Metabolic Alterations in Experimental Models of Depression
Abstract
:Introduction
Materials and Methods
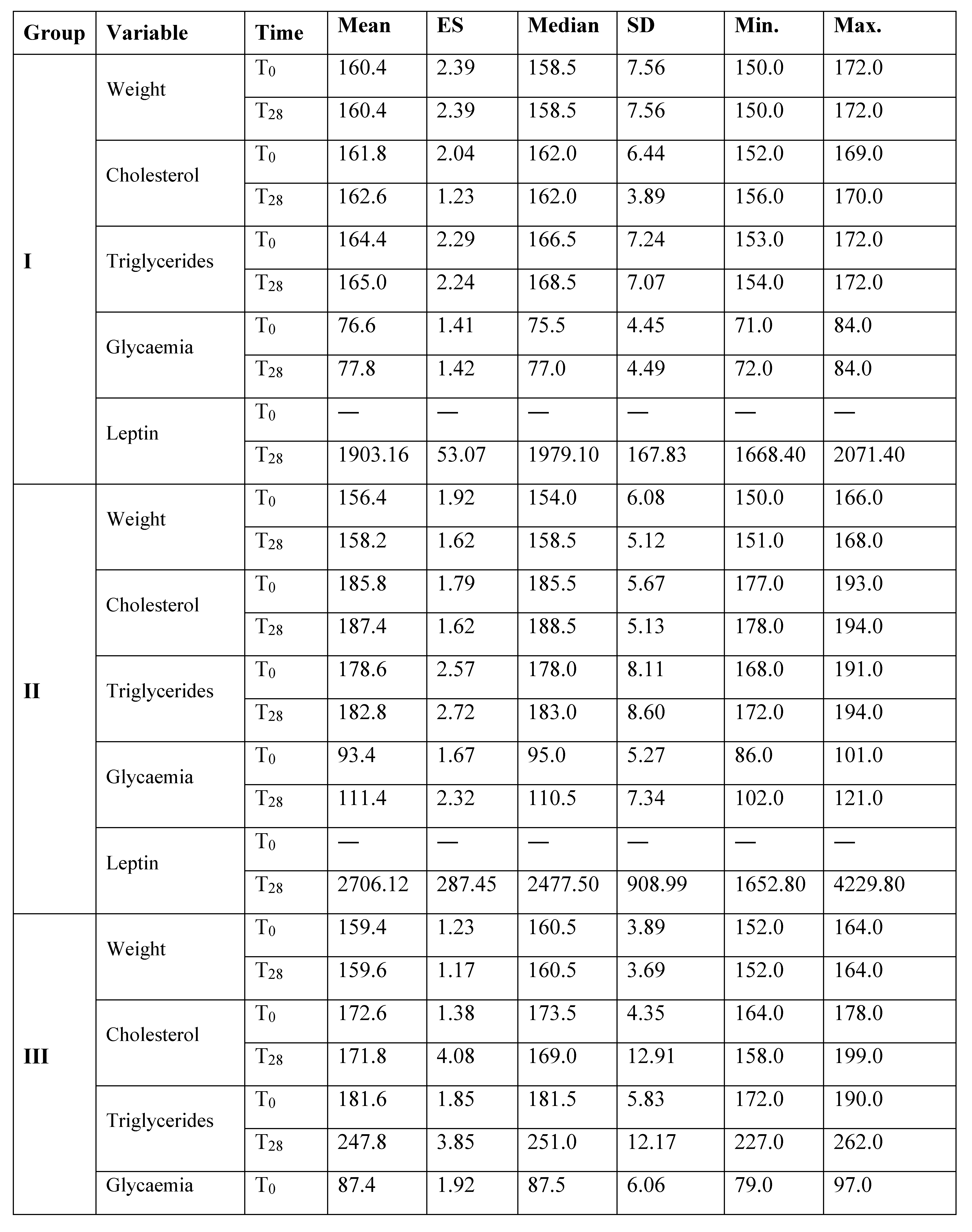
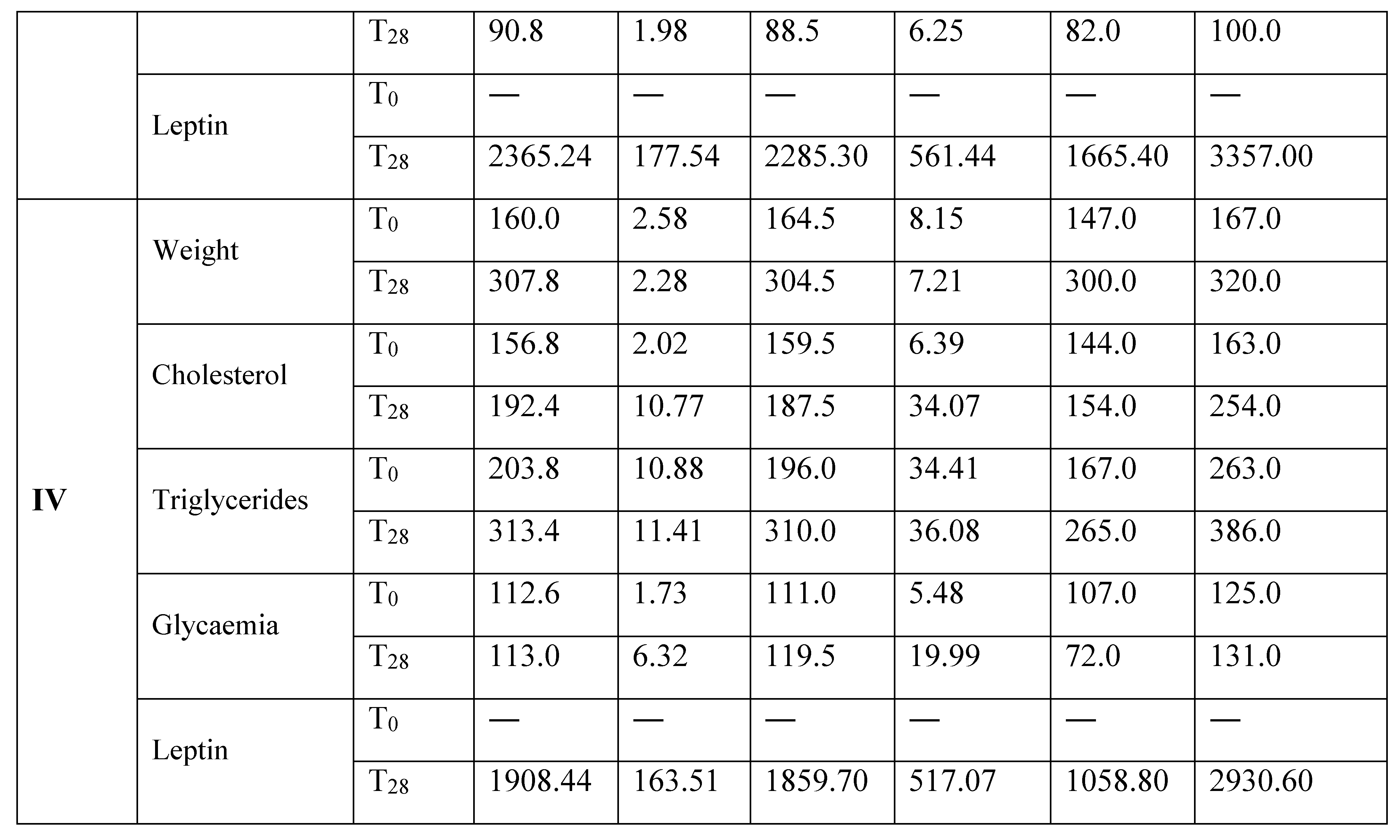
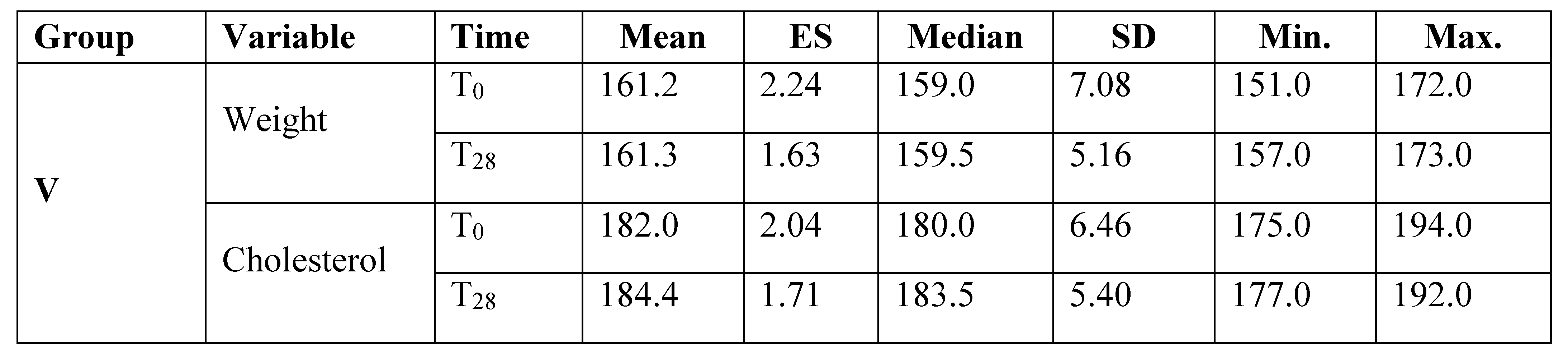
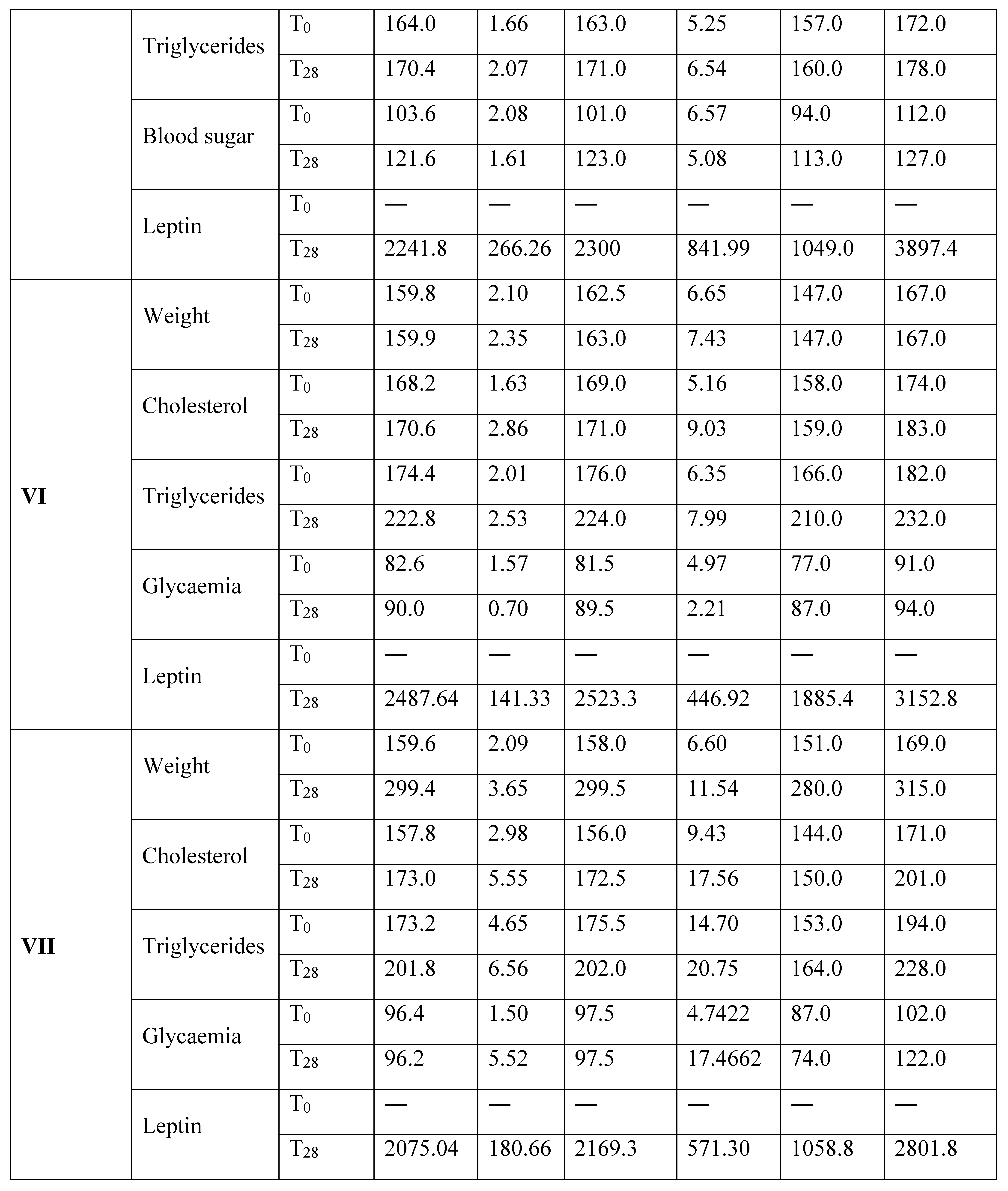
Results
Discussion
 |
Conclusions
References
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; de Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H.; Gorwood, P. Successful Management of Major Depressive Disorder; Evolving Medicine Ltd., Unitec House, 2 Albert Place: London N3 1QB, UK, April 2013. [Google Scholar] [CrossRef]
- Thase, M.E. Using biomarkers to predict treatment response in major depressive disorder: evidence from past and present studies. Dialogues Clin Neurosci. 2014, 16, 539–544. [Google Scholar] [CrossRef]
- Lloyd, C.E.; Hermanns, N.; Nouwen, A.; Pouwer, F.; Underwood, L.; Winkley, K. Katon, W., Maj, M., Sartorius, N., Eds.; The Epidemiology of Depression and Diabetes. In Depression and Diabetes; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 1–27. [Google Scholar]
- Foley, D.L.; Morley, K.I.; Madden, P.A.; Heath, A.C.; Whitfield, J.B.; Martin, N.G. Major Depression and the metabolic syndrome. Twin Res. Hum. Genet. 2010, 13, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Bogers, R.P.; Bemelmans, W.E.; Hoogenveen, R.T.; Boshuizen, H.C.; Woodward, M.; Knekt, P.; van Dam, R.M.; Hu, F.B.; Visscher, T.L.; Menotti, A.; et al. BMI-CHD Collaboration Investigators. