Highlights
- This study suggests that prognosis/survival would be the same irrespective of gender in MSI vs. MSS colo-rectal cancer
- further investigations, with impact on therapeutic strategy, are however necessary
Highlights
- This study suggests that prognosis/ survival would be the same irrespective of gender in MSI vs. MSS colo-rectal cancer
- further investigations, with impact on therapeutic strategy, are however necessary
Abstract
Microsatellite instability (MSI) is a feature of colorectal tumors that develops as a result of inactivation of the DNA mismatch repair system. It is found in about 15% of all colorectal cancers and is an important prognostic molecular marker when assessing patients with colorectal cancer. It can influence prognosis and treatment decisions in both the advanced and early stages. Although in early stages this marker suggests a favorable prognosis and presents an important argument against adjuvant treatment in stage II disease, in metastatic stages it no longer associated with such an optimistic outcome. The present trial is a prospective, single-center study which included 122 colorectal cancer patients who were tested for MSI using immunohistochemistry. The trial included patients with stage II to IV colorectal cancer, treated in the Prof. Dr. Agrippa Ionescu Emergency Hospital, Bucharest, Romania. Follow-up data were collected during a 24-month period. The study attempted to determine whether differences exist in overall survival for MSI (microsatellite instability) vs. MSS (microsatellite stable) colorectal cancer and to ascertain whether sex of the patient influences prognosis in MSI patients, irrespective of stage or treatment. Results demonstrated no significant differences in survival for MSI vs MSS colorectal patients, and patients’ gender proved not to influence the outcome in MSI patients.
Introduction
Microsatellite instability (MSI) is a feature of colorectal tumors that develops as a result of inactivation of the DNA mismatch repair (MMR) system. It is found in about 15% of all colorectal cancers. Approximately 3% of these are a consequence of Lynch syndrome, and almost all Lynch syndromes test positively for microsatellite instability [1].
Another 12% of colorectal cancers have microsatellite instability secondary to the inactivation of the MMR system due to hypermethylation of the promoter of the MLH1 gene. MSI can be considered another important molecular marker which provides clinical information useful in the care of patients with colorectal tumors. As MSI testing techniques are currently accessible in modern laboratories, several researchers have proposed more clinical applications for it [2]. Colorectal cancer is the third most common neoplasm in the world and the fourth cause of mortality [3].
Curative surgery is the primary treatment in non- metastatic stages, followed by adjuvant chemotherapy when necessary. Genetic abnormalities involved in carcinogenesis include changes in the APC, k-ras, and p53 genes, and also microsatellite instability which acts as an important cause in the development of neoplasms [4].
Microsatellite instability is characterized by the existence of a hypermutable phenotype produced by the loss of the DNA's ability to repair copy errors. Enzymes belonging to the mismatch repair system identify and fix the DNA's polymerase error during the cell replication process. When both alleles (from mother and father) are inoperative, functional enzymes cannot be produced to repair these defects, thus giving rise to a hypermutant phenotype. The mutations produced are evident in microsatellite loci and hence the name, microsatellite instability (MSI) [5].
In addition to genetic modifications, the pathological changes for these types of tumors are clearly different from tumors that follow other carcinogenesis patterns: location is more frequently in the right colon (cecum, ascending colon, 1/3 right transverse colon) and the cells are poorly differentiated and atypical. They usually present with mucinous histology with rich peritumoral and intratumoral lymphocyte infiltration [6].
Since the 1990s, the key element of adjuvant chemotherapy in colorectal cancer has been the fluoropirimidines, which have proven effective in patients with stage III colon cancer and stage II and III rectal cancer. Almost all adjuvant treatment regimens involve monotherapy or a combination of 5-fluorouracil with oxaliplatin - FOLFOX protocol [7].
Microsatellite instability first proved its utility as a prognostic factor for the early stages of cancer. A meta- analysis performed on 7642 cases identified the beneficial prognostic role of MSI status compared to patients with MSS for stage II colorectal cancer (CRC) [8].
