Pain in Photodynamic Therapy
Abstract
:Introduction
Discussion
- Mechanism of action
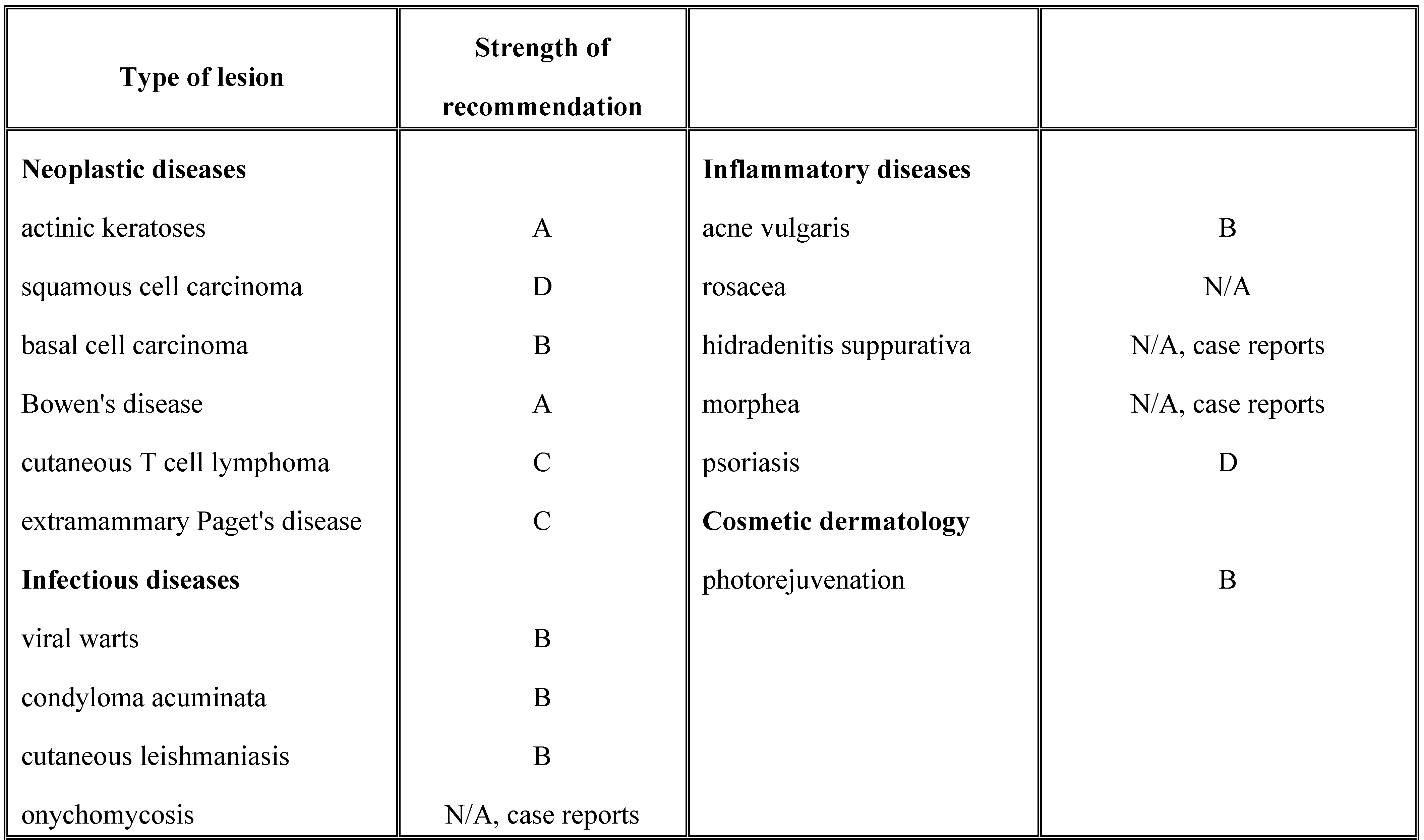
- Applications of photodynamic therapy in dermatology. PDT has several applications in dermatology, including neoplastic diseases, inflammatory diseases, microbial diseases, photoaging and rejuvenation (Table 1). While there is clear evidence on the effectiveness of PDT for actinic keratosis, BCC, especially the superficial type and Bowen's disease, the data regarding the use of PDT for other dermatological disorders is still scarce. However, studies show promising results [11,12,13,14,15]. Contraindications to PDT are porphyria, systemic lupus erythematosus, non- responsive tumors, allergy to the photosensitizing agent and photosensitive dermatoses [13].
