Abstract
Carbamazepine is an early anticonvulsant still used today in the treatment of several forms of epilepsy. An active metabolite in the human body contributes to its pharmacological effect. Carbamazepine metabolism has high inter-individual variability, such that it is relatively difficult to establish a direct link between dose and concentration, or between concentration and pharmacological effect. Carbamazepine is thus a good candidate for therapeutic drug monitoring (TDM). Good UV specific absorbance and high plasmatic concentrations allow for the use of UV detection, which is often more accessible than other methods of detection. This paper presents several methods used for the detection of carbamazepine in plasma, methods that are capable of detecting drug and metabolites at adequate levels/acceptance criteria. These methods have possible application not only in pharmacokinetic, bioequivalence, and permeability studies, but also in the therapeutic drug monitoring of carbamazepine.
Introduction
Epilepsy is a common neurological disorder. The probability of an individual developing the disorder during a lifetime is between 3% and 5%. Newborn, children, and the elderly have the highest risk of developing epilepsy. An epileptic seizure is defined as a paroxysmal discharge of cerebral neurons, followed by obvious clinical phenomena: motor, sensory, and autonomic, with impairment or complete loss of consciousness. Epilepsy is defined as a condition characterized by repeating seizures (colloquially named “fits”). A patient should not be diagnosed with epilepsy until two such non-febrile seizures occur [1].
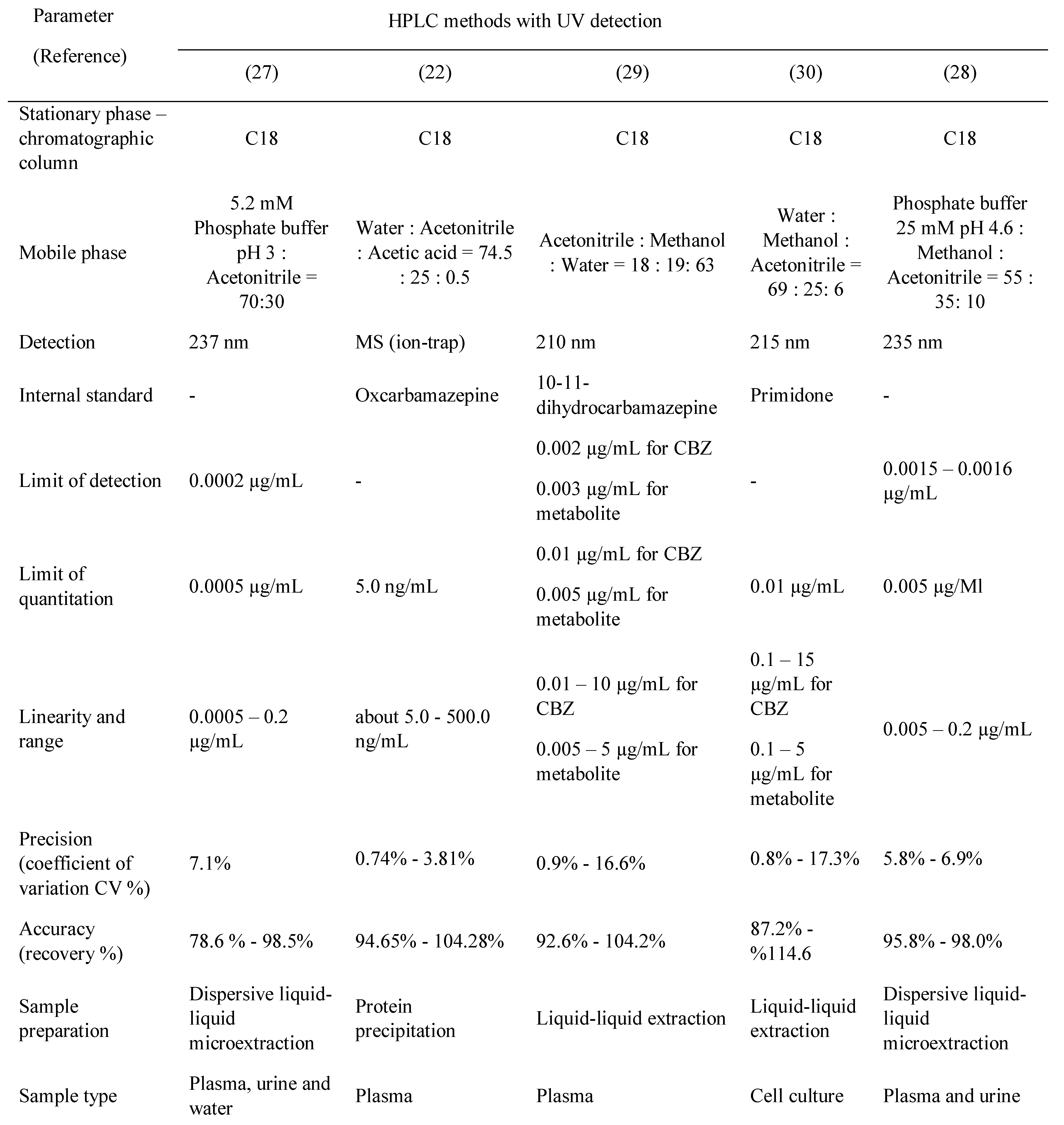
TDM (therapeutic drug monitoring) may be defined as the use of drug or metabolite monitoring in body fluids as an aid to the management of therapy (the term therapeutic drug management is now also employed as an alternative description). Patients may need different doses of a drug to produce optimum pharmacological effects, as individuals vary widely regarding drug absorption, elimination (pharmacokinetics), and effect (pharmacodynamics) [2]. The pharmacokinetics and pharmacodynamics steps involved in drug action are summarized in Figure 1. Most antiepileptic drugs meet the requirements for therapeutic drug monitoring (TDM) and thus are benefitted by this approach.
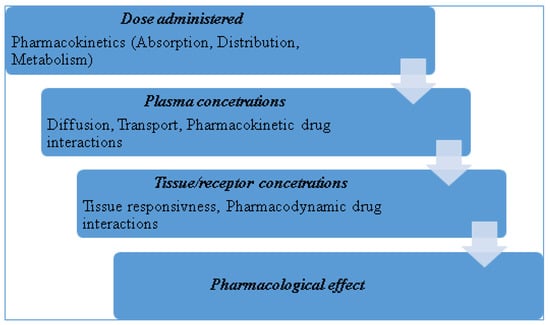
Figure 1.