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300,000 persons. Arch Intern Med. 2007, 167, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin El Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Carney, R.M.; Freedland, K.E.; Miller, G.E.; Jaffe, A.S. Depression is a risk factor for cardiac mortality and morbidity: a review of a potential mechanism. J Psychosom Res. 2002, 53, 897–902. [Google Scholar] [CrossRef]
- Gehi, A.; Hass, D.; Pipkin, S.; Whooley, M.A. Depression and medication adherence in outpatients with coronary heart dissease: findings from heart and Soul Study. Arch Intern Med. 2005, 165, 2508–2513. [Google Scholar] [CrossRef]
- DiMatteo, M.R.; Lepper, H.S.; Croghan, T.W. Depression is a risk factor for noncompliance with medical treatment: meta-analisys of the effect of anxiety and depression on patient adherence. Arch Inter Med. 2000, 160, 2101–2107. [Google Scholar] [CrossRef]
- Nechita, F.; Pirlog, M.C.; Chirita, A. Circadian malfunctions in depression – neurobiological and psychosocial approaches. Rom J Morphol Embryol 2015, 56, 949–955. [Google Scholar]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Mallon, L.; Broman, J.E.; Hetta, J. High incidence of diabetes in men with sleep complains or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care 2005, 28, 2762–2767. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol. 2007, 28, 61–71. [Google Scholar] [CrossRef]