 |
Adverse reactions
- Pain in photodynamic therapy
- Factors contributing to pain in PDT for dermatological disorders
- Pain management
Topical anesthetics
Locally injected anesthetics
Conscious sedation
Other methods
Conclusion
Acknowledgement
References
- Yokoyama, Y.; Shigeto, T.; Miura, R.; Kobayashi, A.; Mizunuma, M.; Yamauchi, A.; Futagami, M.; Mizunuma, H. A Strategy Using Photodynamic Therapy and Clofibric Acid to Treat Peritoneal Dissemination of Ovarian Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 775–779. [Google Scholar]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [PubMed]
- Pervaiz, S.; Olivo, M. Art and science of photodynamic therapy. Clin. Exp. Pharmacol. Physiol. 2006, 33, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Wang, Y.; Li, H.; Jin, Q.; Ji, J. Dual pH-responsive 5-aminolevulinic acid pseudopolyrotaxane prodrug micelles for enhanced photodynamic therapy. Chem. Commun. 2016, 52, 3966–3969. [Google Scholar]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar]
- Katz, S.I.; Gilchrest, B.A.; Paller, A.S.; Leffell, D.J. Fitzpatrick’s Dermatology in General Medicine, 8th ed.; McGrawHill, 2012; ISBN 978-0071669047. [Google Scholar]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar]
- Bacellar, I.O.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef]
- Matei, C.; Tampa, M.; Poteca, T.; Panea-Paunica, G.; Georgescu, S.R.; Ion, R.M.; Popescu, S.M.; Giurcaneanu, C. Photodynamic therapy in the treatment of basal cell carcinoma. J. Med. Life 2013, 6, 50–54. [Google Scholar]
- Morton, C.A.; McKenna, K.E.; Rhodes, L.E. Guidelines for topical photodynamic therapy: Update. Br. J. Dermatol. 2008, 159, 1245–1266. [Google Scholar] [CrossRef]
- Kim, M.; Jung, H.Y.; Park, H.J. Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications. Int. J. Mol. Sci. 2015, 16, 23259–23278. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.T.; Lin, J.Y. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145–163. [Google Scholar]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic Therapy for Infections: Clinical Applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [PubMed]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections– state of the art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [PubMed]
- Sandberg, C.; Stenquist, B.; Rosdahl, I.; Ros, A.M.; Synnerstad, I.; Karlsson, M.; Gudmundson, F.; Ericson, M.B.; Larkö, O.; Wennberg, A.M. Important factors for pain during photodynamic therapy for actinic keratosis. Acta Derm.-Venereol. 2006, 86, 404–408. [Google Scholar]
- Halldin, C.B.; Gillstedt, M.; Paoli, J.; Wennberg, A.M.; Gonzalez, H. Predictors of pain associated with photodynamic therapy: A retrospective study of 658 treatments. Acta Derm.-Venereol. 2011, 91, 545–551. [Google Scholar]
- Crichton, N. Visual analogue scale (VAS). J. Clin. Nurs. 2001, 10, 706-6. [Google Scholar]
- Chaves, Y.N.; Torezan, L.A.; Niwa, A.B.; Sanches, J.A.; Festa, N.C. Pain in photodynamic therapy: Mechanism of action and management strategies. An. Bras. De. Dermatol. 2012, 87, 521–529. [Google Scholar]
- Figure one extracted from: http://www.trialdatasolutions.com/tds/howto/v as. jsp, last accessed 31.01.2016.