Pharmacokinetics and pharmacodynamics steps involved in drug action; [2], adapted.
Carbamazepine, an iminostilbene drug substance from a chemical point of view, is effective against grand mal and all forms of epilepsy except petit mal. Its pharmacological action is similar to phenobarbital, phenytoin, lamotrigine, topiramate, and valproic acid, Figure 2, [3]. It was initially approved in the U.S. for use as an anti-seizure agent in 1974. It has been employed since the 1960’s for the treatment of trigeminal neuralgia and is now considered a primary drug for the treatment of partial and tonic-clonic seizures [4].
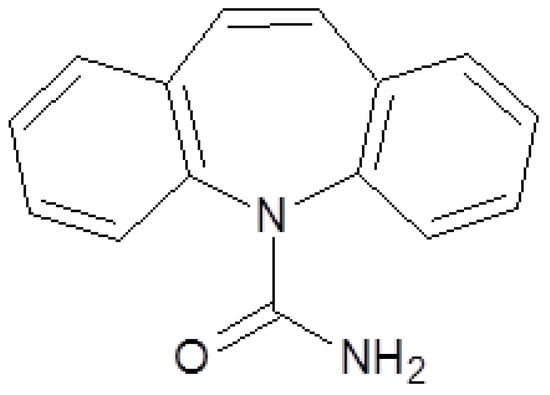
Figure 2.
Carbamazepine chemical structure.
Therapy with antiepileptic drugs should commence only if the risk of future seizures on the patient’s health outweighs the risk of therapy. A premature start of antiepileptic therapy may expose the patient to unnecessary risk tied to the adverse effects of the drugs. Also the social consequences should not be overlooked, especially in the case of diagnostic errors (for example: the loss of the driver’s license) [5]. On the other hand, waiting too long to treat must also be avoided. There are insufficient data to support the contention that seizures affect brain tissue and diminish cognitive capacity, but it is known that status epilepticus is a contributing factor to brain injury and death [1].
However, the most compelling reasons to start antiepileptic treatment are the risk of personal injury, the risk of causing harm to others, and the psychosocial consequences of untreated epilepsy: low self-esteem, anxiety, and domestic and employment difficulties [1].
The choice of the drug used for treatment is determined mainly by its efficacy against the seizures and the potential adverse effects. Monotherapy is preferred.
Carbamazepine (5H- dibenzo [b,f] azepin – 5 - carboxamida) is a popular anticonvulsant and mood- stabilizing drug used in Europe for many years [4]. Reports of bone marrow suppression and leucopenia as adverse effects delayed the release on the US market until 1974, when it was approved for the treatment of trigeminal neuralgia. It has since become a first line drug against temporal lobe epilepsy and other convulsive disorders. It is a derivative of iminostilbene with a carbamyl group at the 5 position. The moiety is essential for the antiepileptic action [6].
Carbamazepine is used mainly in the management of epilepsy, bipolar disorder, trigeminal neuralgia, attention-deficit hyperactivity disorder (ADHD), schizophrenia, complex regional pain syndrome, paroxysmal extreme pain disorder, neuromyotonia, disorder, borderline, and post-traumatic stress disorder [6].
Discussion
- General data
Pharmacokinetics
The pharmacokinetics of carbamazepine are complex. They are influenced by its limited aqueous solubility and by the ability of many anti-seizure drugs, including carbamazepine itself, to increase their conversion to active metabolites by hepatic oxidative enzymes [4].
Carbamazepine is slowly and erratically absorbed after oral administration with great inter-individual variability. Peak concentrations in plasma are usually observed 4-8 hours after oral ingestion, but may be delayed by as much as 24 hours, especially following administration of a large dose. It is moderately protein bound (65–85%) and is rapidly distributed to all tissues. Concentrations in cerebrospinal fluid (CSF) appear to correspond to the concentration of free drug in plasma [4]. The drug exhibits auto-induction, which causes the initial low clearance rate to double or triple with continued exposure [1,4,6].
Clearance is ensured by hepatic biotransformation (>99%) and to a small degree by renal excretion. Carbamazepine is metabolized by the hepatic microsomal enzymes system, through oxidation by the isoforms of cytochrome P450, followed by glucuronidation [3].
Carbamazepine is metabolized in the liver by the isoenzymes CYP1A2, CYP3A4, CYP2C8 and CYP2C9, only two of which can be found in the transformation of other anti-epileptics (CYP3A4 and CYP2C9). The participation of such a high number of CYP isoforms implies numerous interactions with drugs from different therapeutic classes, through cross inhibition or induction [3].
Carbamazepine decreases phenytoin metabolism due to competition for CYP2C9 isoform, but also by enzymatic inhibition, which leads to an increase in plasmatic concentrations, with an increase in toxicity (Figure 3). It also exerts enzymatic induction on the oxidative enzymatic systems dependent on cytochrome P450 and uridine glucuronyl transferase system (UGT). Carbamazepine reduces the effectiveness of valproic acid, clonazepam, ethosuximide, and lamotrigine [3]. It also reduces its own metabolism. Thus, the half-life of carbamazepine is drastically reduced after repeated doses. Usually the half-life is between 12 and 24 hours, observed to be shorter in children than in adults [1].
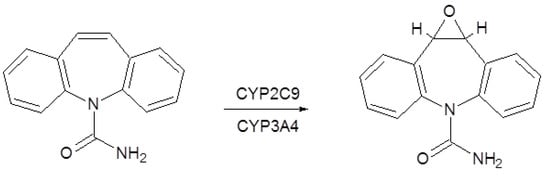
Figure 3.
Carbamazepine metabolism.
The main metabolite of carbamazepine is the pharmacologically active carbamazepine-10,11-epoxide. It is eliminated mainly as metabolites, through renal excretion, with only a small part eliminated through feces. In children carbamazepine clearance is faster with a high accumulation of active metabolites [1,3]. Other metabolites identified in the urine include carbamazepine-N-glucuronide, iminostilbene, and several monohydroxy- and trihydroxy-carbamazepine isomers [7].
Carbamazepine-10,11-epoxide is as active as the parent compound in various animals, and its concentrations in plasma and brain may reach 50% of those of carbamazepine, especially during the concurrent administration of phenytoin or phenobarbital. The 10,11- epoxide is metabolized further to inactive compounds, which are excreted in the urine principally as glucuronides. Carbamazepine is also inactivated by conjugation and hydroxylation [7].
