Highlights
- The gradual and differentiated development of the reactive astrocytes in close relationship with the time from the main trauma event, with specific lesional, perilesional, and far area distribution demonstrates that studying the reactive astrocitose process can constitute as an objective index for evaluating the posttraumatic elapsed time.
- The reaction of the glial cells plays a significant role in the development of cranio-cerebral lesions.
Highlights
- The gradual and differentiated development of the reactive astrocytes in close relationship with the time from the main trauma event, with specific lesional, perilesional, and far area distribution demonstrates that studying the reactive astrocitose process can constitute as an objective index for evaluating the posttraumatic elapsed time.
- The reaction of the glial cells plays a significant role in the development of cranio-cerebral lesions
Abstract
Introduction and objectives. This study aims to investigate whether the cerebral modifications of posttraumatic reactive astrocitose can be considered an objective criterion for determining the age of traumatic cranio-cerebral lesions. Materials and methods. The present study consists of a series of 23 medico-legal cases that underwent autopsy in Teleorman County (Romania) Department of Forensic Medicine during 2007–2016, with full immune-histochemical microscopic examination using GFAP staining. The study consists of two groups, a series of 13 cases with cranio-cerebral trauma with different posttraumatic survival periods and 9 cases as a control group. Results and discussions. We discovered GFAP+ reactive astrocytes even when death occurred immediately after the trauma event and up to 4 months after the traumatic incident. We also discovered an intense positive correlation between the density of the GFAP+ cell from the perilesional area and the posttraumatic survival period. The highest cerebral density of the GFAP+ astrocytes occurred with acute death prior (1 to 24 hours) and the lowest in the chronic period (over 2 weeks). Conclusions. The gradual and differentiated appearance of the reactive astrocytes in close relation with the cerebral posttraumatic interval, with specific lesional and perilesional distribution as well as in surrounding area, clearly demonstrates that the state of the reactive astrocitose may constitute an objective index for evaluation of the elapsed time after the posttraumatic event.
Introduction
Astrocytes are the most known neuroglial components, being five times denser than neurons and being a common part of the central nervous system of all vertebrates. Reactive astrocitose is a specific process in which the astrocytes respond to all cerebral lesions, being a result of structural lesions upon the central nervous system. Reactive astrocitose consists of a broad range of astrocyte transformations, starting with reversible gene expressions or light cellular hypertrophy with integrity of the tissue and cell internal architecture and ending up with definitive glial scar construction through rearrangement of the cerebral histological elements. The immuno-histochemical study of the glial fibrillar acid protein (GFAP) expression is used as a typical marker for identifying the reactive astrocytes, other molecular markers used for this immune- histochemical determination being glutamin-systethase and S100ß [1,2]. The utility of these markers is nevertheless limited because, in contrast to the GFAP, they are not specific to astrocytes cells. GFAP is an extremely stable fibrillar intermediate protein, being also an essential structural component of the astrocyte cell internal cytoskeletal structure that, under normal physiological conditions, cannot be found dispersed in the cytoplasmic fluid. Also, GFAP cannot be detected through immune-histochemical techniques in non- reactive astrocytes. Experimental studies have shown that GFAP is not essential for normal physiological functions of the astrocyte cell, but it is important in reactive astrocitose processes and for glial scar development [3,4].
This study investigates whether the use of immune-histochemical techniques with GFAP staining for cerebral modifications of reactive astrocitose can constitute an objective criterion for determining the age of traumatic cranio-cerebral lesions.
Material and method
This study analyzed the autopsy cases performed in Teleorman County, Romania, Department of Forensic Medicine from 2007–2016. It includes a retrospective observation between 2007 and 2014 and a prospective study between 2015 and 2016. Of the entire lot of autopsies performed, we selected 22 cases for microscopic evaluation, with 2 sub-batches. The first lot included 13 cases with deaths from posttraumatic cranio-cerebral lesions, with variable survival times, ranging from instant death up to 4 months survival time. All selected cases had a violent pattern of trauma onset (4 physical aggressions, 6 road accidents, 1 free fall, 1 fall within the same height, and 1 case of undetermined conditions). We did not include those cases with neurosurgical postoperative lesions, as various postoperative conditions could have interfered with the initial macro- and microscopical cerebral lesions. Based on posttraumatic survival times, these cases were further divided into 4 categories:
- -
- Death within the supra-acute period, of seconds and minutes and up to hours from the initial trauma event: 3 cases;
- -
- Death within the acute period, ranging form1 hour and up to 24 hours after the trauma event: 3 cases;
- -
- Death within the sub-acute period, ranging from 1 day and up to 14 days: 3 cases;
- -
- Death during the chronic period, after 2 weeks post trauma: 4 cases.
