serum albumin concentration lower than 3.5 g/dl was not associated with a risk for TCP. This result can be explained by the weaker impact on liver function that viral infection has in patients undergoing HD.
We found a positive correlation between platelet count and CRP; and a CRP level higher than 10 mg/dl was associated with a lower risk of TCP in patients with ESRD and CVH, explained by thrombocytosis that is secondary to inflammatory processes [22]. Elevated CRP in patients on HD is a known occurrence and is associated with cardiovascular disease mortality and all- cause mortality [23]. Coated platelets are a subgroup of platelets activated by thrombin and collagen; these express high levels of surface procoagulant proteins. Valayodon et al. studied them in patients with ESRD and reported that patients with a 40% or higher proportion of coated platelets had higher CRP levels than other ESRD patients, but no significant difference between the total platelet counts [24].
LDH is known to be elevated in patients on HD, increased by 20%-40% in patients with ESRD compared to normal volunteers [25]. LDH concentrations in both of our groups were above the reference range in our study, but no particular relationship with platelet counts was established.
In viral hepatitis, liver biopsy is the gold standard method to assess fibrosis staging, but it carries with it risks such as hemorrhage, haemobilia, and gallbladder and colonic perforation. ESRD patients have a greater bleeding risk because of the platelet dysfunction and underlying coagulopathy, and the use of heparin for the dialysis procedure. Among non-invasive methods for determining liver fibrosis, elastography has been proven to be valuable in investigating diffuse liver diseases, has a particularly high accuracy in detecting liver cirrhosis (84% sensitivity and 94% specificity in HCV infection, 75% sensitivity and 90% specificity in HBV infection), and has reduced the need for liver biopsies [26,27]. The withdrawal of fluid during HD influences liver stiffness measurement results, even in patients without viral hepatitis infection. Kellner et al. showed that a significant increase in liver stiffness measurement was found after HD in patients with an initial level lower than 7.1 kPa [28]. In our study, for patients with chronic viral hepatitis, F3 stage liver fibrosis was a risk factor for TCP.
Serum HA is a biomarker that has been proven useful in distinguishing between various fibrosis stages. In a previous study, we detected cut-off values for hyaluronic acid that can differentiate between F0/1 (33.46 ng/ml), F1/2 (80.24 ng/ml) and F2/3 (88.54 ng/ml), but none of the F stages determined by HA could be associated with a risk for TCP in our patients [11,29].
Because this was a retrospective study, other factors that influence platelet count, such as splenomegaly, could not be assessed. The small sample size of patients with cirrhosis did not allow us to properly measure the impact of several factors on patients with F4. Liver fibrosis was only detected by non-invasive methods since liver biopsy was not performed because of the high risk for hemorrhage.
Conclusions
TCP is a common occurrence in patients with ESRD undergoing HD. The overlap with chronic hepatitis increases its prevalence even more among this particular group of patients. We found that CVH in ESRD patients does not impact platelet counts, suggesting that the natural course of liver disease slows down in subjects undergoing HD. In these patients, once liver fibrosis reaches F3, they are at a risk for TCP.
Conflicts of Interest
The authors declare that there are no conflicts of interest to be disclosed for this article.
References
- Gauer, R.L.; Braun, M.M. Thrombocytopenia. Am Fam Physician. 2012, 85, 612–622. [Google Scholar] [PubMed]
- Shah, R.; Vachharajani, T.J.; Asif, A.; Aqarwal, A. Thrombocytopenia in ESRD patients: Epidemiology, mechanisms and interventional nephrology perspective. Semin Dial. 2014, 27, 618–625. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Rodrigues de Freitas, M.; Fecury, A.A.; de Almeida, M.K.; Freitas, A.S.; de Souza Guimarães, V.; da Silva, A.M.; da Costa, Y.F.; da Costa, R.A.; Ferreira, P.; Martins, L.C. Prevalence of hepatitis C virus infection and genotypes in patient with chronic kidney disease undergoing hemodialysis. J Med Virol. 2013, 85, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Hsu, P.I.; Yu, H.C.; Lin, C.K.; Tsai, W.L.; Chen, W.C.; Chan, H.H.; Lai, K.H. Factors linked to severe thrombocytopenia during antiviral therapy in patients with chronic hepatitis C and pretreatment low platelet counts. BMC Gastroenterol. 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alric, L.; Bonnet, D. Grazoprevir + elbasvir for the treatment of hepatitis C virus infection. Expert Opin Pharmacother. 2016, 17, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Dahal, S.; Upadhyay, S.; Banjade, R.; Dhakal, P.; Khanal, N.; Bhatt, V.R. Thrombocytopenia in Patients with Chronic Hepatitis C Virus Infection. Mediterr J Hematol Infect Dis. 2017, 9, e2017019. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chen, L.; Yang, J.; Feng, P. Entecavir- Associated Thrombocytopenia in a Decompensated Cirrhotic Patient: A Case Report and Literature Review. Medicine. 2016, 95, e3103. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, B.; Toma, C.L.; Diaconu, C. Bronchiolo- alveolar carcinoma in a young patient: A case report. Archivos Bronconeumol. 2013, 49, 315–316. [Google Scholar]