- Schleyer, V.; Radakovic-Fijan, S.; Karrer, S.; Zwingers, T.; Tanew, A.; Landthaler, M.; Szeimies, R.M. Disappointing results and low tolerability of photodynamic therapy with topical 5-aminolaevulinic acid in psoriasis. A randomized, double-blind phase I/II study. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 823–828. [Google Scholar]
- Warren, C.B.; Karai, L.J.; Vidimos, A.; Maytin, E.V. Pain associated with aminolevulinic acid- photodynamic therapy of skin disease. J. Am. Acad. Dermatol. 2009, 61, 1033–1043. [Google Scholar]
- Gaál, M.; Otrosinka, S.; Baltás, E.; Ócsai, H.; Oláh, J.; Kemény, L.; Gyulai, R. Photodynamic therapy of non-melanoma skin cancer with methyl aminolaevulinate is associated with less pain than with aminolaevulinic acid. Acta Derm.-Venereol. 2012, 92, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Wiegell, S.R.; Stender, I.M.; Na, R.; Wulf, H.C. Pain associated with photodynamic therapy using 5-aminolevulinic acid or 5-aminolevulinic acid methylester on tape-stripped normal skin. Arch. Dermatol. 2003, 139, 1173–1177. [Google Scholar]
- Kasche, A.; Luderschmidt, S.; Ring, J.; Hein, R. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J. Drugs Dermatol. 2006, 5, 353–356. [Google Scholar] [PubMed]
- Rud, E.; Gederaas, O.; Høgset, A.; Berg, K. 5-Aminolevulinic Acid, but not 5-Aminolevulinic acid esters, is transported into adenocarcinoma cells by system BETA transporters. Photochem. Photobiol. 2000, 71, 640–647. [Google Scholar]
- Baglo, Y.; Gabrielsen, M.; Sylte, I.; Gederaas, O.A. Homology Modeling of Human γ-Butyric Acid Transporters and the Binding of Pro-Drugs 5-Aminolevulinic Acid and Methyl Aminolevulinic Acid Used in Photodynamic Therapy. PLoS ONE. 2013, 8, e65200. [Google Scholar]
- Zeitouni, N.C.; Paquette, A.D.; Housel, J.P.; Shi, Y.; Wilding, G.E.; Foster, T.H.; Henderson, B.W. A Retrospective Review of Pain Control by a Two-Step Irradiance Schedule During Topical ALA-Photodynamic Therapy of Non- melanoma Skin Cancer. Lasers Surg. Med. 2013, 45, 89–94. [Google Scholar]
- Zeitouni, N.C.; Sunar, U.; Rohrbach, D.J.; Paquette, A.D.; Bellnier, D.A.; Shi, Y.; Henderson, B.W. A Prospective Study of Pain Control by a Two- Step Irradiance Schedule During Topical Photodynamic Therapy of Non-melanoma Skin Cancer. Dermatol. Surg. 2014, 40, 1390–1394. [Google Scholar] [PubMed]
- Radakovic-Fijan, S.; Blecha-Thalhammer, U.; Kittler, H.; Hönigsmann, H.; Tanew, A. Efficacy of 3 different light doses in the treatment of actinic keratosis with 5-aminolevulinic acid photodynamic therapy: A randomized, observer-blinded, intrapatient, comparison study. J. Am. Acad. Dermatol. 2005, 53, 823–827. [Google Scholar]
- Morton, C.A.; Whitehurst, C.; Moore, J.V.; MacKie, R.M. Comparison of red and green light in the treatment of Bowen’s disease by photodynamic therapy. Br. J. Dermatol. 2000, 143, 767–772. [Google Scholar] [CrossRef]
- Babilas, P.; Knobler, R.; Hummel, S.; Gottschaller, C.; Maisch, T.; Koller, M.; Landthaler, M.; Szeimies, R.M. Variable pulsed light is less painful than light-emitting diodes for topical photodynamic therapy of actinic keratosis: A prospective randomized controlled trial. Br. J. Dermatol. 2007, 157, 111–117. [Google Scholar]
- Kessels, J.P.; Nelemans, P.J.; Mosterd, K.; Kelleners-Smeets, N.W.; Krekels, G.A.; Ostertag, J.U. Laser-mediated Photodynamic Therapy: An Alternative Treatment for Actinic Keratosis? Acta Derm. Venereol. 2016, 96, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Wiegell, S.R.; Hædersdal, M.; Philipsen, P.A.; Eriksen, P.; Enk, C.D.; Wulf, H.C. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br. J. Dermatol. 2008, 158, 740–746. [Google Scholar]