- Pulkki-Raback, L.; Elovainio, M.; Kivimaki, M.; Mattsson, N.; Raitakari, O.T.; Puttonen, S.; Marniemi, J.; Viikari, J.S.; Keltikangas-Jarvinen, L. Depressive symptoms and the metabolic syndrome in childhood and adulthood: a prospective cohort study. Health Psychol. 2009, 28, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kinder, L.S.; Carnethon, M.R.; Palaniappan, L.; King, A.C.; Fortmann, S.P. Depression and the metabolic syndrome in young adults: findings from the Third National Health and Nutrition Examination Survey. Psychosom Med. 2004, 66, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; McClure, C.; Johnson, B.D.; Sheps, D.S.; Bittner, V.; Rutledge, T.; Shaw, L.J.; Sopko, G.; Olson, M.B.; Krantz, D.S.; et al. Depression, the metabolic syndrome and cardiovascular risk. Psychosom Med. 2008, 70, 40–48. [Google Scholar] [CrossRef]
- Agil, A.; Navarro-Alarcon, M.; Ruiz, R.; Abuhamadah, S.; El Mir, M.Y.; Vasquez, G.F. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res. 2011, 50, 207–212. [Google Scholar] [CrossRef]
- Bartness, T.J.; Demas, G.E.; Song, C.K. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med. 2002, 227, 363–376. [Google Scholar] [CrossRef]
- Yanagihara, H.; Ando, H.; Hayashi, Y.; Obi, Y.; Fujimura, A. High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol Int. 2006, 23, 905–914. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Cano, P.; Jimenez-Ortega, V.; Esquifino, A.I. Melatonin and metabolic syndrome. Physiopathologic and therapeutical implications. Neuroendocrinology 2011, 93, 133–142. [Google Scholar] [CrossRef]