A second lot consisted of 9 cases selected for comparison as a control group, with no cranio-cerebral events and no cerebral lesions detected during autopsy. We did not include in this lot cases with known cerebral vascular diseases. These cases had both violent (5 cases) and non-violent (4 cases) mechanisms of death onset from the following: mechanical asphyxia through strangulation, phosphorous-organic acute intoxication, underwater mechanical asphyxia, hypothermia, myocardial fibrosis, acute myocardial infarction, pulmonary thromboembolism, toxic-septic shock, and electrocution.
- Techniques employed by the study
For each case, we harvested during autopsy cerebral tissue samples of approx. 3 by 2 cm that were later immersed in fixation dye based on a 10% buffered formalin solution and hermetically sealed in recipients should this be receptacles? with a 5 times larger volume of formalin solution than the tissue mass, for a period of 2 weeks. The tissue samples were later embedded in paraffin blocks, each of them with 3 serial sections of 1.5-2 µm thickness. These sections were stripped of paraffin and rehydrated and dyed using a standard Hematoxilin-Eozine (HE) staining. The final step consisted of a manual technique for immuno- histochemical staining.
For the GFAP-revealing immune-histochemical staining, we employed the following steps: as a first measure we blocked the intrinsic peroxidase activity by treating the sections with Peroxidazed-1 for 5 minutes at room temperature. Then, the revealing of the GFAP antigen was achieved by the Heat Induced Epitope Retrieval method (HIER) with boiling at 950C for 45 minutes with the aid of a Decloaking Chamber. Blocking of the non-specific staining was achieved by treating the sections with Background Sniper solution (Biocare Medical©) for 10 minutes at room temperature. Applying the primary GFAP(M) antibody was achieved by delivering the GA-5 clone mice-produced monoclonal anti-GFAP(M) antibody on the sections (Biocare Medical©) with the factory recommended dilution of 1:100 for 60 minutes at room temperature. Detection of the probe/polymer was achieved by applying first the probe for 10 minutes then the universal polymer for 20 minutes. The antibodies were identified by means of the MACH 4 Universal HRP-Polymer detection system (Biocare Medical©) which can detect both anti-rabbit and anti-mice antibodies. After each of these steps, the blades were washed with buffer solution with a pH of 7.6 (TBS Plus, by Biocare Medical©). Applying the DAB chromogen was achieved by fixing the blades Betazoid DAB solution (Biocare Medical©) for 2 minutes at room temperature. Later, we washed them for 1 minute with deionized water and after this step the overcoloring was done with CUT Hematoxylin, followed by dehydration, clarifying, and mounting the sections on the final lamellas.
- Microscopical examination
Brain fragments were examined with light microscopy and different magnifying lenses provided by the Leica ICC 50W transmitted light microscope with an attached photography kit. For the cranio-cerebral trauma cases, we performed a regional quantitative analysis of the GFAP-positive cells determining the final mean number of cells at the level of lesion, adjacent area, and far surroundings. Two independent observers each analyzed 5 microscopic fields with a 20x lens for 3 blades of the same case by employing a standard viewport grill and therefore obtaining the mean number of GFAP-positive cells distributed at the level of the lesion, the adjacent zone, and the far surrounding area. We report them as an arithmetic mean between the determinations of each case. The final number of GFAP- positive cells for the corresponding areas was determined as a mean computation between the figures reported by the two independent observers. The GFAP- positive cell density at the level of each area was determined by reporting the final mean number of cells per square mm of brain tissue.
- Statistical analysis
Data were parsed with the SPSS analysis software (SPSS for Microsoft Windows, ver. 17.0). Statistical analysis focused on brain density of the GFAP-positive cells, as a continuous variable, described both globally as well as on layers, on subgroups of interest, by the central trend (mean, median) and dispersion (standard deviation). During the analytical stage, comparison of the subgroups and validation of the hypothesis were achieved using the t-test for independent samples and with the non-parametric Mann-Witney U test when the assumption of a normal distribution was not met. ANOVA as well as the non-parametric Kruskal-Wallis test were used for establishing differences between various parameters within the studied groups. Differences between the 2 lots of cases were considered significant using p of 0.05. Data were then transformed into standard graphical representations.