Pharmacodynamics
Carbamazepine stabilizes the neuronal membrane (blocking depolarization and hyperpolarization) by blocking the sodium channels, thereby increasing the excitability and convulsive threshold. It also reduces the discharge of pathologically modified neurons and limits cell depolarization inside the epileptic center [3].
This effect appears to be mediated by a slowing of the rate of recovery of voltage-activated sodium channels from inactivation. These effects of carbamazepine are evident at concentrations in the range of therapeutic drug levels in the CSF of humans. The effects of carbamazepine are selective at these concentrations, in that there are no effects on spontaneous activity or on responses to iontophoretically applied GABA or glutamate. The carbamazepine metabolite, 10,11-epoxycarbamazepine, also limits sustained repetitive firing at therapeutically relevant concentrations, suggesting that this metabolite may contribute to the anti-seizure efficacy of carbamazepine [4].
Toxicology
Carbamazepine is chemically related to tricyclic antidepressants, like imipramine; thus it exhibits similar adverse effects: cholinergic effects, convulsions, and cardiac conduction anomalies. In severe intoxications, myoclonus, hypothermia, coma, and respiratory arrest may occur. Atrioventricular block and bradycardia have also been reported. It is highly hepatotoxic and can present teratogenicity (spina bifida and cardiac malformations) [3].
During long-term therapy, the more frequent untoward effects of the drug include drowsiness, vertigo, ataxia, diplopia, and blurred vision. The frequency of seizures may increase, especially with overdose. Other adverse effects include nausea, vomiting, serious hematological toxicity (aplastic anemia, agranulocytosis), and hypersensitivity reactions (dangerous skin reactions, eosinophilia, lymphadenopathy, splenomegaly). A late complication of therapy with carbamazepine is retention of water, with decreased osmolality and concentration of Na+ in plasma, especially in elderly patients with cardiac disease [4].
Carbamazepine induces hypersensitivity reactions that frequently involve the skin. Approximately 5-10% of patients taking carbamazepine experience cutaneous adverse drug reactions. Carriers of specific HLA variants are known to be susceptible to carbamazepine-induced hypersensitivity reactions. Therefore, HLA testing of patients can identify those at-risk individuals so that an alternative drug can be used [8].
Therapy and posology
In epilepsy, carbamazepine is administered in doses of 0.8-1.2 g/day for 2-3 times a day for an adult and 0.1- 1 g/day (10-25 mg/kg/day) for children between 1 and 15 years. The posology can be optimized by therapeutic drug monitoring (TDM) due to the good correlation between plasmatic concentration and therapeutic effect [3].
Monitoring plasmatic concentration is feasible when deemed appropriate from clinical data. The therapeutic concentration interval is around 4 to 12 µg/mL [9]. Because determination of free carbamazepine is more reliable, concentration in saliva and tears (where carbamazepine occurs in unbound form) has also been determined [1].
However, there may be no simple relationship between the dose of carbamazepine and concentrations of the drug in plasma. Therapeutic concentrations are reported to be 6-12 μg/mL, although considerable variation occurs. Side effects referable to the CNS are frequent at concentrations above 9 μg/mL [4]. During maintenance therapy carbamazepine absorption and clearance are highly variable and its elimination half-life may be longer than previously suspected. Sex and racial differences in clearance may contribute to variable dosing requirements and clinical response [10].
Discontinuing treatment with carbamazepine is achieved by steadily reducing the dosage so as to avoid severe seizures [3].
- 2.
- Therapeutic drug monitoring (TDM)
Pharmacological effects can be measured with the use of markers—the response of the patient to the treatment. In clinical practice this is straightforward when the response is readily measurable. The response can be a clinical measure (e.g. blood pressure in the case of antihypertensive drugs) or may use an appropriate laboratory marker (e.g., glucose determination for hypoglycemic agents or cholesterol/triglycerides levels for lipid-lowering drugs) [2].
Dose adjustment is much more difficult (but no less necessary) when drug response cannot be rapidly assessed clinically, which is the case for antidepressants. Under certain conditions and suitable analytical methods, plasma concentration of a drug and/or metabolite can be determined and may serve as an effective and clinically useful surrogate marker of the response. However, TDM is not just about the analytical result; rather it assumes multiple determinations at different moments, with the analytical results interpreted in order to make the best decision for the patient [2].
The AGNP (Arbeitsgemeinschaft für Neuropsycho- pharmakologie und Pharmakopsychiatrie) provides a consensus guideline regarding optimal use of TDM in a clinical context for psychotropic drugs. They note that the development of new, sensitive analytical techniques have shown that drug concentrations vary widely due to high inter-individual variability in the pharmacokinetics and pharmacodynamics of these drugs by correlating plasmatic concentration with dosage and adverse effects [11]. The high inter-individual variability of plasma concentrations is also reported for different drugs, such as methotrexate, tamoxifen, methadone, and tramadol [12,13,14], as well as for metabolites used as biomarkers of exposure (i.e., cotinine). Such findings have encouraged clinicians to use TDM in combination with pharmacogenetic testing in order to demonstrate genetically determined metabolism [15,16,17].
If plasma drug concentrations are to be a useful surrogate marker of response, two premises must be satisfied:
- -
- The drug concentration in plasma must accurately reflect the concentration at the site of action (the receptor).
- -
- The drug concentration at the receptor should provide an accurate indication of the pharmacological response.
The essential criteria for TDM to be clinically useful may be summarized as follows [2]:
- Poor correlation existing between the dose given and the plasma concentration obtained in different patients (wide inter-individual pharmacokinetic variability).
- Good correlation existing between plasma
- concentration and pharmacological effect in different patients (low inter-individual pharmacodynamic variability); this also implies an established therapeutic range of plasma concentrations or a good relationship between plasma concentration and effect.
- Clinically relevant only for drugs that show significant toxic or undesirable effects at plasma concentrations only slightly above those required for useful effect (low therapeutic index).
- TDM is redundant for drugs where there is a better
- clinical marker of both the desired effect and any associated adverse effects.
- Treatment is expected to be long-term.
For effective TDM practice, analytical methods of adequate sensitivity are necessary to correctly evaluate the drug concentration levels in body fluids. Selectivity is also an issue due to co-medication (drugs administered simultaneously may interfere with the assay). Because carbamazepine is metabolized to an active compound, the challenge of developing a suitable method is even greater. In order to have all the information to assess pharmacological and toxicological effects, both the parent compound and the metabolite must be correctly assayed in order to make good decisions regarding patient therapy.
