Determination of Tramadol in Human Plasma by HPLC with Fluorescence Detection
Abstract
Introduction
Materials and method
Chemicals and apparatus:
- Tramadol hydrochloride (Sigma Aldrich) Reagents:
- Methanol Chromosolv for HPLC (Merck)
- Acetonitrile Chromosolv for HPLC (Merck)
- Formic acid (Merck)
- Water for chromatogtaphic use (obtained with Barnstead®EasypureRoDi system)
Materials
- Solid phase extraction cartridges, DSC 18 (Supelco)
- Chromatographic column: Kromasil®, 150 mm x 4.6 mm, 5 μm; C18stationary phase
Apparatus:
Biological material
- Human plasma obtained from peripheral venous blood provided by a Hematology Institute
Methodology
Chromatographic conditions
- Mobile phase: 0.1% formic acid: acetonitrile (20:80)
- Flow rate: 1 mL/min
- Column temperature: 22°C
- Sample temperature: 20°C
- Fluorescence detector: λ ex= 280 nm and λem= 310 nm
- Elution: isocratic
- Injection volume: 20 µL
- Analysis time : 6.5 minutes
Preparation of solutions
Control samples
Tramadol extraction procedure from plasma
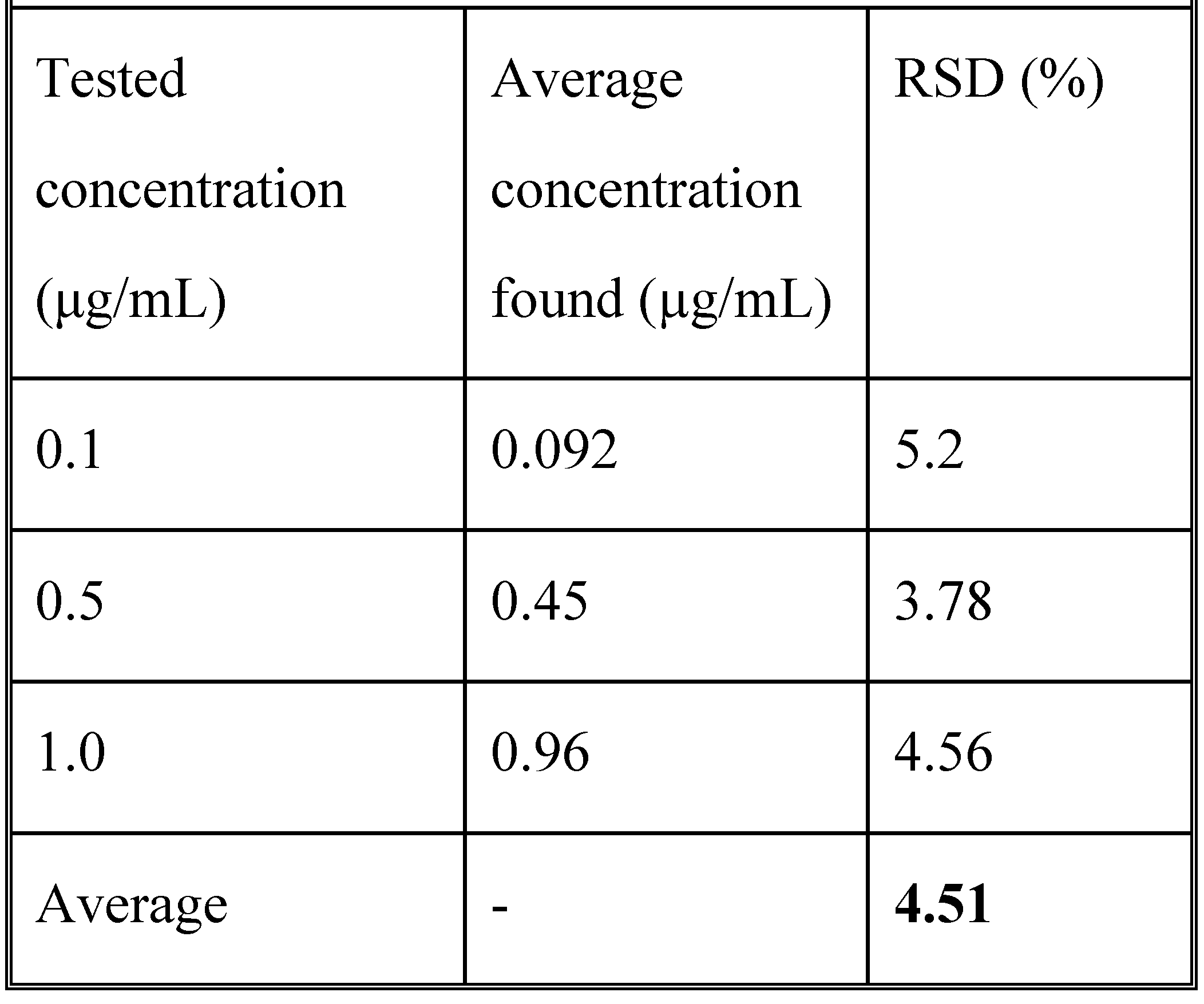
Analytical method validation
Results
Development and validation of the method
Discussions
Tramadol plasma dosing method in patients treated with tramadol

Conclusions
- ✓ Considering the native fluorescence of tramadol, and based on the literature data, we developed and validated a new HPLC method with fluorescence detection for the determination of tramadol in human plasma.
- ✓ The method uses simple experimental conditions: Kromasil column (C18 bound phase), mobile phase acetonitrile: 0.1% formic acid, the fluorescence detection, λex/em = 280/310 nm for 6.5 minutes analysis time, applying SPE procedure from plasma on C18 cartridge.