Results
In the supra-acute posttraumatic period, we detected GFAP-positive cells with light to moderate hypertrophy. During the acute, sub-acute, and chronic intervals, the GFPA-positive astrocytes developed severe hypertrophy. Development of the glial scars was noted starting with 1 to 2 months after the trauma event.
During the supra-acute post-traumatic period for the GFAP-positive astrocytes with light hypertrophy, GFAP staining was observed mostly in the body of the astrocytes, and lighter at the outer limits of the cells. As for the GFAP-positive astrocytes with moderate hypertrophy, GFAP staining was immuno- histochemically present at both the body of the cell (that looks inflated) as well as in the outer limits of the cells that appear thickened. (Figure 1, Figure 2, A-B).
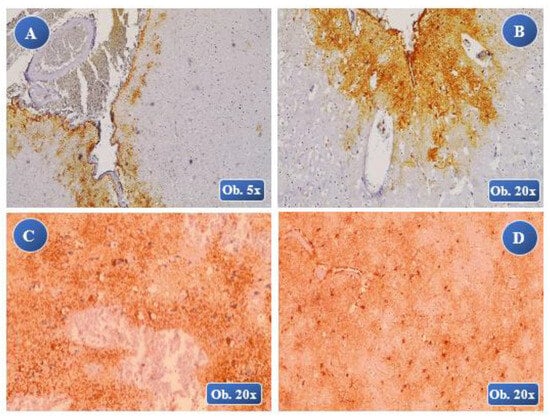
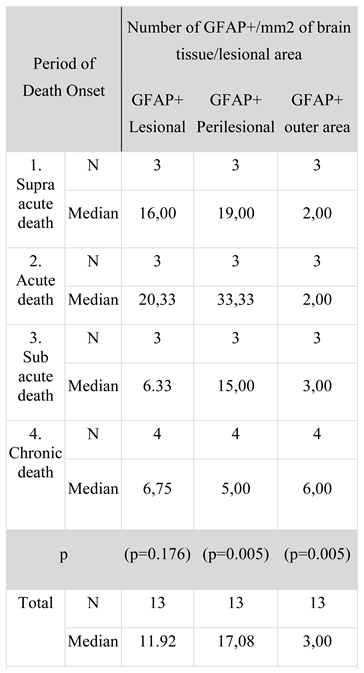
Figure 1.
Immediate posttraumatic death. A, B: (lesional/perilesional), low immunopositivity reaction of the GFAP-positive staining with growth of the GFAP+ cells at the lesional and perilesional levels; astrocytes slightly hypertrophic in aspect, mostly in the body of the cells, extensions of the astrocytes with a filament-like aspect. At 25 minutes posttraumatic: C (lesional-perilesional), D (perilesional) – positive reduced immunoreaction of GFAP+ with a light hypertrophy aspect of the astrocyte, ballooning of the cell body with thin cellular extensions and a perineuronal tree-like aspect in the areas of ischemic perilesional zones.
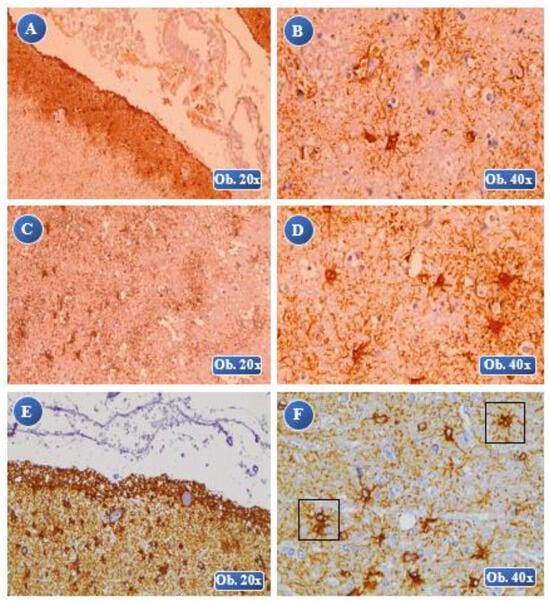
Figure 2.