Plasma or serum samples are generally used for TDM. Chromatography is the most widely used method, high performance liquid chromatography (HPLC) or gas-chromatography (GC), coupled with various detectors: UV (although not very sensitive), fluorescence, MS, MS-MS, or nitrogen-phosphorous detectors for GC. Also, there is need for sample clean- up—atime-consuming step—in order to remove matrix effects.
Regarding the methods used in bioanalysis, the FDA (US Food and Drug Administration) has issued a guideline (Guidance for Industry – Bioanalytical method validation) in which general recommendations regarding method validation are presented. The information in the guidance applies to bioanalytical procedures and immunological and microbiological procedures that are performed for the quantitative determination of drugs and/ or metabolites as well as therapeutic proteins in biological matrices, such as blood, serum, plasma, urine, tissue, and skin [18].
Validating bioanalytical methods includes performing all the procedures that demonstrate that a particular method used for quantitative measurement of analytes in a given biological matrix (e.g., blood, plasma, serum, or urine) are reliable and reproducible for the intended use. Fundamental parameters for this validation include the following: accuracy, precision, selectivity, sensitivity (limits of quantitation), reproducibility, and stability [18].
- 3.
- Bioanalytical methods for determination of carbamazepine and metabolite
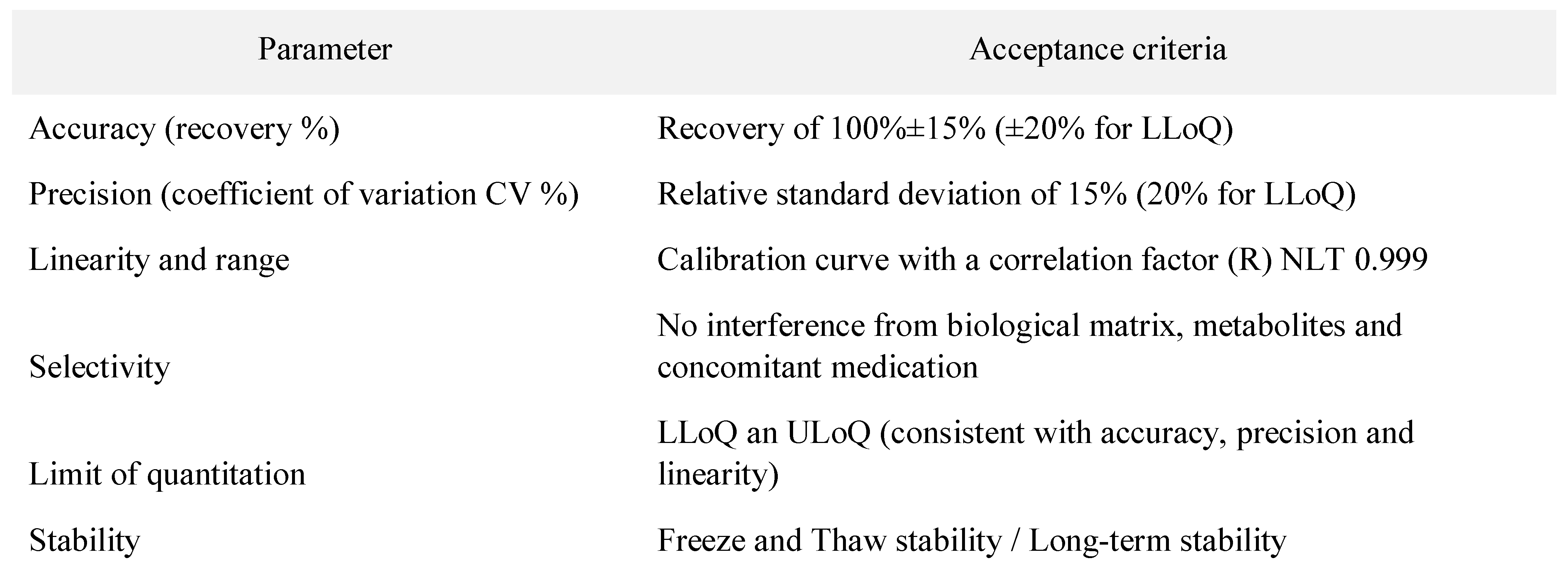
In Table 1 the acceptance criteria for validation parameters of bioanalytical methods are presented. These criteria follow the Guidance for Industry – Bioanalytical method validation Draft Guidance from 2013 provided by the FDA/CDER [18].

Table 1.
Acceptance criteria for method validation parameters according to FDA/CDER Guidance for Industry (DRAFT) [18].
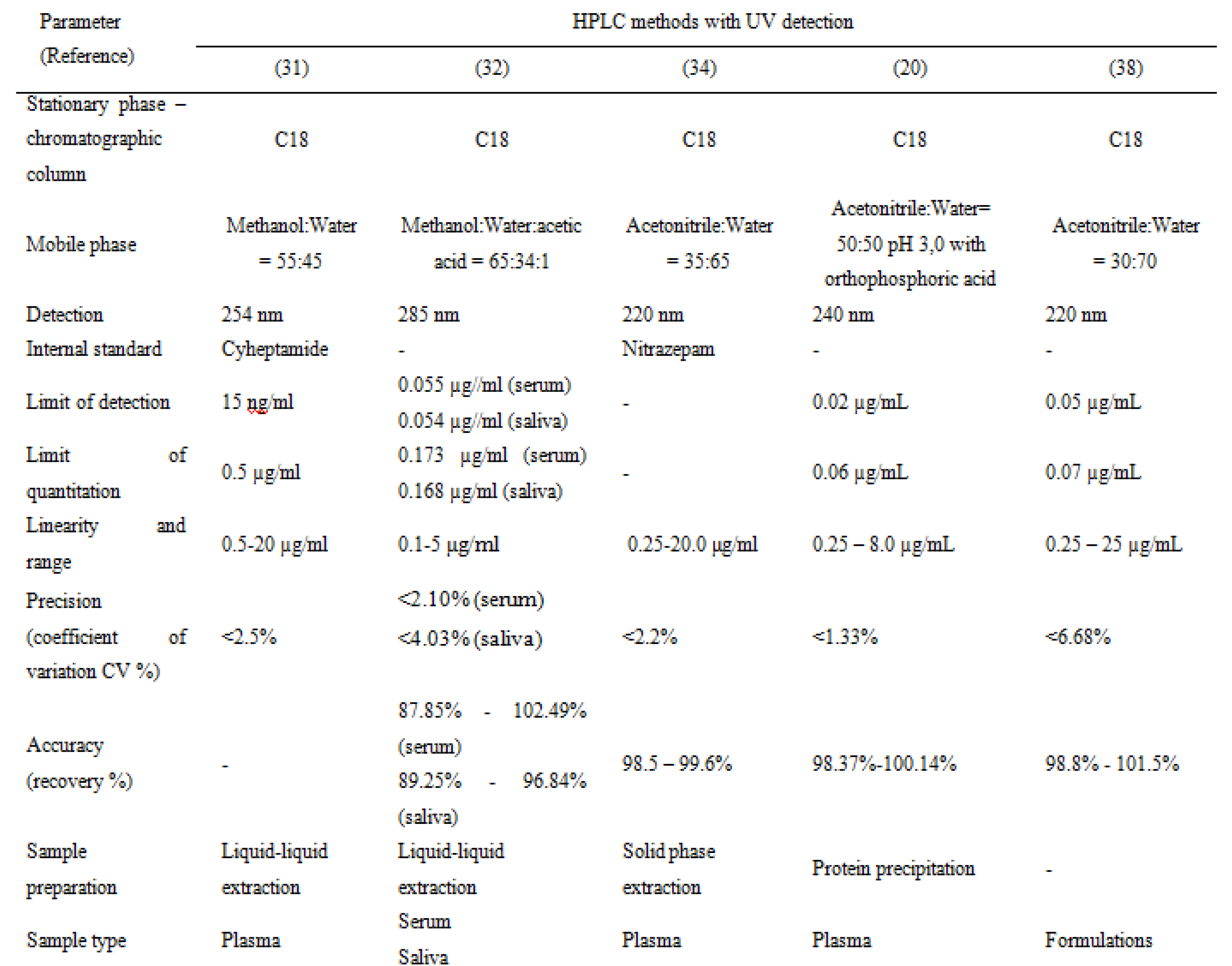
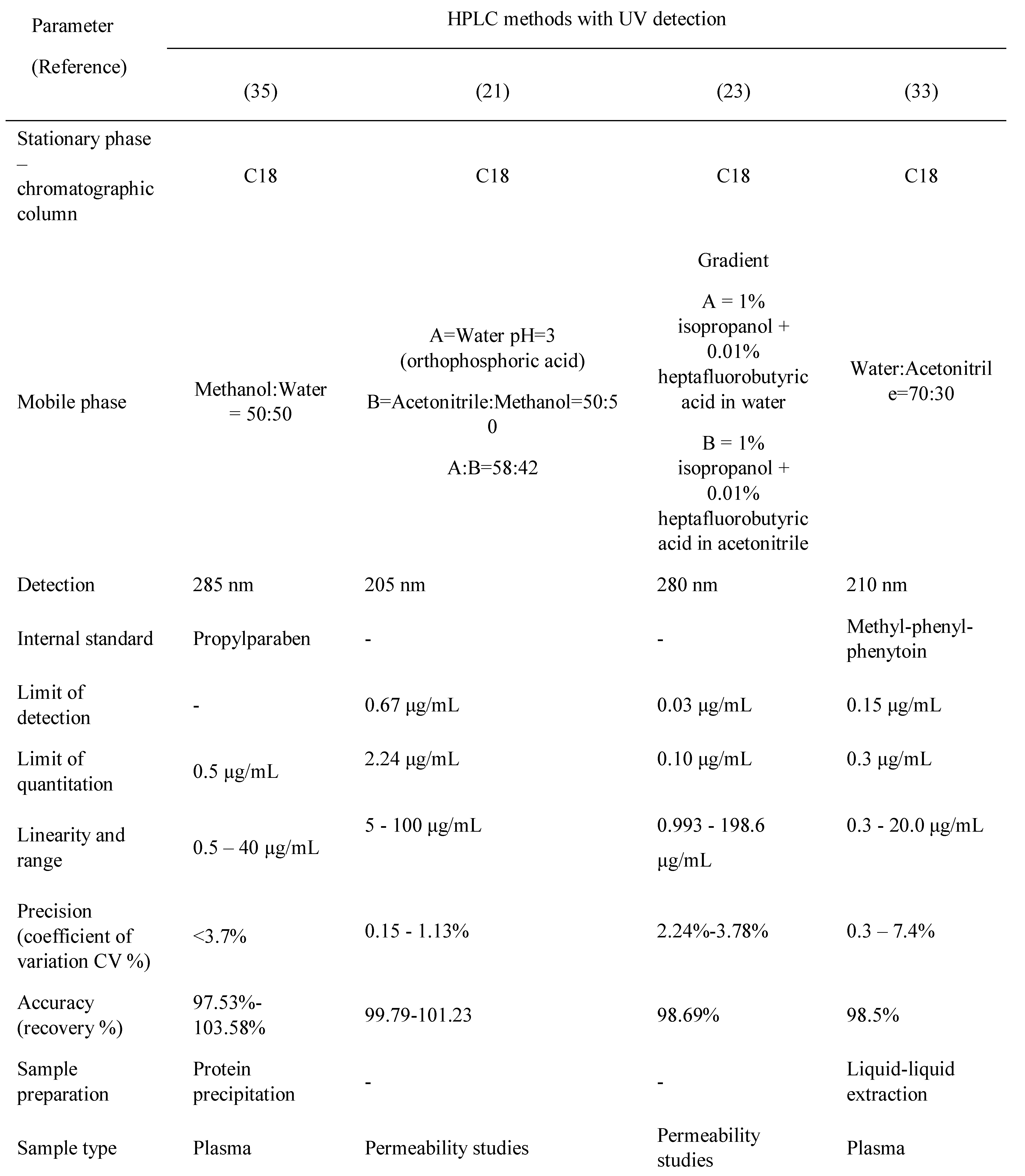
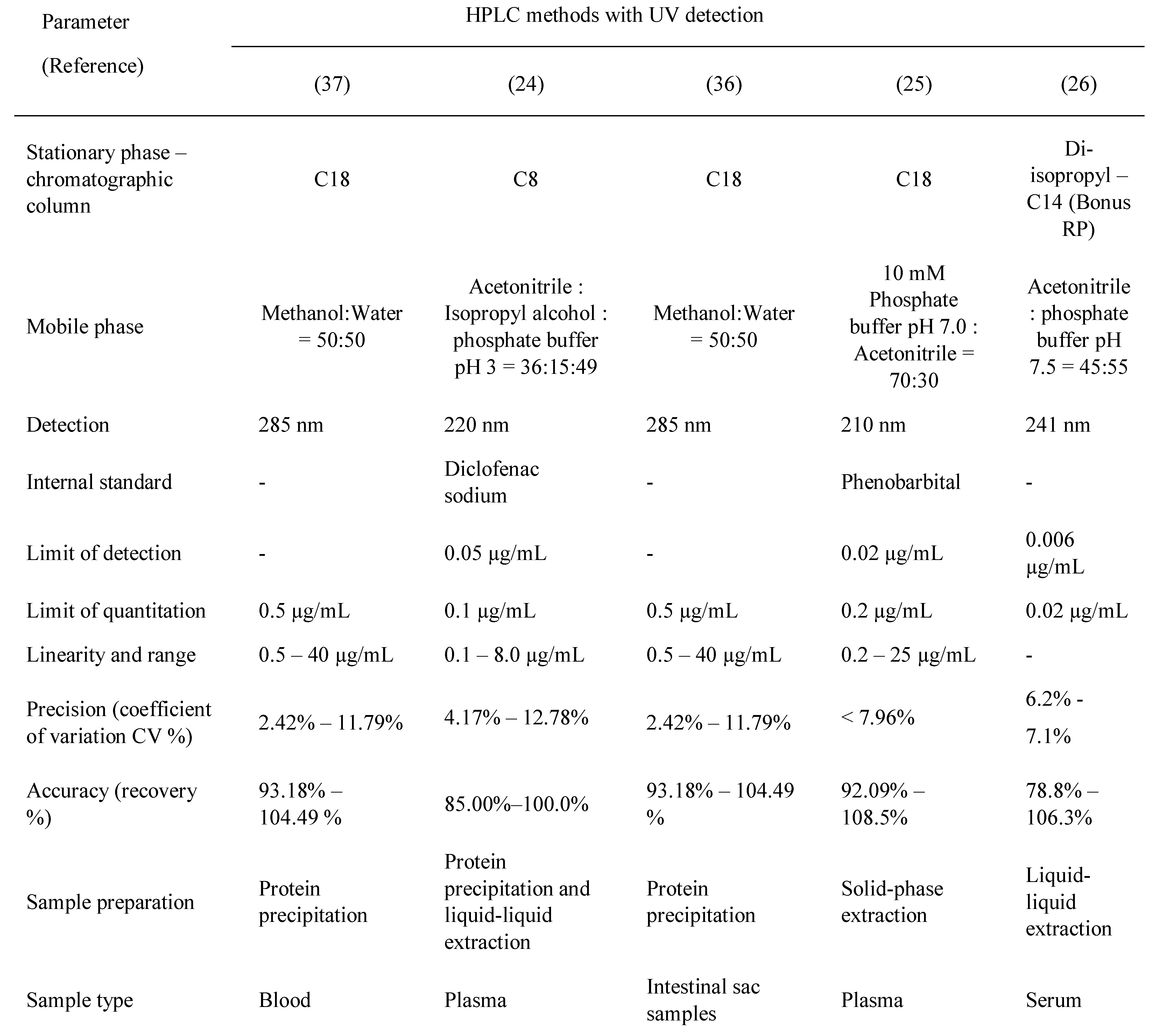
