Protective Role of S-Adenosylmethionine Against Fructose-Induced Oxidative Damage in Obesity
Abstract
:Introduction
Materials and Methods
Animal models
Blood and tissue collection
Biochemical Analyses
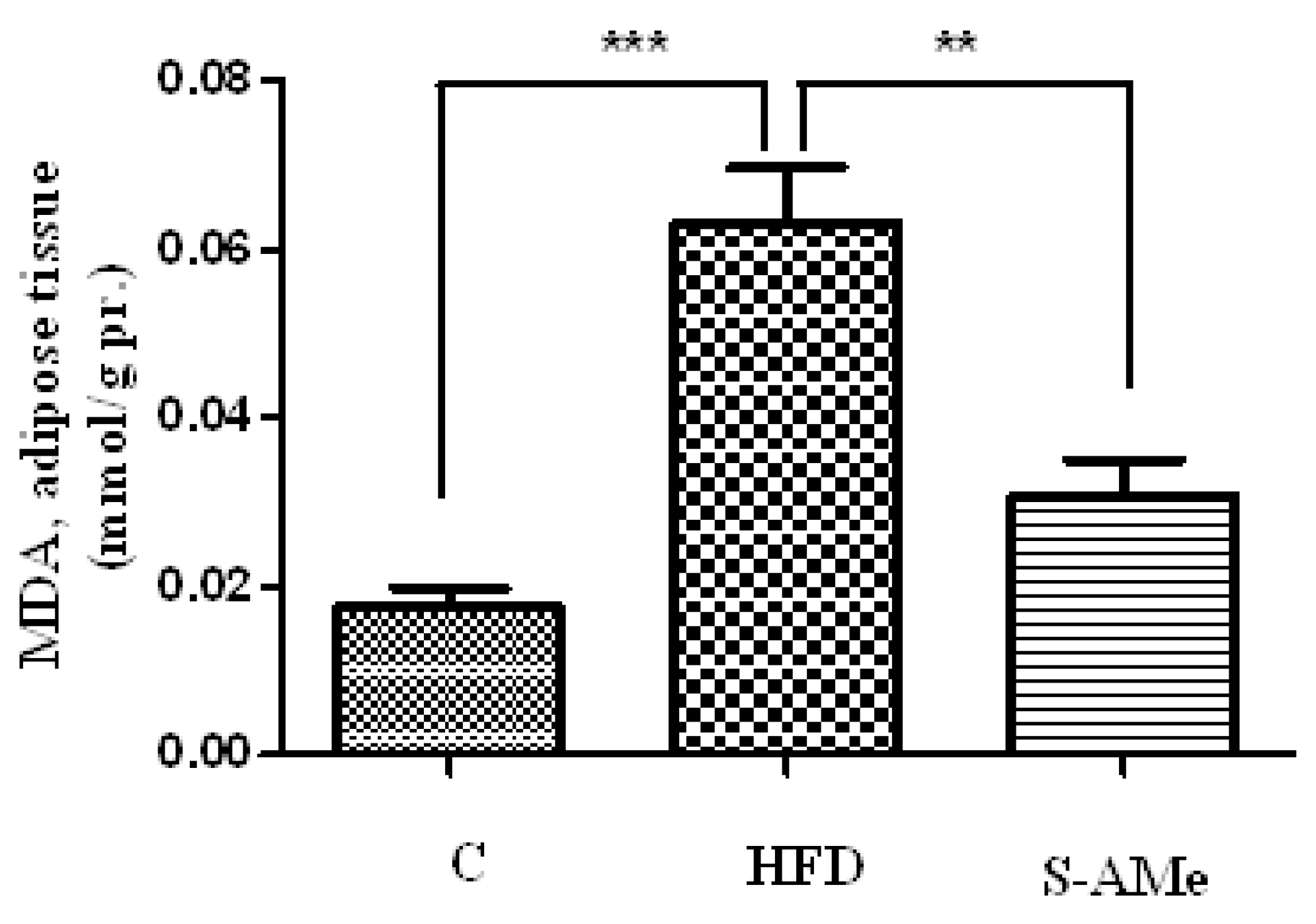
- Determination of lipid peroxides. Membrane lipid peroxidation was assayed by MDA (malondialdehyde) measured by its thiobarbituric acid (TBA) (Merk, Germany) reactivity in adipose tissue homogenates using the method detailed by Porter et al. [13]. Results were expressed in nmol MDA per g tissue and were determined using the extinction coefficient of MDA–TBA complex at 532 nm= 1.56 – 10-5 cm-1 M-1 solution.