At 45 minutes posttraumatic: A (lesional-perilesional), B (perilesional) moderate immuno-positivity of the GFAP reaction with multiple astrocyte cells moderately hypertrophied, both a lesional and perilesional levels, with an 8-times growth in volume of the cell body and cell extension thickening, without any astrocyte overlapping. At 1 hour posttraumatic: C, D (perilesional) moderate GFAP positive reaction, moderate hypertrophy of the astrocyte cells, cell body with an increased volume and with numerous thickened cell extensions, rare overlapping zones. At 3 hours posttraumatic: E (lesional), F (perilesional) a high positive reactivity of the GFAP reaction, numerous astrocytes with moderate hypertrophy, cell body with a considerable volume growth and thickened cell extensions with lots of overlapping zones (F).
During the acute and sub-acute posttraumatic periods at the level of astrocytes with severe hypertrophy, GFAP staining revealed a volumetric growth of the body and outer limits of the astrocytes as well as fragmentation of the astrocyte pseudopods (ranging from light fragmentation during the acute period to severe fragmentation in the sub-acute timing) (Figure 2: C and D; Figure 3; Figure 4: A and B).
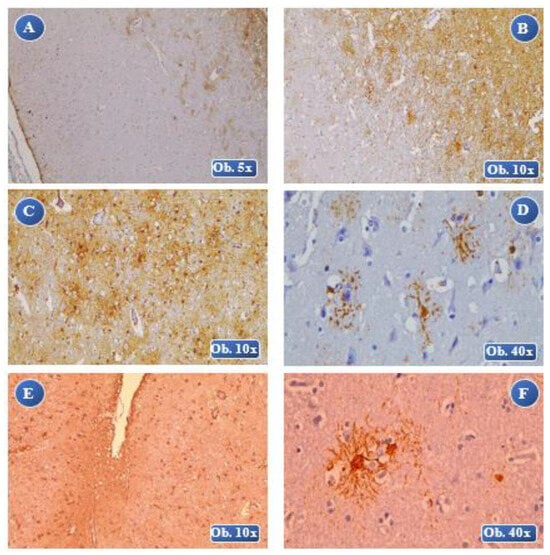
Figure 3.
At 6 hours posttraumatic: A (lesional), B (lesional-perilesional) an increased immuno-positivity of the GFAP reaction, numerous GFAP-positive astrocyte cells at the perilesional level. GFAP-positive astrocytes presented severe hypertrophic modifications, cell body with increased volume, thickened cell extensions, rare areas of overlapping. At 1 day posttraumatic: C, D (perilesional) an increased immunopositivity of the GFAP reaction, severe astrocyte hypertrophy, fragmentation of the astrocyte cells, increased cell volume that appears separated from the adjacent cell extensions. At 6 days posttraumatic: E (lesional-perilesional), F (perilesional) an increased immunopositivity of the GFAP reaction, low GFAP-positive cell density, especially at the lesional level. Astrocytes with severe hypertrophic modifications, increased volume of the body, multiple thickened extensions, lots of overlapping astrocyte areas.
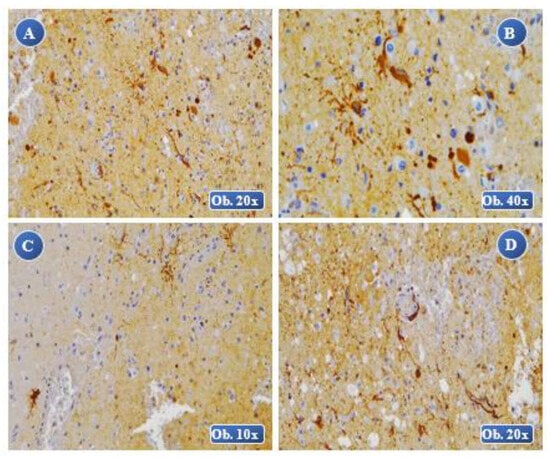
Figure 4.
At 8 days posttraumatic: A, B (lesional) an increased immunopositivity of the GFAP reaction, a reduced number of GFAP-positive cells with alteration of the cellular morphology, cell body with a very large volume that appears separated from the astrocyte extensions. At 2 weeks posttraumatic: C, D (lesional) an increased immunopositivity of the GFAP reaction, with severe hypertrophy and morphological alterations of the cell body, extended lesions of cell fragmentation, dispersed aspect of the body and cell extensions.