- ✓ The developed method is selective, is linear on concentration range of 100 ng - 1 μg/ mL, corresponding to both therapeutic and the toxic/lethal tramadol levels, and has the detection/quantification limitsof 0.010 μg/mL and 0. 100μg/mL respectively. In addition, the useof the fluorescence detector contributes, in itself, to increasing the selectivity of the method.
- ✓ The applicability of HPLC-FL method was verified for determination of tramadol in plasma in patients treated with tramadol. Thus,the utility of the method for quantification of therapeutic levels of tramadol has been demonstrated.
References
- Bahrami, G.; Mohammadi, B. Enhancement of Fluorescence Intensity of Tramadol and Its Main Metabolites in LC Using Pre- Column Derivatization with 9-Fluorenylmethyl Chloroformate. Chromatographia 2008, 68, 935–940. [Google Scholar] [CrossRef]
- Ceccato, A.; Vanderbist, F.; Pabst, J.Y.; Streel, B. Enantiomeric determination of tramadol and its main metabolite O- desmethyltramadol in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000, 748, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; Ho, P.C. Enantiomeric separation of tramadol hydrochloride and its metabolites by cyclodextrin-mediated capillary zone electrophoresis. J Chromatogr B Biomed Sci Appl. 1998, 707, 287–94. [Google Scholar] [CrossRef] [PubMed]
- Curticapean, A. ; MunteanD; CurticapeanM; DogaruM; VariC Optimized HPLC method for tramadol and O-desmethyl tramadol determination in human plasma. J. Biochem. Biophys. Methods 2008, 70, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, Z.A.J.; Bălălău, D.; Baconi, D.L.; Guţu, C.M.; Ilie, M. HPTLC method for the assay of tramadol and pentazocine from mixtures. Farmacia 2011, 59, 381–387. [Google Scholar]
- Ebrahimzadeh, H.; Yamini, Y.; Sedighi, A.; Rouini, M.R. Determination of tramadol in human plasma and urine samples using liquid phase microextraction with back extraction combined with high performance liquid chromatography. J Chromatogr B AnalytTechnol Biomed Life Sci. 2008, 863, 229–34. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Fawcett, J.P. Improved HPLC method for the simultaneous determination of tramadol and O-desmethyltramadol in human plasma. Journal of Chromatography B. 2005, 821, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Moffat, A.C.; Osselton, M.D.; Widdop, B. Clarke’s Analysis of Drugs and Poisons in pharmaceuticals, body fluids and postmortem material, 4th ed.Pharmaceutical Press, 2011. [Google Scholar]
- Nobilis, M.; Kopecky, J; Kvetina, J; Svoboda, Z. High-performance liquid chromatographic determination of tramadol and its O-desmethylated metabolite in blood plasma. Application to a bioequivalence study in humans. Journal of Chromatography A. 2002, 949, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Nobilis, M.; Pastera, J.; Anzenbacher, P.; Svoboda, D.; Kopecký, J.; Perlík, F. High- performance liquid chromatographic determination of tramadol in human plasma. J Chromatogr B Biomed Appl. 1996, 681, 177–83. [Google Scholar] [CrossRef] [PubMed]
- Pospísilová, M.; Polásek, M.; Jokl, V. Determination of tramadol in variousdosageformsbycapillary isotachophoresis. J PharmBiomed Anal. 1998, 18, 777–83. [Google Scholar]
- Solarino, B.; Rießelmann, B.; Buschmann, C.T.; Tsokos, M. Multidrug poisoning involving nicotine and tramadol. Forensic Science International 2010, 194, e17–19. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.; Garrido, M.J.; Pavon, J.M.; Calvo, R.; Troconiz, I.F. Pharmacokinetic- pharmacodynamic modeling of the antinociceptive effects of main active metabolites of tramadol, (+)-O- desmethyltramadol and (-)-O-desmethyltramadol, in rats. J. Pharmacol. Exp. Ther. 2000, 293, 646–653. [Google Scholar] [CrossRef] [PubMed]
- *** - FDA Guidance for Industry - Bioanalytical Method Validation, DRAFT guidance. 2013. Available online: www.fda.gov.



© 2016 by the authors. 2016 Daniela Baconi, Miriana Stan, Zainab Abdul Jalil Ebrahim, Cristian Tuchila, Cristian Balalau. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baconi, D.; Stan, M.; Abdul Jalil Ebrahim, Z.; Tuchila, C.; Balalau, C. Determination of Tramadol in Human Plasma by HPLC with Fluorescence Detection. J. Mind Med. Sci. 2016, 3, 55-64. https://doi.org/10.22543/2392-7674.1033
Baconi D, Stan M, Abdul Jalil Ebrahim Z, Tuchila C, Balalau C. Determination of Tramadol in Human Plasma by HPLC with Fluorescence Detection. Journal of Mind and Medical Sciences. 2016; 3(1):55-64. https://doi.org/10.22543/2392-7674.1033
Chicago/Turabian StyleBaconi, Daniela, Miriana Stan, Zainab Abdul Jalil Ebrahim, Cristian Tuchila, and Cristian Balalau. 2016. "Determination of Tramadol in Human Plasma by HPLC with Fluorescence Detection" Journal of Mind and Medical Sciences 3, no. 1: 55-64. https://doi.org/10.22543/2392-7674.1033
APA StyleBaconi, D., Stan, M., Abdul Jalil Ebrahim, Z., Tuchila, C., & Balalau, C. (2016). Determination of Tramadol in Human Plasma by HPLC with Fluorescence Detection. Journal of Mind and Medical Sciences, 3(1), 55-64. https://doi.org/10.22543/2392-7674.1033