- Kraus, T.; Haack, M.; Schuld, A.; Hinze-Selch, D.; Pollmacher, T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology 2001, 73, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, M.; Kuloglu, M.; Tezcan, E.; Ustundag, B.; Gecici, O.; Firidin, B. Serum leptin and cholesterol values in suicide attempters. Neuropsychobiology 2002, 45, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.T.; Rhodes, M.E.; Czambel, R.K. Sexual diergism of baseline plasma leptin and leptin suppression by arginine vasopressin in major depressives and matched controls. Psychiatry Res. 2002, 113, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Esel, E.; Ozsoy, S.; Tutus, A.; Sofuoglu, S.; Kartalci, S.; Bayram, F.; Kokbudak, Z.; Kula, M. Effects of antidepressant treatment and of gender on serum leptin levels in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 2005, 29, 565–570. [Google Scholar] [CrossRef]
- Zeman, M.; Jirak, R.; Jachymova, M.; Vecka, M.; Tvrzicka, E.; Zak, A. Leptin, adiponectin, leptin to adiponectin ratio and insulin resistance in depressive women. Neuro Endocrinol. Lett. 2009, 30, 387–395. [Google Scholar]
- Lu, X.Y.; Kim, C.S.; Frazer, A.; Zhang, W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA 2006, 103, 1593–1598. [Google Scholar] [CrossRef]