During the chronic posttraumatic survival period, we noticed severe astrocyte hypertrophy, the body of the cells being overinflated with numerous thickened extensions and overlapping of the pseudopods, thus creating glial scars (Figure 4- C and D; Figure 5). The GFAP-positive astrocyte response observed in most of the control cases was diffuse in the brain tissue, with light hypertrophy of the cell`s body, without any hypertrophic modifications of the astrocyte extension and with no fragmentation or overlapping of the astrocyte pseudopods (Figure 6). In only one case from the control group did we notice severe reactive astrocitose, a high number of GFAP-positive cells being present in the perivascular area with a diffuse disposition indicating a general glial reaction, a characteristic for a toxic encephalitic process?
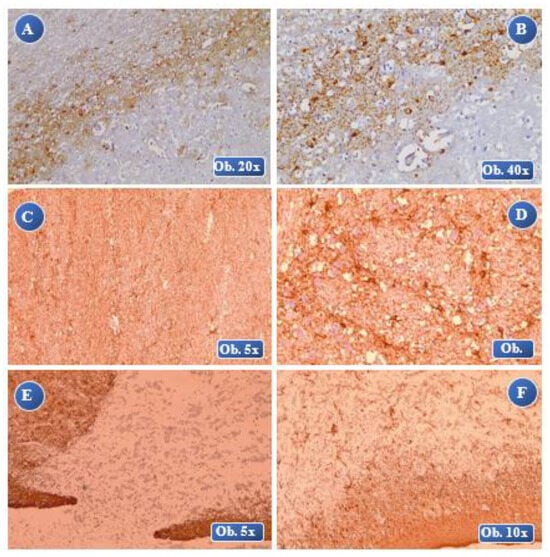
Figure 5.
At 1 month posttraumatic: A (lesional-perilesional), B (lesional) an increased immunopositivity of the GFAP reaction, numerous GFAP-positive astrocyte cells at the perilesional level, cell body with increased volume, thickened cell extensions, severe reactive astrocytosis with glial scar formation. At 2 months posttraumatic: C, D (lesional) laminar necrosis with gliosis, an increased immunopositivity of the GFAP reaction, severe hypertrophy, many overlapping areas of the cell extensions and forming of extended glial scars. At 4 months posttraumatic: E, F (lesional-perilesional) an increased immunopositivity of the GFAP reaction, a reduced number of GFAP-positive cells with alteration of the cellular morphology, numerous astrocyte bodies separated by astrocyte extensions and many overlapping areas.
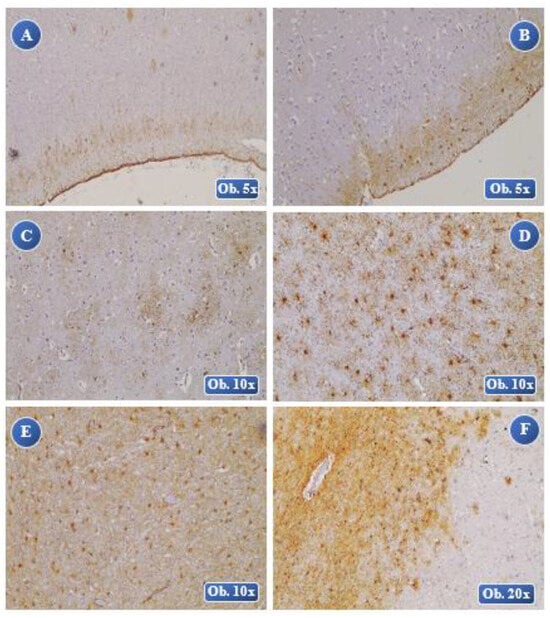
Figure 6.
Analysis of the control group. Reduced intensity of the immunohistochemical GFAP staining, rare astrocytes with light hypertrophy and diffuse dispersion in the brain tissue, a slightly larger cell body with lots of thin cell extensions in the case of organo-phosphorus intoxication (A), pulmonary thromboembolism (B) and strangling (C). Moderate GFAP staining, moderate cell count and slight hypertrophy with homogenous dispersion, ballooning of the cell body with filament-like astrocyte pseudopods in myocardial-fibrosis (D) and hypothermia (E). Moderate GFAP reaction, increased expression of the GFAP-positive cells with light hypertrophy in diffuse focal arrangement near the blood vessels in electrocution (F). A balloon-like cell body with thin cell extensions with no overlapping areas of the astrocytes.
Statistical analysis on the density of the GFAP- positive brain cells revealed significant effects (p < 0.05) between the two groups. The density of the brain GFAP- positive cells was higher in both the amplitude and in the central trend for the cases in the study group (that included the cranio-cerebral trauma cases with variable posttraumatic survival times) compared to the control group (that showed a history of cranio-cerebral trauma with different causes for death) (Chart 1).