- Determination of total thiols. GSH levels in adipose tissue and sera were defined as the total number of sulfhydryl groups (SH groups) in the sample. SH groups were determined by the method of Hu [14], based on the absorption of the color complex between thiol groups and 5,5'-dithiobis-(2- nitrobenzoic acid (DTNB) (Merk, Germany) at 412 nm. Standard solutions of reduced glutathione were used to calculate the concentration of thiol groups.
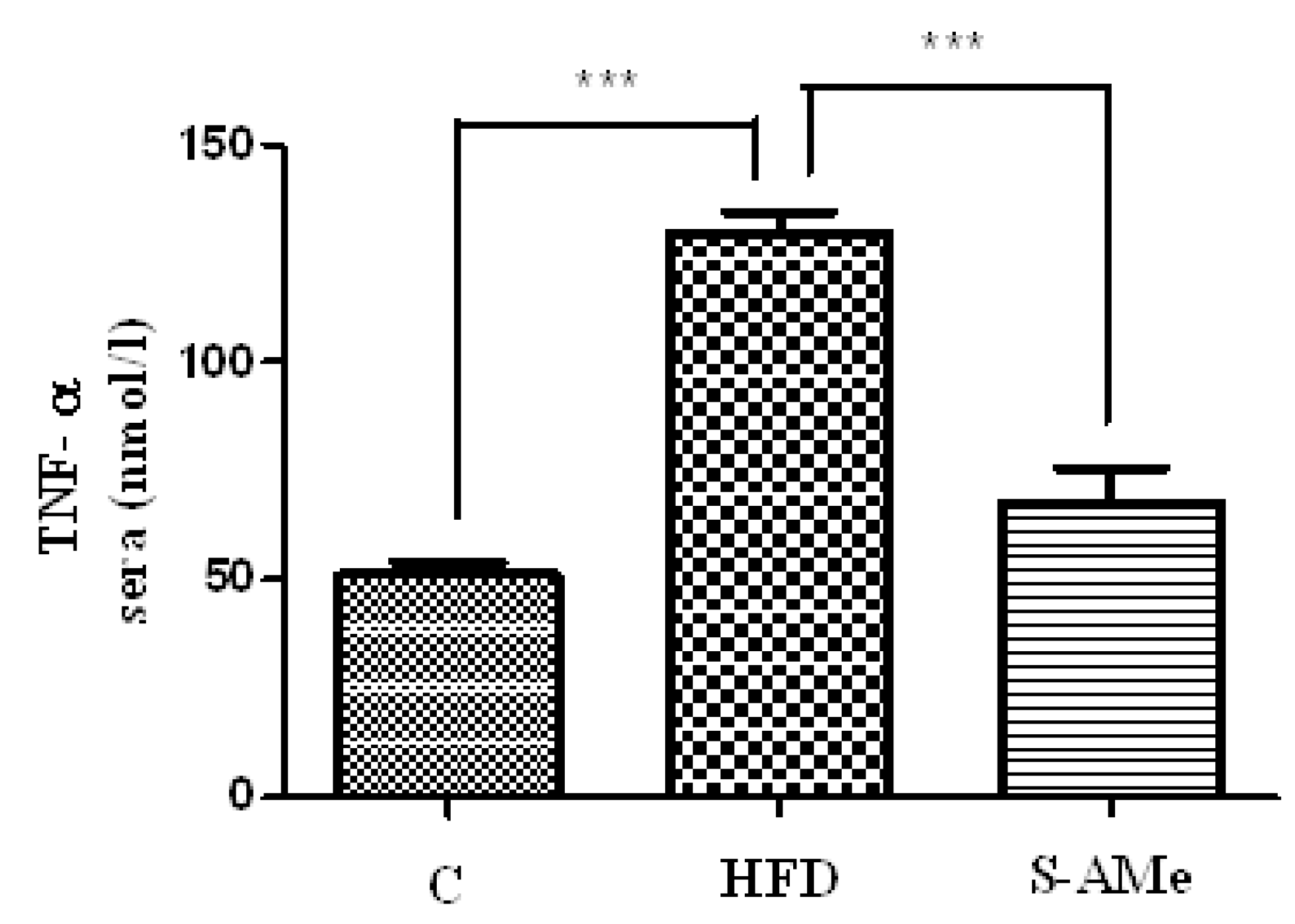
- Determination of TNF-α levels. Concentrations of TNF-α cytokine (IBL International) were measured in sera using the commercially available ELISA kit.