Our study attempted to determine whether MSI colorectal cancer is more frequent in male or female patients, whether sex of the patient has an impact on survival of MSI patients, and whether the survival of MSI vs. MSS patients is different irrespective of stage or consequent treatment.
Materials and methods
This trial was a prospective study that included 122 patients with colorectal cancer who were operated upon or biopsied in the General Surgery Department of the Emergency Clinical Hospital, Prof. Dr. Agrippa Ionescu, Bucharest, Romania. The inclusion period lasted for 2 years (January 1, 2013 to January 1, 2015). Informed consent for participation was obtained from patients or their relatives as appropriate.
Inclusion in the study occurred only if sufficient biological material was available for MSI testing through immunohistochemistry. Staging information was reported according to the standard method of colorectal cancer classification (TNM). Other data collected included the degree of cell differentiation, age, and gender, and these were obtained by reviewing observation sheets, operator protocols, and histopathological reports. Follow-up data sources included the national health electronic archives (SIUI system), direct interviews with patients or their physicians, clinical observation sheets, and death reports.
The technique used to identify microsatellite instability was immunohistochemistry (IHC). Tumors with high microsatellite instability (MSI-H, classified only if at least 30% of the markers were positive) and with low microsatellite instability (MSI-L if at least one marker tested positively in tumor tissue) were tested for the presence of 4 immunohistochemical markers (by the use of antibodies): MLH1, PMS2, MSH2, MSH6.
Statistical analysis
Statistical analysis was performed using GraphPad 6 Prism and MedCalc 14.
Results
The patient characteristics are presented below.
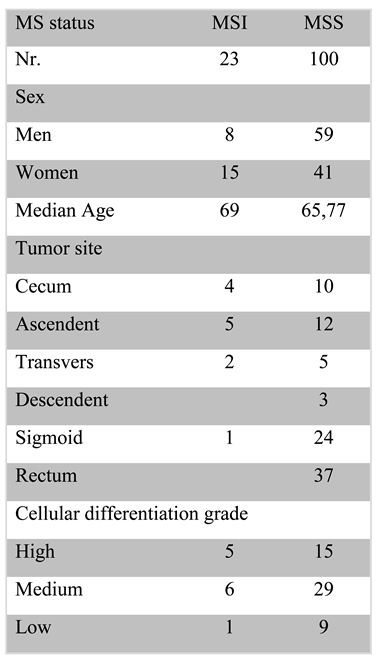
18.6% of the cases tested positive for MSI, of which 5.6% were stage IV. The stage with the highest positivity for MSI was stage 3 (8.9%). Table 1 contains the biological characteristics of the investigated patients in which the majority were observed; the patients with microsatellite instability were more likely male, with a more frequent location at the level of the cecum and with an average degree of differentiation.

Table 1.
Patient characteristics.
Median survival was 34 months for MSI patients and 27 months for MSS patients (p=0.396). As the Kaplan Meyer curve (Figure 1) for these two groups shows, the difference in survival was not significant. Thus, in the first 35 months, survival appears the same, although after this interval MSS patients appear to have lower survival. At 39 months, survival of MSS patients is 33.3% and MSI is 28%; in contrast, at 48 months twice the number of patients with MSI have survived.
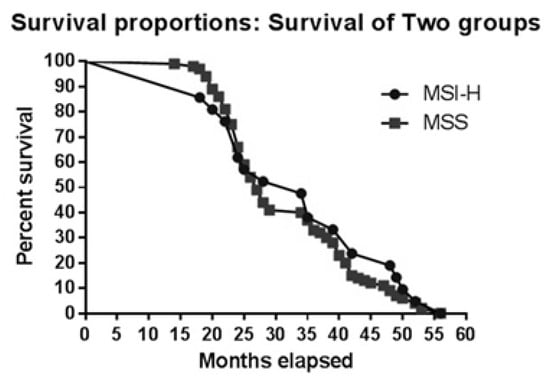
Figure 1.
Kaplan-Meier curve for MSI vs. MSS patients.