The methods reported in the literature are mostly RP-HPLC (reversed-phase high performance liquid chromatography). The chromatographic columns, stationary phases, are C18 (octadecylsilane) and the mobile phases used are usually simple, polar, binary mixtures between water and an organic solvent (methanol or acetonitrile). Acidic modifiers are added in some methods: formic acid [19], phosphoric acid [20,21], acetic acid [22], or heptafluorobutyric acid [23]. The elution system is isocratic, with one method reported to have gradient elution [23]. Phosphate buffers of different pH are used as an aqueous mobile phase in some methods [24,25,26,27,28].
Ternary mixtures are also widely used as a mobile phase[29,30].One method reported the use of a diisopropyl-C14 column which is suitable for analyzing very basic and polar compounds [26]. Gas- Chromatography methods are not suitable for carbamazepine determination due to its susceptibility to thermal degradation.
Detection is achieved by the use of spectrophotometric methods (UV) with various wavelengths, between 210 nm and 285 nm. MS-MS detection is also used in two of the methods [19,22]. UV detection is sufficient as the therapeutic ranges of carbamazepine and its metabolite are well within the sensitivity limits of UV detection. However, if a very specific method is required, MS detection can be employed.
Regardless of the detection method used, sample preparation is a very important step. Sample cleanup is essential to lower the noise obtained in the chromatogram and to reduce potential damage done to various parts of the equipment. Methods used for sample preparation vary from liquid-liquid extraction [31,32,33], solid phase extraction [34], and protein precipitation [35,36,37]. All methods presented use plasma or other biological samples, although one method is used for carbamazepine determination in pharmaceutical formulations. This method however could be applied to biological fluids [38].