- Braathen, L.R. Daylight photodynamic therapy in private practice in Switzerland: Gain without pain. Acta Derm.-Venereol. 2012, 92, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Collins, P. Randomized, double-blind, placebo-controlled prospective study of the efficacy of topical anaesthesia with a eutetic mixture of lignocaine 2.5% and prilocaine 2.5% for topical 5-aminolaevulinic acid–photodynamic therapy for extensive scalp actinic keratoses. Br. J. Dermatol. 2006, 154, 146–149. [Google Scholar] [PubMed]
- Holmes, M.V.; Dawe, R.S.; Ferguson, J.; Ibbotson, S.H. A randomized, double-blind, placebo-controlled study of the efficacy of tetracaine gel (Ametop®) for pain relief during topical photodynamic therapy. Br. J. Dermatol. 2004, 150, 337–340. [Google Scholar]
- Skiveren, J.; Haedersdal, M.; Philipsen, P.A.; Wiegell, S.R.; Wulf, H.C. Morphine gel 0.3% does not relieve pain during topical photodynamic therapy: A randomized, double- blind, placebo-controlled study. Acta Derm.-Venereol. 2006, 86, 409–411. [Google Scholar]
- Touma, D.; Yaar, M.; Whitehead, S.; Konnikov, N.; Gilchrest, B.A. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch. Dermatol. 2004, 140, 33–40. [Google Scholar]
- Paoli, J.; Halldin, C.; Ericson, M.B.; Wennberg, A.M. Nerve blocks provide effective pain relief during topical photodynamic therapy for extensive facial actinic keratoses. Clin. Exp. Dermatol. 2008, 33, 559–564. [Google Scholar]
- Borelli, C.; Herzinger, T.; Merk, K.; Berking, C.; Kunte, C.; Plewig, G.; Degitz, K. Effect of subcutaneous infiltration anesthesia on pain in photodynamic therapy: A controlled open pilot trial. Dermatol. Surg. 2007, 33, 314–318. [Google Scholar]
- Cabete, J.; Campos, S.; Lestre, S. Conscious sedation with inhaled 50% nitrous oxide/oxygen premix in photodynamic therapy sessions for vulvar lichen sclerosus treatment. An. Bras. Dermatol. 2015, 90, 120–122. [Google Scholar]
- Gholam, P.; Fink, C.; Uhlmann, L.; Enk, A. Pain reduction in patients after applying a nitrous oxide/oxygen mixture (Livopan) during photodynamic therapy: Study protocol for an observational study (Livopan study). BMJ Open 2015, 5, e006412. [Google Scholar] [CrossRef] [PubMed]
- Halldin, C.B.; Paoli, J.; Sandberg, C.; Ericson, M.B.; Wennberg, A.M. Transcutaneous electrical nerve stimulation for pain relief during photodynamic therapy of actinic keratoses. Acta Derm.-Venereol. 2008, 88, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Wiegell, S.R.; Haedersdal, M.; Wulf, H.C. Cold water and pauses in illumination reduces pain during photodynamic therapy: A randomized clinical study. Acta Derm.-Venereol. 2009, 89, 145–149. [Google Scholar] [PubMed]
© 2016 by the authors. 2016 Mircea Tampa, Maria Isabela Sârbu, Mădălina-Irina Mitran, Cristina-Iulia Mitran, Adrian Dumitru, Vasile Benea, Simona-Roxana Georgescu
Share and Cite
Tampa, M.; Sârbu, M.I.; Mitran, M.-I.; Mitran, C.-I.; Dumitru, A.; Benea, V.; Georgescu, S.-R. Pain in Photodynamic Therapy. J. Mind Med. Sci. 2016, 3, 19-30. https://doi.org/10.22543/2392-7674.1038
Tampa M, Sârbu MI, Mitran M-I, Mitran C-I, Dumitru A, Benea V, Georgescu S-R. Pain in Photodynamic Therapy. Journal of Mind and Medical Sciences. 2016; 3(1):19-30. https://doi.org/10.22543/2392-7674.1038
Chicago/Turabian StyleTampa, Mircea, Maria Isabela Sârbu, Mădălina-Irina Mitran, Cristina-Iulia Mitran, Adrian Dumitru, Vasile Benea, and Simona-Roxana Georgescu. 2016. "Pain in Photodynamic Therapy" Journal of Mind and Medical Sciences 3, no. 1: 19-30. https://doi.org/10.22543/2392-7674.1038
APA StyleTampa, M., Sârbu, M. I., Mitran, M.-I., Mitran, C.-I., Dumitru, A., Benea, V., & Georgescu, S.-R. (2016). Pain in Photodynamic Therapy. Journal of Mind and Medical Sciences, 3(1), 19-30. https://doi.org/10.22543/2392-7674.1038