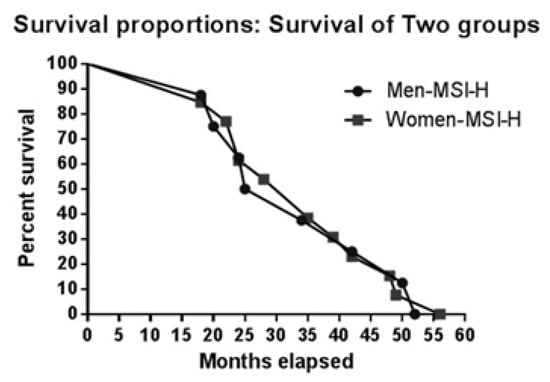
To determine whether sex has an impact on survival, we first analysed median survival: 35 months for women with MSI, 26 months for women with MSS; 26 months for men with MSI, and 28 months for men with MSS. Figure 2 and Figure 3 illustate survival proportions for each sex group. However, the Kaplan Meyer curve for both showed no significant differences (p=0.99) in survival rates.
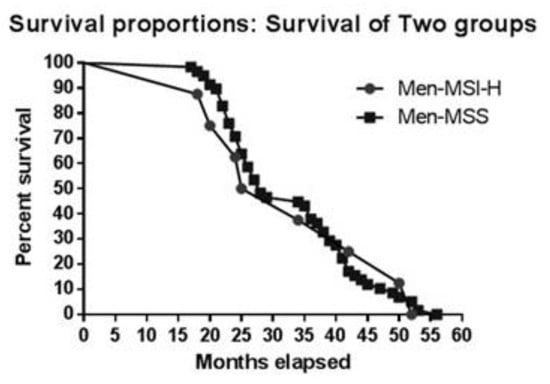
Figure 2.
Kaplan-Meier curve for MSI vs. MSS in men.
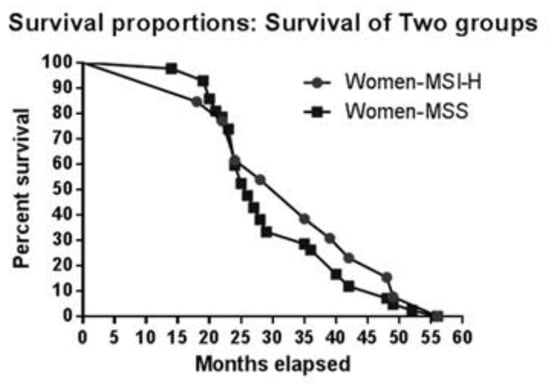
Figure 3.
Kaplan-Meier curve for MSI vs. MSS in women.
Discussion
MSI testing
The current "gold standard" for DNA evaluation of mismatch repair mutations is PCR (polymerase chain reaction) [9]. The tissue to be analyzed is extracted from both tumor tissue and normal tissue, and the DNA from the chromosome microsatellite—more precisely some of the loci from the arms of the chromosomes—is amplified by the polymerase, the DNA fragments are separated by size, and they are compared to the nucleotide pairs of the normal and tumor tissues. Microsatellite instability present in 2 of the 5 markers tested (30% of the markers tested) defines the tumor as MSI-H. The procedure is laborious, costly, and time- consuming. The time interval from diagnosis to surgery is generally insufficient to obtain the test results, results that could influence the course of intervention.
For the above-mentioned considerations in our clinic, identification of MSI markers is achieved through IHC. In interpreting the results, the sensitivity parameters of the technique should be taken into account. Sensitivity is: 80-91% for MLH1 or MSH2 mutations while the specificity is 90%. For MSH6 or PMS2 mutations, the sensitivity is 55-77% while the specificity is 90%. The global IHC sensitivity of the MMR gene is 83% and the specificity is 89% [10] .
The age of patients testing positive for microsatellite instability in the study group was high, with a median of 69 years (range = 41-90), data consistent with reports by Yiu et.al and Yearsley et al. [11].