Statistical analysis
Results
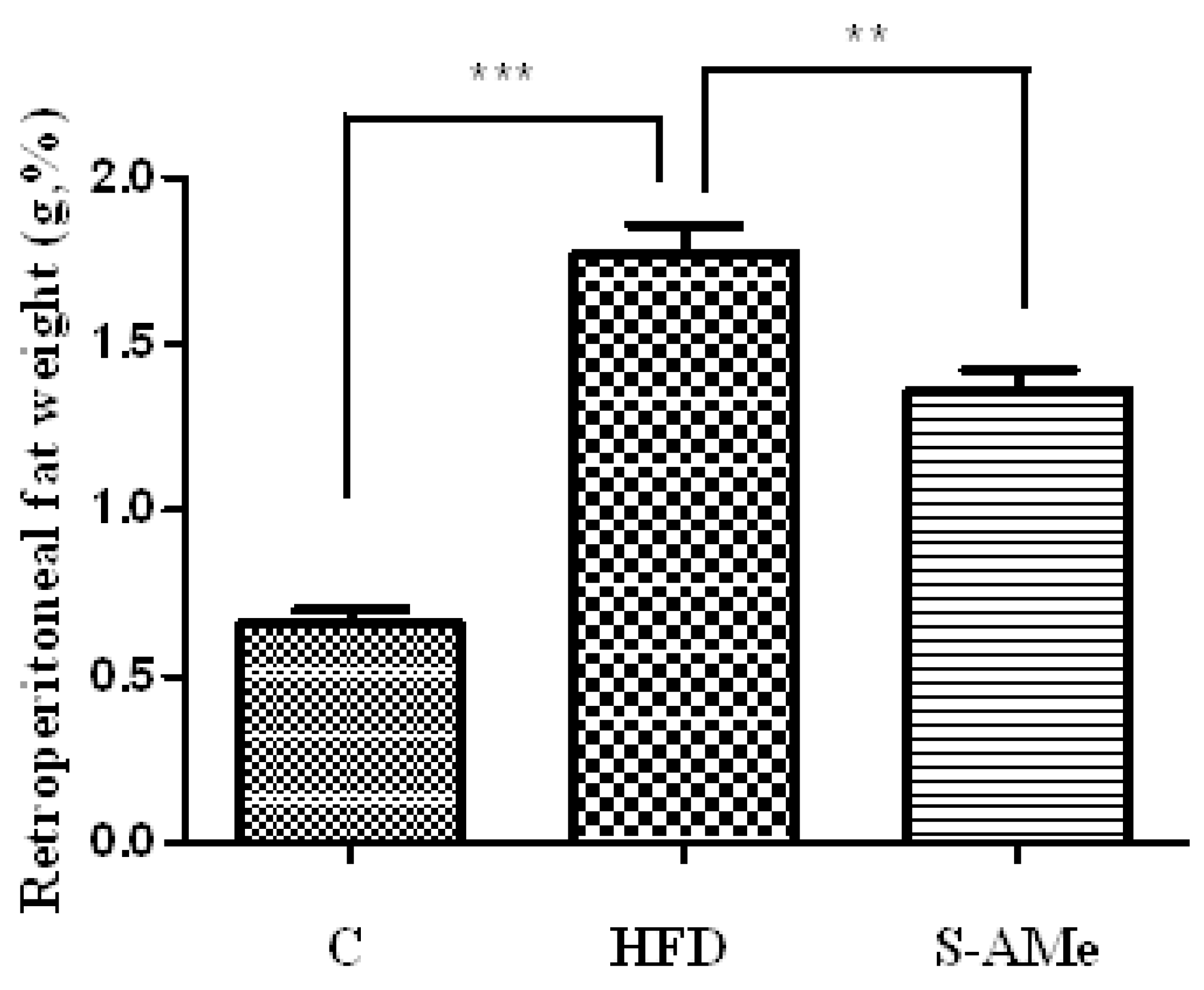
Changes in body weight and weight of visceral adipose tissue.