Some methods have used internal standards for the evaluation of carbamazepine assay in the biological fluid, which is usually plasma. Some methods were used for cell permeability studies.Another chromatographic method reported for the assay of carbamazepine is HPTLC (high performance thin-layer chromatography), used for quantitation in formulation and for in-vitro dissolution studies [39].
UV spectrophotometric methods have been reported for carbamazepine in pharmaceutical formulations [40,41]. These methods are not applicable to biological samples because they fall short with regards to specificity: interference from sample matrix or co-medication is highly possible and differentiation between carbamazepine and metabolites is not possible.
Immunoassays have also been reported for determination in plasma [31]. In one case immunoassays were used to generate mathematical equations for determination of carbamazepine and its metabolite in plasma [19].
Table 2, Table 3, Table 4 and Table 5 present a summary of the chromatographic methods presented in this article. The parts left blank (with a dash) indicate that the validation parameter was either not performed or not reported.

Table 2.
Carbamazepine and metabolite determination by HPLC (I).

Table 3.
Carbamazepine and metabolite determination by HPLC (II).

Table 4.
Carbamazepine and metabolite determination by HPLC (III).

Table 5.
Carbamazepine and metabolite determination by HPLC (IV).
Also, where results reported in the articles did not match the parameters reported in the present tables, calculations were performed to standardize the values presented, in order to allow an objective comparison of the methods (e.g., if results for precision were presented as standard deviation and the average was also reported, a simple calculation was performed in order to report relative standard deviation in the tables).
Results for accuracy are presented as minimum and maximum values obtained for inter-day and intra-day, where applicable.