This presence of MSI-H at an advanced age is due most likely to sporadic/spontaneous rather than inherited genetic changes. In this category of patients with colorectal cancer (CRC), the incidence of MSI-H is 7-15% (>30 years), data confirmed in our study which placed the incidence at 9% (the majority of patients being above 60 years old) and within the norms in the
literature. In patients under 30 with colorectal cancer, the incidence of MSI-H is above 50%, most likely denoting an inherited component rather than sporadic mutations [12,13,14,15,16]. Patient age is the most important factor in establishing the type of deficiency. Even in the hereditary setting, MMR deficiency does not guarantee CRC. In patients with Lynch syndrome, each mutation is associated with a different incidence. The development of CRC by the age of 70 for these patients is as follows: 48-77% for MSH2, 41-79% for MLH1, 12-50% for MSH6 and 15-20% for PMS 2 [17]. MMR inactivation has some differences in the sporadic mutation setting, the most important being BRAF hotspot activation. Positive testing for BRAF V600E mutation certifies the sporadic character of MSI in a patient.
This mutation was, unfortunately, unavailable for testing in the present group of patients, as it would have provided beneficial information, especially for the younger group of patients and would have made family genetic counseling easier [18]. If MMR fails to recognize and correct errors (insertions, deletions, misincorporations) generated by the DNA polymerase, a high number of peptides with wrong c-terminal aminoacids results. These peptides are intensely immunogenic and are considered neoantigens. Their presence leads to high intratumoral T lymphocyte infiltration [19,20]. Our study reports similar survival in the MSI vs. MSS group, which is consistent with reports in the literature [21].
The molecular profile of a tumor will very likely determine the patient's subsequent progression and survival [22]. This is particularly important in cases of locally advanced colorectal tumors (Stage II and Stage III) where patients can be cured by surgery, and adjuvant therapy has a lower impact [23]. The identification of clearly visible tumor markers that can influence the patient's incidence in a certain risk category is very useful. However, when adjuvant tratment containing fluoropyrimidines and oxaliplatin is necessitated, long- term toxicity becomes the main issue. The possibility of targeting cancer cells with these drugs by using liposomes has been recently studied, although the efficacy of this method of administering chemotherapy on toxicity is still a matter of debate [24]. Several studies have addressed the role microsatellite instability plays in colorectal cancer patients [25,26,27]. Although many studies have reported good results in patients diagnosed with microsatellite instability, estimating the prognostic value of this molecular marker represents an extensive variation [28].
Why MSI is not a positive prognosis factor in the long run is controversial. The high intratumoral lymphocyte infiltration may be interpreted as a favorable prognostic factor, at least in the early stages of CRC. Some arguments for efficient immune response in the early stages include: the high levels of co- stimulatory molecules necessary in the T lymphocyte activation, which also attracts CD4+ lymphocytes at the setting, and low levels of regulatory lymphocytes which usually inhibit the activity of cytotoxic LT. Nevertheless, MSI tumors can undergo an immunoediting processes at some point in their evolution. After the initial immune response, some of the neoantigens fail to be recognized as non-self. On the other hand, MSS tumors which evolve in a less immunogenic environment are more sensitive to a late immune attack [29].
Other immune-related factors can predict survival in CRC, independent of MMR status. One of the most cited is the lymphocyte tumor infiltration percentage. In MSS CRC, for example, the presence of high levels of CD8+TL is a factor with substantial impact on survival [30].
CRC immunogenicity can also be related to the presence of HLA class I antigens, that leads to LT activation. HLA class I antigens are seldom found on the surface of MSI CRC cells, making them invulnerable to natural killer lymphocyte activity [31].
The link between MSI and CRC immunogenicity can be exploited for therapeutic purposes. The most studied therapeutic option is represented by the immune checkpoint inhibitors. Pembrolizumab, an IgG4 monoclonal antibody, is well known for its efficacy in treating malignant melanoma and non-small cell lung cancer. Its mechanism of action consists of blocking PD- 1and thus preventing the interaction with PDL-1 and PDL-2 [32].