Markers of oxidative stress - levels of MDA and SH groups.
3. Marker of inflammation.
Discussion
Conclusions
References
- Oron-Herman, M.; Kamari, Y.; Grossman, E.; Yeger, G.; Peleg, E.; Shabtay, Z.; Shamiss, A.; Sharabi, Y. Metabolic syndrome: comparison of the two commonly used animal models. Am J Hypertens. 2008, 21, 1018–22. [Google Scholar] [CrossRef] [PubMed]
- Rudich, A.; Kanety, H.; Bashan, N. Adipose stress- sensing kinases: linking obesity to malfunction. Trends Endocrinol Metab. 2007, 18, 291–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.C.; Mori, T.A.; Burke, V.; Newnham, J.; Stanley, F.J.; Landau, L.I.; Kendall, G.E.; Oddy, W.H.; Beilin, L.J. Synergy between adiposity, insulin resistance, metabolic risk factors, and inflammation in adolescents. Diabetes Care. 2009, 32, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Das, M.; Mora, A.; Zhang, Z.; Jun, J.Y.; Ko, H.J.; Barrett, T.; Kim, J.K.; Davis, R.J. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008, 322, 1539–43. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P. From fat to inflammation. Gastroenterology 2006, 130, 207–10. [Google Scholar] [CrossRef] [PubMed]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005, 54, 2939–45. [CrossRef] [PubMed]
- Wolf, A.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004, 323, 630–5. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001, 1930–5. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004, 114, 1752–61. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Rock, C.L.; Eldridge, A.L.; Kristal, A.R.; Patterson, R.E.; Cooper, D.A.; Neumark-Sztainer, D.; Cheskin, L.J.; Thornquist, M.D. Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J Nutr. 2001, 131, 2184–91. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, A.C.; Drăgoi, C.M.; Ceaușu, I.; Poalelungi, C.; Iliescu, D.; Arsene, A.L. Clinical implications of the indolergic system and oxidative stress in physiological gestational homeostasis. Farmacia. 2015, 63, 46–51. [Google Scholar]
- Andringa, K.K.; King, A.L.; Eccleston, H.B.; Mantena, S.K.; Landar, A.; Jhala, N.C.; Dickinson, D.A.; Squadrito, G.L.; Bailey, S.M. Analysis of the liver mitochondrial proteome in response to ethanol and S- adenosylmethionine treatments: novel molecular targets of disease and hepatoprotection. Am J Physiol Gastrointest Liver Physiol. 2010, 298, G732–45. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.A.; Nixon, J.; Isaac, J. Cyclic peroxidase and thiobarbituric assay. Biochim Biophys Acta 1976, 441, 596–599. [Google Scholar] [PubMed]
- Hu, M. Measurment of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–5. [Google Scholar] [PubMed]
- Melanson, K.J.; Angelopoulos, T.J.; Nguyen, V.; Zukley, L.; Lowndes, J.; Rippe, J.M. High-fructose corn syrup, energy intake, and appetite regulation. Am J Clin Nutr. 2008, 88, 1738S–1744S PMID: 19064539. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab. 2012, 37, 233–40. [Google Scholar] [CrossRef] [PubMed]
- Botezelli, J.D.; Mora, R.F.; Dalia, R.A.; Moura, L.P.; Cambri, L.T.; Ghezzi, A.C.; Voltarelli, F.A.; Mello, M.A. Exercise counteracts fatty liver disease in rats fed on fructose-rich diet. Lipids Health Dis. 2010, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Busserolles, J.; Gueux, E.; Rock, E.; Mazur, A.; Rayssiguier, Y. High fructose feeding of magnesium deficient rats is associated with increased plasma triglyceride concentration and increased oxidative stress. Magnes Res. 2003, 16, 7–12. [Google Scholar] [PubMed]