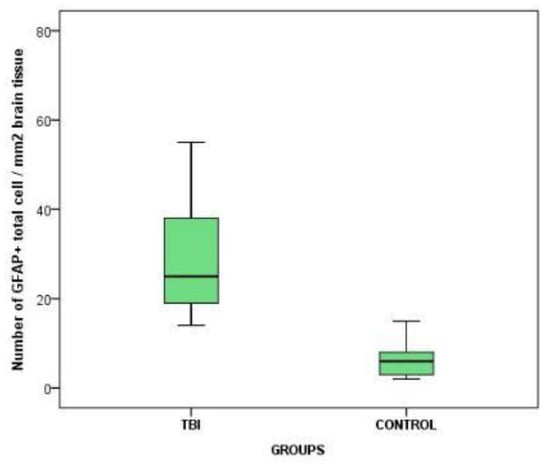
Chart 1.
The variation of GFAP-positive cerebral cell density between the 2 groups.
In the immediate posttraumatic death and up to 25 minutes of survival time after the trauma event, we noticed the same density of GFAP-positive cells (25 cells per square mm of brain tissue), with a doubling at the 45 minutes and 1-hour posttraumatic marks. At the 3-hour posttraumatic survival mark, we recorded the highest density of GFAP-positive cells, at 78 cells per square mm of brain tissue, followed by a steep decline in density at 6 hours posttrauma with a 38 cell per square mm value, followed by a slow descent of up to 1 month after the traumatic event, with a count of 15 cells per square mm of brain tissue.
At the two-month posttraumatic mark, we registered a slight elevation in GFAP-positive cell density at both lesional and peri-lesional level, with 24 cells per square mm. At 4 months of posttraumatic survival time we noticed the lowest GFAP-positive brain cells density (Chart 2).
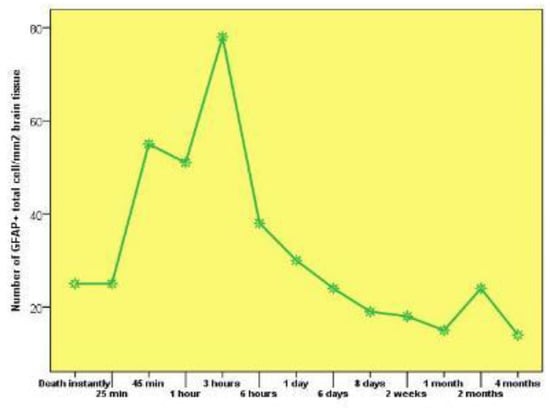
Chart 2.
The variation of GFAP-positive cerebral cell density in connection with the survival posttraumatic time between the 2 groups.
At 3-hours posttraumatic index, the study revealed the highest density of GFAP-positive cells at both lesional and peri-lesional levels, of about 35 cells per square mm and 40 cells per square mm of brain tissue. At 4 months, no GFAP-positive cells were seen at the lesional level, this being their lowest density. Between 1 and 4 months posttrauma, we also noticed a reduced density of GFAP-positive cells at the level of the lesion, with values ranging from 2 to 5 cells per square mm of brain tissue.
The GFAP-positive cells situated far from the cerebral lesions were not detected during the first hour following the trauma event. The highest density of GFAP-positive cells situated away from the lesional site was recorded at 4 months post trauma, with 9 cells per square mm of brain tissue (Chart 3).
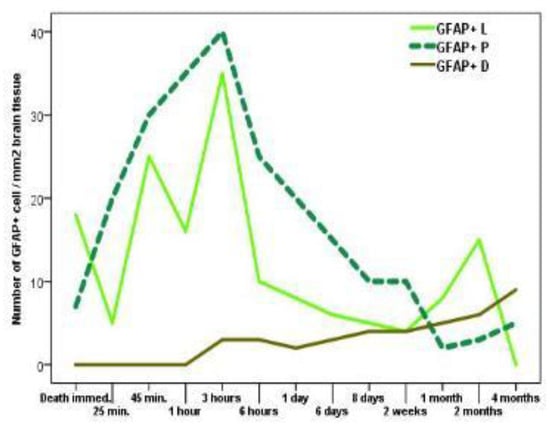
Chart 3.