In 2015, pembrolizumab made medical history when it became the first FDA approved oncology drug, not targeted to a specific cancer site, but rather to any tumor harboring MSI [33]. Focus on pembrolizumab was inevitable in CRC. One of the first phase 2 clinical trials that certified the efficacy of pembrolizumab in MSI CRC enrolled 41 patients, of which 21 were CRC- MMR proficient, 11 were CRC-MMR deficient, and 9 had other primary sites with MMR deficiency. All patients received 10 mg/kg pembrolizumab every 2 weeks. Two endpoints were studied: the immune related response rate, which was superior in the other-than CRC histology subgroup (71%), whereas the CRC MMR deficient group had a response rate of 41%. None of the
MMR proficient CRC patients responded. The second end-point was the 20 week immune related progression free survival (PFS), which clearly favored the CRC MMR deficient subgroup. This trial demonstrated that MSI is a valuable factor in influencing CRC response to immune-check point inhibitors [34].
Nivolumab, another PD-1 targeting monoclonal antibody, was also studied in this setting. One phase 2 trial compared nivolumab monotherapy with a nivolumab/ipilimumab combination in MMR deficient and MMR proficient metastatic CRC patients. In the MMR deficient group, PFS was 5.3 months for monotherapy but was not yet reached for the combination. However, MMR proficient patients had PFS of just 1.4 months on monotherapy [35].
Therefore, MSI in the CRC metastatic setting is also an important factor in the therapeutic decision, enabling the choice of immunotherapy as the second or third line of treatment. Other therapeutic options that may have immunogenic potential consist of neoantigen vaccines, developed by analyzing the tumor genome and predicting which neopeptides encoded by mutant genes have most immunogenic potential. These epitopes can then be used to develop autologus T cell transplants, gene-modified cell therapies, or other vaccines [36].
Conclusions
Our study demonstrates that survival is the same irrespective of gender in MSI vs. MSS colo-rectal cancer. In addition, no notable differences in survival were found between stage IV MSI vs MSS patients, but a superior survival in MSS patients after 36 months should be taken into consideration. Other recent studies confirm our findings, but further research on gender differences is needed.
Acknowledgment
All authors have equal contribution to this article and have no conflicts of interest to disclose; no grants were received for the present study.
References
- Boland, C.R. Evolution of the nomenclature for the hereditary colorectal cancer syndromes. Fam Cancer. 2005, 4, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.F.; Loda, M.; Gaida, G.M.; Lipman, J.; Mishra, R.; Goldman, H.; Jessup, J.M.; Kolodner, R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997, 57, 808–811. [Google Scholar] [PubMed]
- Weitz, J.; Koch, M.; Debus, J.; Hohler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Hamelin, R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res. 2002, 62, 2447–2454. [Google Scholar] [PubMed]
- Hemminki, A.; Peltomaki, P.; Mecklin, J.P.; Jarvinen, H.; Salovaara, R.; Nystrom-Lahti, M.; de la Chapelle, A.; Aaltonen, L.A. Loss of the wild type MLH1 gene is a feature of hereditary nonpolyposis colorectal cancer. Nat Genet. 1994, 8, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite Instability in Colorectal Cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; Tabah-Fisch, I.; de Gramont, A.; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, Fluorouracil, and leucovorinas adjuvant treatment for colon cancer. New Engl J Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Hamelin, R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res. 2002, 62, 2447–2454. [Google Scholar] [PubMed]