- Fischbach, F.; Dunning, M.B. A manual of laboratory and diagnostic tests. ISBN/ISSN, 2015; 379–382, 624–644ISBN 9781451190892. ISSN 9781451190892. [Google Scholar]
  |
 |
  |
© 2016 by the author. 2016. Maria-Gabriela Puiu, Mihnea C. Manea, George L. Paraschiv, Traian Purnichi, Ecaterina Ionescu, Simona Tache, Ioana Paunica, Mirela Manea
Share and Cite
Puiu, M.-G.; Manea, M.C.; Paraschiv, G.L.; Purnichi, T.; Ionescu, E.; Tache, S.; Paunica, I.; Manea, M. Metabolic Alterations in Experimental Models of Depression. J. Mind Med. Sci. 2016, 3, 172-181. https://doi.org/10.22543/2392-7674.1042
Puiu M-G, Manea MC, Paraschiv GL, Purnichi T, Ionescu E, Tache S, Paunica I, Manea M. Metabolic Alterations in Experimental Models of Depression. Journal of Mind and Medical Sciences. 2016; 3(2):172-181. https://doi.org/10.22543/2392-7674.1042
Chicago/Turabian StylePuiu, Maria-Gabriela, Mihnea C. Manea, George L. Paraschiv, Traian Purnichi, Ecaterina Ionescu, Simona Tache, Ioana Paunica, and Mirela Manea. 2016. "Metabolic Alterations in Experimental Models of Depression" Journal of Mind and Medical Sciences 3, no. 2: 172-181. https://doi.org/10.22543/2392-7674.1042
APA StylePuiu, M.-G., Manea, M. C., Paraschiv, G. L., Purnichi, T., Ionescu, E., Tache, S., Paunica, I., & Manea, M. (2016). Metabolic Alterations in Experimental Models of Depression. Journal of Mind and Medical Sciences, 3(2), 172-181. https://doi.org/10.22543/2392-7674.1042