- Cortez-Pinto, H.; Chatham, J.; Chacko, V.P.; Arnold, C.; Rashid, A.; Diehl, A.M. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA 1999, 282, 1659–64. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; Patel, J.M.; Johnson, R.J. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006, 290, F625–31. [Google Scholar] [CrossRef] [PubMed]
- Talior I, Tennenbaum T, Kuroki T. Eldar-Finkelman H. PKC-delta-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: role for NADPH oxidase. Am J Physiol Endocrinol Metab. 2005, 288, E405–11. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Maecker, H.L.; Johnson, R.S.; Giaccia, A.J. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002, 2, 331–41. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, Z.; Oron-Herman, M.; Grozovski, M.; Rosenthal, T.; Pappo, O.; Link, G.; Sela, B.A. Fructose- induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005, 45, 1012–8. [Google Scholar] [CrossRef] [PubMed]
- Kanellis, J.; Watanabe, S.; Li, J.H.; Kang, D.H.; Li, P.; Nakagawa, T.; Wamsley, A.; Sheikh-Hamad, D.; Lan, H.Y.; Feng, L.; Johnson, R.J. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003, 41, 1287–93. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, S.K.; Lee, I.K.; Johnson, R.J. Uric acid- induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005, 16, 3553–62. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kitada, T.; Yamada, T.; Sakaguchi, H.; Nakatani, K.; Wakasa, K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002, 37, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Tzaneva, M.; Bratoeva, K.; Kotzev, I.; Radanova, M. Heme-oxygenase-1 upregulated by s- adenosylmethionine. Potential protection against non-alcoholic fatty liver induced by high fructose diet. Farmacia 2017, 6, 262–7. [Google Scholar]
- Gustafson, B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010, 17, 332–41. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Moon MK, Kim M, Chung SS, Lee HJ, Koh SH, Svovoda P, Jung MH, Cho YM, Park YJ, Choi SH, Jang HC, Park KS, Lee HK. S-Adenosyl-l-methionine ameliorates TNFalpha-induced insulin resistance in 3T3-L1 adipocytes. Exp Mol Med 2010, 42, 345–352. [CrossRef] [PubMed]
- Bekyarova, G.; Atanasova, M.; Tzaneva, M.; Dimitrova, A. Melatonin modulates inflammatory response and suppresses burn-induced apoptotic injury. J Mind Med Sci. 2017, 4, 59–66. [Google Scholar] [CrossRef]
- Cordero, P.; Gomez-Uriz, A.M.; Campion, J.; Milagro, F.I.; Martinez, J.A. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013, 8, 105–13. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. 2017 Kameliya Zh Bratoeva, Mariya A. Radanova, Albena V. Merdzhanova, Ivan S. Donev
Share and Cite
Bratoeva, K.Z.; Radanova, M.A.; Merdzhanova, A.V.; Donev, I.S. Protective Role of S-Adenosylmethionine Against Fructose-Induced Oxidative Damage in Obesity. J. Mind Med. Sci. 2017, 4, 163-171. https://doi.org/10.22543/7674.42.P163171
Bratoeva KZ, Radanova MA, Merdzhanova AV, Donev IS. Protective Role of S-Adenosylmethionine Against Fructose-Induced Oxidative Damage in Obesity. Journal of Mind and Medical Sciences. 2017; 4(2):163-171. https://doi.org/10.22543/7674.42.P163171
Chicago/Turabian StyleBratoeva, Kameliya Zh, Mariya A. Radanova, Albena V. Merdzhanova, and Ivan S. Donev. 2017. "Protective Role of S-Adenosylmethionine Against Fructose-Induced Oxidative Damage in Obesity" Journal of Mind and Medical Sciences 4, no. 2: 163-171. https://doi.org/10.22543/7674.42.P163171
APA StyleBratoeva, K. Z., Radanova, M. A., Merdzhanova, A. V., & Donev, I. S. (2017). Protective Role of S-Adenosylmethionine Against Fructose-Induced Oxidative Damage in Obesity. Journal of Mind and Medical Sciences, 4(2), 163-171. https://doi.org/10.22543/7674.42.P163171