- Lindor, N.M.; Burgart, L.J.; Leontovich, O.; Goldberg, R.M.; Cunningham, J.M.; Sargent, D.J.; Walsh-Vockley, C.; Petersen, G.M.; Walsh, M.D.; Leggett, B.A.; Young, J.P.; Barker, M.A.; Jass, J.R.; Hopper, J.; Gallinger, S.; Bapat, B.; Redston, M.; Thibodeau, S.N. Immunohistochemistry Versus Microsatellite Instability Testing in Phenotyping Colorectal Tumors. J Clin Oncol. 2002, 20, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Yiu, R.; Qiu, H.; Lee, S.H.; Garcia-Aguilar, J. Mechanisms of microsatellite instability in colorectal cancer patients in different age groups. Dis Colon Rectum. 2005, 48, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Yearsley, M.; Hampel, H.; Lehman, A.; Nakagawa, H.; de la Chapelle, A.; Frankel, W.L. Histologic features distinguish microsatellite-high from microsatellite- low and microsatellite- stable colorectal carcinomas, but do not differentiate germline mutations from methylation of the MLH1 promoter. Hum Pathol. 2006, 37, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Farrington, S.M.; Petersen, G.M.; Hamilton, S.R.; Parsons, R.; Papadopoulos, N.; Fujiwara, T.; Jen, J.; Kinzler, K.W.; Wyllie, A.H.; Vogelstein, B.; Dunlop, M.G.; et al. Genetic instability occurs in the majority of young patients with colorectal cancer. Nat Med. 1995, 1, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Farrington, S.M.; Lin-Goerke, J.; Ling, J.; Wang, Y.; Burczak, J.D.; Robbins, D.J.; Dunlop, M.G. Systematic analysis of hMSH2 and hMLH1 in young colon cancer patients and controls. Am J Hum Genet. 1998, 63, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Alling, K.C.; French, A.J.; McDonnell, S.K.; Burgart, L.J.; Schaid, D.J.; Peterson, B.J.; Moon-Tasson, L.; Mahoney, M.R.; Sargent, D.J.; O'Connell, M.J.; Witzig, T.E.; Farr GHJr Goldberg, R.M.; Thibodeau, S.N. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999, 91, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, L.A.; Salovarra, R.; Kristo, P.; Canzian, F.; Hemminki, A.; Peltomäki, P.; Chadwick, R.B.; Kääriäinen, H.; Eskelinen, M.; Järvinen, H.; Mecklin, J.P.; de la Chapelle, A. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998, 338, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Bonadona, V.; Bonaiti, B.; Olschwang, S.; Grandjouan, S.; Huiart, L.; Longy, M.; Guimbaud, R.; Buecher, B.; Bignon, Y.J.; Caron, O.; Colas, C.; Noguès, C.; Lejeune- Dumoulin, S.; Olivier-Faivre, L.; Polycarpe-Osaer, F.; Nguyen, T.D.; Desseigne, F.; Saurin, J.C.; Berthet, P.; Leroux, D.; Duffour, J.; Manouvrier, S.; Frébourg, T.; Sobol, H.; Lasset, C.; Bonaïti-Pellié, C.; French Cancer Genetics, Network. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011, 305, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Laiho, P.; Ollikainen, M.; Pinto, M.; Wang, L.; French, A.J.; Westra, J.; Frebourg, T.; Espín, E.; Armengol, M.; Hamelin, R.; Yamamoto, H.; Hofstra, R.M.; Seruca, R.; Lindblom, A.; Peltomäki, P.; Thibodeau, S.N.; Aaltonen, L.A.; Schwartz, S., Jr. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet. 2004, 41, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Abdulovic, A.L.; Hile, S.E.; Kunkel, T.A.; Eckert, K.A. The in vitro fidelity of yeast DNA polymerase δ and polymerase ɛ holoenzymes during dinucleotide microsatellite DNA synthesis. DNA Repair (Amst). 2011, 10, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Dolcetti, R.; Viel, A.; Doglioni, C.; Russo, A.; Guidoboni, M.; Capozzi, E.; Vecchiato, N.; Macrì, E.; Fornasarig, M.; Boiocchi, M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosisin colorectal carcinomas with microsatellite instability. Am J Pathol 154, 1805–1813. [CrossRef] [PubMed]