The variation in density of GFAP- positive cerebral cells lesional/perilesional in regards with the posttraumatic survival time. Legend: GFAP+L: GFAP+ lesional cells; GFAP+P: GFAP+ perilesional cells; GFAP+D: GFAP+ cells at the outer limits of the lesional area.
Regarding the lesional area, the density of GFAP- positive cells at the site of the lesion and adjacent area was significant larger (p = 0.0221 and 0.0277 respectively) compared with those situated farther away.
The maximum values for GFAP-positive cells occurred at the perilesional site (median of 17.08 cells per square mm of brain tissue) followed by GFAP- positive cells at the lesional site (median of 11.92 cells per square mm of brain tissue); the lowest value was recorded for GFAP-positive cells being the farthest away from the lesion (median of 3 cells per square mm of brain tissue) (Table 1).

Table 1.
Comparison between the cerebral density of the GFAP-positive cells in regards with the lesional area and the elapsed posttraumatic time.
If the analysis examines the lesional area and the period of death, the density of GFAP-positive cells differs significantly in comparison to the lesional site situated far away (p = 0.005).
The maximum density of GFAP-positive cells from the level of the lesion was encountered during the sub- acute death. The maximum density of the GFAP- positive cells at the perilesional area occurred during the acute death, and the lowest during the sub-acute death.
In the case of the supra-acute, acute, and sub-acute death, perilesional GFAP-positive cells were closer to the outer limits, with each statistically significant (p = 0.0462, 0.0023 and 0.0151 respectively) (Table 1).
Regarding the lesional area and the posttraumatic survival time, results showed a strong positive correspondence between the cerebral density of GFAP- positive cells from the lesional, perilesional, and outer limits as well as negative correspondence between the cerebral densities of the GFAP-positive cells from the perilesional and the outer limit areas.
In the control group with cranio-cerebral lesions, the GFAP-positive cells placed at the outer limits of the lesional points, density was directly related with survival posttraumatic time; the greater the density, the longer was the time.
Discussions
Experimental studies conducted on mice by Kalayci and Woiciechowskyha have identified specific modifications of reactive astrocytosis beginning with the 24-hour posttraumatic marker, consisting in a hypertrophic astrocyte reaction with a maximum at 3-4 days post trauma. These researchers also showed that diffuse cranio-cerebral trauma can lead to light astrogliosis with moderate diffusion, the GFAP- positive astrocytes presenting different grades of hypertrophy, without registering losses in the fields of the astrocytes and without the constitution of glial scars [5,6].
The studies of Zhonghua et al., based on the experimental induction of lesions at the cerebral trunk in mice, revealed typical reactive astrocytosis beginning at 15 minutes and up to 15 days post-trauma. The first reactive astrocytosis modifications recorded at 15 minutes consisted in bloating of the astrocyte cells with reduced immunopositivity of GFAP staining. Between 1 and 3 hours post trauma, the astrocytes displayed significant hyperplasic modifications, and between the 1-hour to 6-hour markers, the study showed astrocyte hyperplasic with moderate hypertrophy, with a relatively low intensity of the staining. Between 24- hours and 96-hours, an elevated number of moderate hypertrophic astrocytes and a high intensity of the staining were evident. Between 10 to 15 days after the trauma, their study revealed both hypertrophy and hyperplasic processes, with the most intense coloration of the GFAP staining [7].
Haussman et al. have investigated the immunohistochemical reactions of the reactive astrocytosis processes that take place in the brain tissue in the first 30 weeks after a traumatic event. Between the 1st day and the 4th week, numerous GFAP-positive hypertrophic astrocytes were found in the surrounding areas of some cortical brain concussions [8]. Summarizing the results of the experimental data regarding the reactive astrocytosis process with the aid of the GFAP staining, we can conclude that the earliest presence of the GFAP-positive cells is 15 minutes after a trauma event and this lasts up to a month posttrauma.
In this study we identified GFAP-positive astrocyte cells immediately after death and up to 4 months after the traumatic event, with direct correlations between the brain density of the GFAP-positive hypertrophic astrocytes and the posttraumatic time lapsed, in the first 3 hours a progressive growth of the GFAP-positive brain cells with a later slight downturn in their numbers up to the 4-month marker.
The majority of the published experimental data regarding the reactive astrocytosis processes has revealed an increased expression of the GFAP-positive cells at the lesional and peri-lesional level, but none of those studies investigated the reactive astrocitose phenomenon in the outer limits areas, away from the initial brain injuries [9].