Conclusions
The pharmacokinetics of carbamazepine are complex and are influenced by multiple factors such as its limited aqueous solubility and the ability of many anti-seizure drugs (including carbamazepine itself) to increase their conversion to active metabolites by hepatic oxidative enzymes.
The methods described above have adequate sensitivity, capable of detecting carbamazepine and epoxy-metabolite at adequate levels, and fulfill all validation acceptance criteria. These methods should have applications not only in pharmacokinetic, bioequivalence and permeability studies, but also in the therapeutic drug monitoring.
References
- Kang, J.Q. Defects at the crossroads of GABAergic signaling in generalized genetic epilepsies. Epilepsy Res. 2017, 137, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.; Alves, G.; Llerena, A.; Falcão, A. Therapeutic Drug Monitoring of Fluoxetine, Norfluoxetine and Paroxetine: A New Tool Based on Microextraction by Packed Sorbent Coupled to Liquid Chromatography. J Anal Toxicol. 2017, 41, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, M.; Pineschi, R.; Bonanni, P.; Pellegri, A.; Clementi, E. Precipitation of Carbamazepine- Controlled Seizures Due to Low-Dose Risperidone in a Child: A Conspiracy to Unbalance Blood Electrolytes. J Clin Psychopharmacol. 2016, 36, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann-Eden, B.; Marson, A.G.; Noack-Rink, M.; Ramirez, F.; Tofighy, A.; Werhahn, K.J.; Wild, I.; Trinka, E. Comparative effectiveness of levetiracetam, valproate and carbamazepine among elderly patients with newly diagnosed epilepsy: subgroup analysis of the randomized, unblinded KOMET study. BMC Neurol. 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Moagar-Poladian, S.; Folea, V.; Paunica, M. Competitiveness of EU member states in attracting EU funding for research and innovation. Romanian Journal of Economic Forecasting. 2017, 20, 150–167. [Google Scholar]
- Tolbert, D.; Cloyd, J.; Biton, V.; Bekersky, I.; Walzer, M.; Wesche, D.; Drummond, R.; Lee, D. Bioequivalence of oral and intravenous carbamazepine formulations in adult patients with epilepsy. Epilepsia 2015, 56, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Gierbolini, J.; Giarratano, M.; Benbadis, S.R. Carbamazepine-related antiepileptic drugs for the treatment of epilepsy - a comparative review. Expert Opin Pharmacother. 2016, 17, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Sitges, M.; Chiu, L.M.; Reed, R.C. Effects of Levetiracetam, Carbamazepine, Phenytoin, Valproate, Lamotrigine, Oxcarbazepine, Topiramate, Vinpocetine and Sertraline on Presynaptic Hippocampal Na(+) and Ca(2+) Channels Permeability. Neurochem Res. 2016, 41, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, M.; Paksu, M.S.; Guzel, A.; Alacam, A.; Alacam, F. Severe Carbamazepine Intoxication in Children: Analysis of a 40-Case Series. Med Sci Monit 2016, 22, 4729–4735. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Birnbaum, A.; Leppik, I.; Conway, J.M.; Musib, L.C.; Brundage, R.C.; Ramsay, R.E.; Pennell, P.B.; White, J.R.; Gross, C.R.; Rarick, J.O.; Mishra, U.; Cloyd, J.C. Steady-state Carbamazepine Pharmacokinetics following Oral and Stable-Labeled Intravenous Administration in Epilepsy Patients: Effect of Race and Sex. Clin Pharmacol Ther. 2012, 91, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Hiemke, C.; Ulrich, S.; Gaertner, I.; Rao, M.; Eckermann, G.; Zernig, G. Therapeutic Monitoring of Psychotropic Drugs - An Outline of the AGNP- TDM Expert Group Consensus Guideline. Ther Drug Monit. 2004, 26, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Bârcă, M.; Ilie, M.; Baconi, D.L.; Ciobanu, A.M.; Bălălău, D.; Burcea, G.T. Spectrofluorimetric methotrexate assay in human plasma. Farmacia 2010, 58, 95–10. [Google Scholar]
- Bălălău, C.; Voiculescu, Ș.; Motofei, I.; Scăunașu, R.V.; Negrei, C. Low Dose Tamoxifen as Treatment of Benign Breast Proliferative Lesions. Farmacia 2015, 63, 371–375. [Google Scholar]
- Kisanga, E.R.; Gjerde, J.; Guerrieri-Gonzaga, A.; Pigatto, F.; Pesci-Feltri, A.; Robertson, C.; Serrano, D.; Pelosi, G.; Decensi, A.; Lien, E.A. Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res. 2004, 10, 2336–2343. [Google Scholar] [CrossRef] [PubMed]
- Vasile, R.D.; Baconi, D.; Hudiţă, C.; Bârcă, M.; Bălălău, C.; Ciobanu, A.M. Methadone plasma levels in heroin addict patients during substitution therapy. Farmacia; 2014, 62, 1202–1212. [Google Scholar]
- Baconi, D.L.; Stan, M.; Ebrahim, Z.A.J.; Tuchila, C.; Balalau, C. Determination of Tramadol in human plasma by HPLC with fluorescence detection. Journal of Mind and Medical Sciences 2016, 3, 55–64. [Google Scholar] [CrossRef]
- Vlăsceanu, A.M.; Petraru, C.; Baconi, D.; Ghica, M.; Arsene, A.; Popa, L.; Nicolae, A.; Drăgoi, C.; Pavalache, G. Quantitative relationships of urinary cotinine levels in smoking diabetic patients. Farmacia 2015, 63, 349–356. [Google Scholar]
- FDA Guidance for Industry - Bioanalytical Method Validation, 2013, Retrieved from FDA Guidances: http://www.fda.gov/Drugs/GuidanceComplianceReg ulatoryInformation/Guidances/default.htm.