- Deschoolmeester, V.; Baay, M.; Marck, E.V.; Weyler, J.; Vermeulen, P.; Filip Lardon, J.; Vermorken, R. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; Srivastava, S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Tangen, C.M.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; Veeder, M.H.; Mailliard, J.A. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: A final report. Ann Intern Med. 1995, 122, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Galateanu, B.; Hudita, A.; Negrei, C.; Ion, R.M.; Costache, M.; Stan, M.; Nikitovic, D.; Hayes, A.W.; Spandidos, D.A.; Tsatsakis, A.M.; Ginghina, O. Impact of multicellular tumor spheroids as an in vivo-like tumor model on anticancer drug response. Int J Oncol. 2016, 48, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Samowitz, W.S.; Curtin, K.; Ma, K.N.; Schaffer, D.; Coleman, L.W.; Leppert, M.; Slattery, M.L. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001, 10, 917–923. [Google Scholar] [PubMed]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent- Puig, P.; Gryfe, R.; Shepherd, L.E.; Tu, D.; Redston, M.; Gallinger, S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003, 349, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M.; Smith, E.J.; Behling, C.A.; Nguyen, L.; Tajima, A.; Doctolero, R.T.; Cabrera, B.L.; Goel, A.; Arnold, C.A.; Miyai, K.; Boland, C.R. Use of 5- fluorouracil and survival in patients with microsatellite unstable colorectal cancer. Gastroenterology 2004, 126, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Constantin, V.D.; Socea, B.; Popa, F.; Carâp, A.C.; Popescu, G.; Vlădescu, T.; Ceauşu, Z.; Berteşteanu, Ş.V.; Ceauşu, M.C. A histopathological and immunohistochemical approach of surgical emergencies of GIST. An interdisciplinary study. Rom J Morphol Embryol 2014, 55(2 Suppl), 619-27. [Google Scholar]
- Ishikawa, T.; Fujita, T.; Suzuki, Y.; Okabe, S.; Yuasa, Y.; Iwai, T.; Kawakami, Y. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 2003, 63, 5564–5572. [Google Scholar] [PubMed]
- Baker, K.; Zlobec, I.; Tornillo, L.; Terracciano, L.; Jass, J.R.; Lugli, A. Differential significance of tumour infiltrating lymphocytes in sporadic mismatch repair deficient versus proficient colorectal cancers: a potential role for dysregulation of the transforming growth factor-beta pathway. Eur J Cancer. 2007, 43, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Speetjens, F.M.; de Bruin, E.C.; Morreau, H.; Zeestraten, E.C.; Putter, H.; van Krieken, J.H.; van Buren, M.M.; van Velzen, M.; Dekker-Ensink, N.G.; van de Velde, C.J.; Kuppen, P.J. Clinical impact of HLA class I expression in rectal cancer. Cancer Immunol Immunother. 2008, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Târtea, E.A.; Florescu, C.; Donoiu, I.; Pirici, D.; Mihailovici, A.R.; Albu, V.C.; Bălşeanu, T.A.; Iancău, M.; Badea, C.D.; Vere, C.C.; Sfredel, V. Implications of inflammation and remodeling of the enteric glial cells in colorectal adenocarcinoma. Rom J Morphol Embryol. 2017, 58, 473–480. [Google Scholar] [PubMed]
- Zheng, J.; Huang, B.; Nie, X.; Zhu, Y.; Han, N.; Li, Y. The clinicopathological features and prognosis of tumor MSI in East Asian colorectal cancer patients using NCI panel. Future Oncol 2018. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with Missmatch-Repair Deficiency. N Engl J Med 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; Sawyer, M.B.; Hendlisz, A.; Neyns, B.; Svrcek, M.; Moss, R.A.; Ledeine, J.M.; Cao, Z.A.; Kamble, S.; Kopetz, S.; André, T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 2018. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Westdorp, H.; Fennemann, F.L.; Weren, R.D.; Bisseling, T.M.; Ligtenberg, M.J.; Figdor, C.G.; Schreibelt, G.; Hoogerbrugge, N.; Wimmers, F.; de Vries, I.J. Opportunities for immunotherapy in microsatellite instable colorectal cancer. Cancer Immunol Immunother. 2016, 65, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. 2018 Adrian Tulin, Iulian Slavu, Raluca Tulin, Lucian Alecu, Radu C. Jecan, Cristina Orlov, Cristian I. Iaciu, Dana L. Stanculeanu, Razvan Hainarosie, Silviu Pituru, Anca Pantea Stoian, Cornelia Nitipir