This study revealed that the GFAP-positive cells are mostly located at the lesion site and the perilesional areas, with a reduced density in the outer limits of the brain injury. We also noticed a strong positive correlation between the GFAP-positive cell density from the perilesional areas and the outer limits in close relationship with the posttraumatic survival time of the case.
Conclusions
The study of the reactive astrocitose processes in the cranio-cerebral trauma, investigated through GFAP immunohistochemical staining, has identified a broad spectrum of astrocyte cell transformations in close relationship with the posttraumatic elapsed time. During the supra-acute period, the study revealed light hypertrophic and moderate astrocitose processes with a reversible potential, along with a growth in volume of the cell body as well as the cell extensions, variable fragmentation of the astrocyte pseudopods, without altering of the tissue architecture. During the acute, sub-acute, and chronic post-traumatic periods, the study revealed irreversible modifications consisting of severe hypertrophy with numerous areas of astrocyte overlapping and glial scar developing along with rearranging of the brain histological elements beginning with 1 month and up to 2 months posttrauma. The highest cerebral density of the GFAP-positive astrocytes was noticed in the acute period of death (1 to 2 hours) and the lowest in the chronic period (over 2 weeks).
The gradual and differentiated development of the reactive astrocytes in close relationship with the time from the main trauma event, with specific lesional, perilesional, and far area distribution demonstrates that studying the reactive astrocitose process can constitute as an objective index for evaluating the posttraumatic elapsed time.
The reaction of the glial cells plays a significant role in the development of cranio-cerebral lesions, during the early stage (when the clinical landscape is dominated by the primary traumatic lesions) and also during the late stage (when the clinical evolution is influenced mostly by the secondary cerebral injuries).
Acknowledgments
This scientific material is a part of a larger paper, a Ph. D thesis, currently under development by Duncea- Borca Roxana-Maria, M.D., Ph. D student at Carol Davila University of Medicine and Pharmacy, Bucharest, with Prof. Vladimir Belis, M.D., PhD., as thesis coordinator. The thesis has the following title: `The role of forensic investigation in diagnosis, prognosis and estimation of posttraumatic interval in TBI`.
References
- Goncalves, C.A.; Leite, M.C.; Nardin, P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem. 2008, 41, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D. Distribution of glutamine synthetase in the rat central nervous system. J Histochem Cytochem. 1979, 27, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.E.; Imura, T.; Song, B.; Qi, J.; Ao, Y.; Nguyen, T.K.; Korsak, R.A.; Takeda, K.; Akira, S.; Sofroniew, M.V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008, 28, 7231–7243. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Leveen, P.; Pekna, M.; Eliasson, C.; Berthold, C.H.; Westermark, B.; Betsholtz, C. Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO J. 1995, 14, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, M.; Unal, M.M.; Gul, S.; Acikgoz, S.; Kandemir, N.; Hanci, V.; Edebali, N.; Acikgoz, B. Effect of coenzyme Q10 on ischemia and neuronal damage in an experimental traumatic brain-injury model in rats. BMC Neurosci. 2011, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Woiciechowsky, C.; Schoning, B.; Stoltenburg- Didinger, G.; Stockhammer, F.; Volk, H.D. Brain-IL-1 beta triggers astrogliosis through induction of IL-6: inhibition by propranolol and IL-10. Med Sci Monit. 2004, 10, BR325–BR330. [Google Scholar] [PubMed]
- Li, R.; Fujitani, N.; Jia, J.T.; Kimura, H. Immunohistochemical indicators of early brain injury: an experimental study using the fluid-percussion model in cats. Am J Forensic Med Pathol. 1998, 19, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, R.; Riess, R.; Fieguth, A.; Betz, P. Immunohistochemical investigations on the course of astroglial GFAP expression following human brain injury. Int J Legal Med. 2000, 113, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Zorilă, A.L.; Zorilă, M.V.; Marinaş, M.C.; Ţolescu, R.Ş.; Zorilă, G.L.; Florou, C.; Neamţu, M.C.; Knieling, A.; Busuioc, C.J. Evaluation of brain injuries in children deceased due to head trauma. Rom J Morphol Embryol. 2017, 58, 1417–1428. [Google Scholar] [PubMed]
© 2018 by the author. 2018 Roxana M. Duncea-Borca, Vladimir Belis, Mihnea Costescu, Relu G. Calota, Reka Kutasi, Cosmin A. Moldovan