- Vidales-Braz, B.M.; da Silva, N.M.; Lobato, R.; Germano, F.N.; da Mota, L.D.; Barros, E.J.; de Martinez, A.M. Detection of hepatitis C virus in patients with terminal renal disease undergoing dialysis in southern Brazil: Prevalence, risk factors, genotypes, and viral load dynamics in hemodialysis patients. Virol J. 2015, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Bonder, A. Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014, 16, 372. [Google Scholar] [CrossRef] [PubMed]
- Orasan, O.H.; Sava, M.; Iancu, M.; Cozma, A.; Saplonţai- Pop, A.; Sarlea Ţărmure, S.; Lungoci, C.; Orăşan, R.A.; Patiu, I.M.; Dumitraşcu, D.L. Serum hyaluronic acid in chronic viral hepatitis B and C: A biomarker for assessing liver fibrosis in chronic hemodialysis patients. Int Urol Nephrol. 2015, 47, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Uto, H.; Oketani, M.; Tokunaga, K.; Nosaki, T.; Fukumoto, M.; Oku, M.; Sogabe, A.; Moriuchi, A.; Ido, A.; Tsubouchi, H. Clinical significance of alanine aminotransferase levels and the effect of ursodeoxycholic acid in hemodialysis patients with chronic hepatitis C. J Gastroenterol. 2010, 45, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Iwamoto, Y.; Suda, A.; Tsuchiya, K.; Nihei, H. New insights into the thrombopoietic status of patients on dialysis through the evaluation of megakaryocytopoiesis in bone marrow and of endogenous thrombopoietin levels. Blood. 2011, 97, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; El-Sherif, A. Interaction between blood and dialysis membrane in hepatitis-C virus-infected patients: A comparative study. Saudi J Kidney Dis Transpl. 2016, 27, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Bat, T.; Bat, B.E.; El-Moghraby, A.; Patel, S.; Feng, X.; Dunbar, C.E.; Sarac, E. Thrombopoietic status of patients on haemodialysis. Br J Haematol. 2016, 172, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Yamagata, K.; Iseki, K.; Tsubakihara, Y. Different impact of hemodialysis vintage on cause- specific mortality in long-term hemodialysis patients. Nephrol Dial Transplant. 2016, 31, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Sette LHAlmeida Lopes, E.D. Liver enzymes serum levels in patients with chronic kidney disease on hemodialysis: A comprehensive review. Clinics. 2014, 69, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Orasan, R.A.; Patiu, I.M.; Anghel, D.; Bejan, C.; Iosub, L.; Totolici, C.; Pop, M.; Turcea, C.; Teodoru, C.; Orasan, O.H.; Kacso, I.M.; Gherman Caprioara, M. Variation of clinical and laboratory features in chronic dialysis patients treated with high-flux hemodialysis after switching to online hemodiafiltration. Int Urol Nephrol. 2013, 45, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Rowland, D.L.; Motofei, I.G.; Popa, F.; Constantin, V.D.; Vasilache, A.; Păunică, I.; Bălălău, C.; Păunică, G.P.; Banu, P.; Păunică, S. The postfinasteride syndrome; an overview. J Mind Med Sci. 2016, 3, 99–107. [Google Scholar] [CrossRef]
- Osada, M.; Kaneko, M.; Sakamoto, M.; Endoh, M.; Takigawa, K.; Suzuki-Inoue, K.; Inoue, O.; Satoh, K.; Enomoto, N.; Yatomi, Y.; Ozaki, Y. Causes of thrombocytopenia in chronic hepatitis C viral infection. Clin Appl Thromb Hemost. 2012, 18, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Zucker, M.L.; Hagedorn, C.H.; Murphy, C.A.; Stanley, S.; Reid, K.J.; Skikne, B.S. Mechanism of thrombocytopenia in chronic hepatitis C as evaluated by the immature platelet fraction. Int J Lab Hematol. 2012, 34, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.Z.; Streja, E.; Kovesdy, C.P.; Budoff, M.J.; Nissenson, A.R.; Krishnan, M.; Anker, S.D.; Norris, K.C.; Fonarow, G.C.; Kalantar-Zadeh, K. High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr. 2011, 94, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, J.; Zhang, F.; Huang, C.; Wu, Y.; Han, Y.; Zhang, W.; Zhao, Y. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: A systematic review and meta-analysis. J Nephrol. 2013, 26, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Valaydon, Z.S.; Lee, P.; Dale, G.L.; Januszewski, A.S.; Rowley, K.G.; Nandurkar, H.; Karschimkus, C.; Best, J.D.; Lyons, T.J.; Jenkins, A.J. Increased coated-platelet levels in chronic haemodialysis patients. Nephrology (Carlton). 2009, 14, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, R.; Lafrance, J.P.; Leblanc, M. Altered laboratory findings associated with end-stage renal disease. Semin Dial. 2006, 19, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; Hancox, S.H.; Howlett, D.C. Liver biopsy: Past, present and future. Br J Hosp Med. 2016, 77, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.C.; Carvalho-Filho, R.J.; Stern, C.; Djpumpo, A.; Giuily, N.; Ripault, M.P.; Asselah, T.; Boyer, N.; Lada, O.; Castelnau, C.; Martinot-Peignoux, M.; Vaila, D.C.; Bedossa, P.; Mercellin, P. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012, 32, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Kellner, P.; Anadol, E.; Hüneburg, R.; Hundt, F.; Bös, D.; Klein, B.; Woitas, R.P.; Spengler, U.; Sauerbruch, T.; Trebicka, J. The effect of hemodialysis on liver stiffness measurement: A single-center series. Eur J Gastroenterol Hepatol. 2013, 25, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Orasan, O.H.; Orasan, R.A.; Patiu, I.M.; Dumitrascu, D.L. Hyaluronic acid in end-stage renal disease treated by hemodialysis. Ren Fail. 2015, 37, 1531–1532. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. 2018 Olga Hilda Orasan, Laura Urian, George Ciulei, Iulia Breaban, Andreea Maria Stefan, Sorina Cezara Secara, Adela-Sitar Taut, Simina Tarmure Sarlea, Vasile Negrean, Dorel Sampelean, Ioan Mihai Patiu, Remus Aurel Orasan and Angela Cozma