- McMillin, G.A.; Juenke, J.M.; Tso, G.; Dasgupta, A. Estimation of Carbamazepine and Carbamazepine- 10,11-Epoxide Concentrations in Plasma Using Mathematical Equations Generated With Two Carbamazepine Immunoassays. Am J Clin Pathol. 2010, 133, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Arayne, M.S.; Ali, S.N. An Ultra-Sensitive LC Method for the Simultaneous Determination of Paracetamol, Carbamazepine, Losartan and Ciprofloxacin in Bulk Drug, Pharmaceutical Formulation and Human Serum by Programming the Detector. Am J Anal Chem, 2013; 4, 24–33. [Google Scholar]
- Patil, S.; Kumar, L.; Kohli, G.; Bansal, A. Validated HPLC Method for Concurrent Determination of Antipyrine, Carbamazepine, Furosemide and Phenytoin and its Application in Assessment of Drug Permeability through Caco-2 Cell Monolayers. Sci Pharm. 2012, 80, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Abd Al Haleem, E.N.; Khaleel, S.A.; Sallam, A.S. Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacol Rep. 2017, 69, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Qin, X.; Teraoka, I.; Gross, R.A. Comparison of retention behavior of oligolysine and oligoarginine in ion-pairing chromatography using heptafluorobutyric acid. Anal Bioanal Chem. 2013, 405, 9739–9746. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, T.; Shi, M.; Zhang, Y.; Zhao, X.; Yang, Y.; Gu, J. Simultaneous determination of ten antiepileptic drugs in human plasma by liquid chromatography and tandem mass spectrometry with positive/negative ion-switching electrospray ionization and its application in therapeutic drug monitoring. J Sep Sci. 2016, 39, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Dzodic, P.; Zivanovic, L.; Protic, A.; Ivanovic, I.; Velickovic-Radovanovic, M.; Spasic, M.; Zivanovic, S. Development and validation of a solid phase extraction-HPLC method for the determination of carbamazepine and its metabolites, carbamazepine epoxide and carbamazepine trans-diol, in plasma. J Serb Chem Soc. 2012, 77, 1–21. [Google Scholar] [CrossRef]
- Powell, G.; Saunders, M.; Marson, A.G. Immediate- release versus controlled-release carbamazepine in the treatment of epilepsy. Cochrane Database Syst Rev 2010, 1, CD007124. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, H.; Abroomand-Azar, P.; Saber-Tehrani, M.; Husain, S. Rapid Determination of Carbamazepine in Human Urine, Plasma Samples and Water Using DLLME followed by RP-LC. Chromatographia 2010, 71, 517–521. [Google Scholar] [CrossRef]
- Borowicz-Reutt, K.K.; Banach, M.; Piskorska, B. Mexiletine and its Interactions with Classical Antiepileptic Drugs: An Isobolographic Analysis. Neurochem Res. 2016, 41, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.K.; Ban, E.; Woo, J.S.; Kim, C.K. Analysis of carbamazepine and its active metabolite, carbamazepine-10,11-epoxide, in human plasma using high-performance liquid chromatography. Anal Bioanal Chem. 2006, 386, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Rodrigues, M.; Falcao, A.; Alves, G. HPLC–DAD Method for the Quantification of Carbamazepine, Oxcarbazepine and their Active Metabolites in HepaRG Cell Culture Samples. Chromatographia 2016, 79, 581–590. [Google Scholar] [CrossRef]
- Mihaly, W.G.; Phillips, J.A.; Louis, W.J.; Vajda, F.J. Measurement of Carbamazepine and Its Epoxide Metabolite by High-Performance Liquid Chromatography, and a Comparison of Assay Techniques for the Analysis of Carbamazepine. Clin Chem. 1977, 23, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, A.C.; Arsene, A.L.; Vuță, V.; Popa, D.E.; Sîrbu, C.A.; Burcea Dragomiroiu, G.T.A.; Dumitrescu, I.B.; Velescu, B.Ș.; Gofiță, E.; Drăgoi, C.M. In vitro P-GP expression after administration of CNS active drugs. Farmacia. 2016, 64, 844–850. [Google Scholar]
- Sanches, C.; Lopez, K.; Omosako, C.; Bertoline, M.; Pereira, M.; Santos, S. Micromethod for Quantification of Carbamazepine, Phenobartital and Phenytoin in Human Plasma by HPLC-UV Detection for Therapeutic Drug Monitoring Application. Latin American Journal of Pharmacy. 2008, 27, 485–491. [Google Scholar]
- Zhu, Y.; Chiang, H.; Wulster-Radcliffe, M.; Hilt, R.; Wong, P.; Kissinger, C.B.; Kissinger, P.T. Liquid chromatography/tandem mass spectrometry for the determination of carbamazepine and its main metabolite in rat plasma utilizing an automated blood sampling system. J Pharm Biomed Anal. 2005, 38, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Mowafy, H.A.; Alanazi, F.K.; El Maghraby, G.M. Development and validation of an HPLC–UV method for the quantification of carbamazepine in rabbit plasma. Saudi Pharm J. 2012, 20, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Devandla, A.; Yamasani, M. Development and Validation of and UPLC Method for the Quantification of Carbamazepine in Intestinal Sac Samples. International Journal of Pharmacy and Biological Science. 2015, 5, 145–152. [Google Scholar]
- Guerrero Garduño, Ó.; González-Esquivel, D.F.; Escalante-Membrillo, C.; Fernández, Á.; Rojas-Tomé, I.S.; Jung Cook, H.; Castro, N. Comparison of a high- performance liquid chromatography method for quantification of carbamazepine with chemiluminescent microparticle immunoassay. Biomed Chromatogr. 2016, 30, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Dzodić, P.L.j.; Zivanović, L.J.; Protić, A.D.; Zećević, M.L.; Jocić, B.M. Determination of carbamazepine and its impurities iminostilbene and iminodibenzyl in solid dosage form by column high-performance liquid chromatography. J AOAC Int. 2010, 93, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Borhade, V.B.; Nair, H.A.; Hegde, D.D.; Barhate, C.R. Development and validation of HPTLC method for estimation of tacrolimus in formulations. Drug Dev Ind Pharm. 2009, 35, 440–448. [Google Scholar] [CrossRef]
- Zadbuke, N.; Shahi, S.; Jadhav, A.; Borde, S. Development and Validation of UV-Visible Spectrophotometric Method for Estimation of Carbamazepine in Bulk and Tablet Dosage Form. Int J Pharmacy and Pharmaceutical Sci. 2016, 8, 234–238. [Google Scholar]
- Tambe, H.; Mulgund, S. UV Spectrophotometric Estimation of Carbamazepine in Bulk and Tablet Dosage Form Using Area Under Curve Method. World Journal of Pharmacy and Pharmaceutical Sciences. 2015, 4, 1200–1206. [Google Scholar]
© 2017 by the author. 2017 Cristian Tuchila, Daniela L. Baconi, Cristina D. Pirvu, Denisa O. Balalau, Ana M. Vlasceanu, Miriana Stan, Cristian Balalau