Spontaneous Rupture of a Splenic Artery Aneurysm with Hemoperitoneum—Case Presentation
Abstract
Introduction
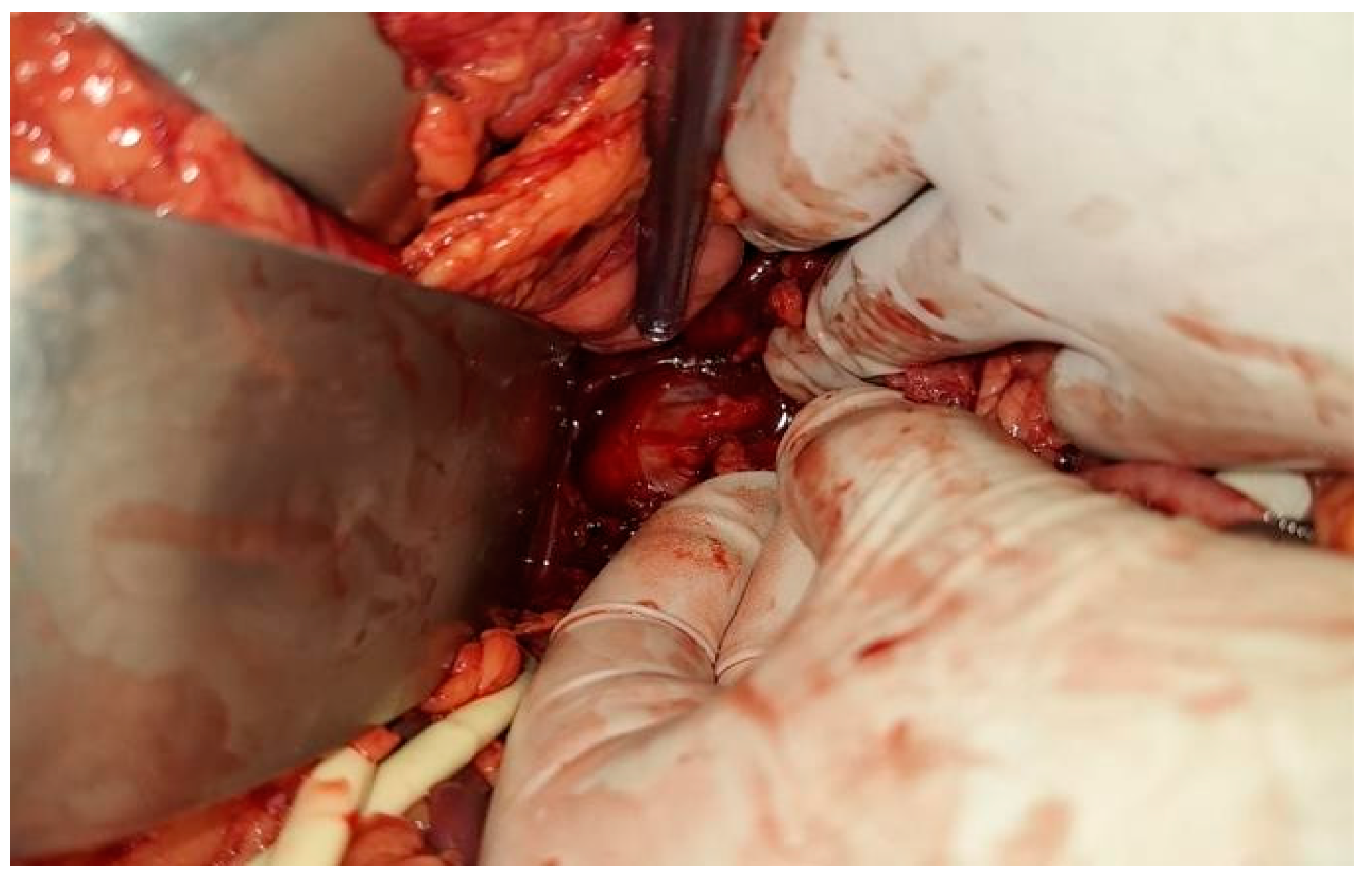
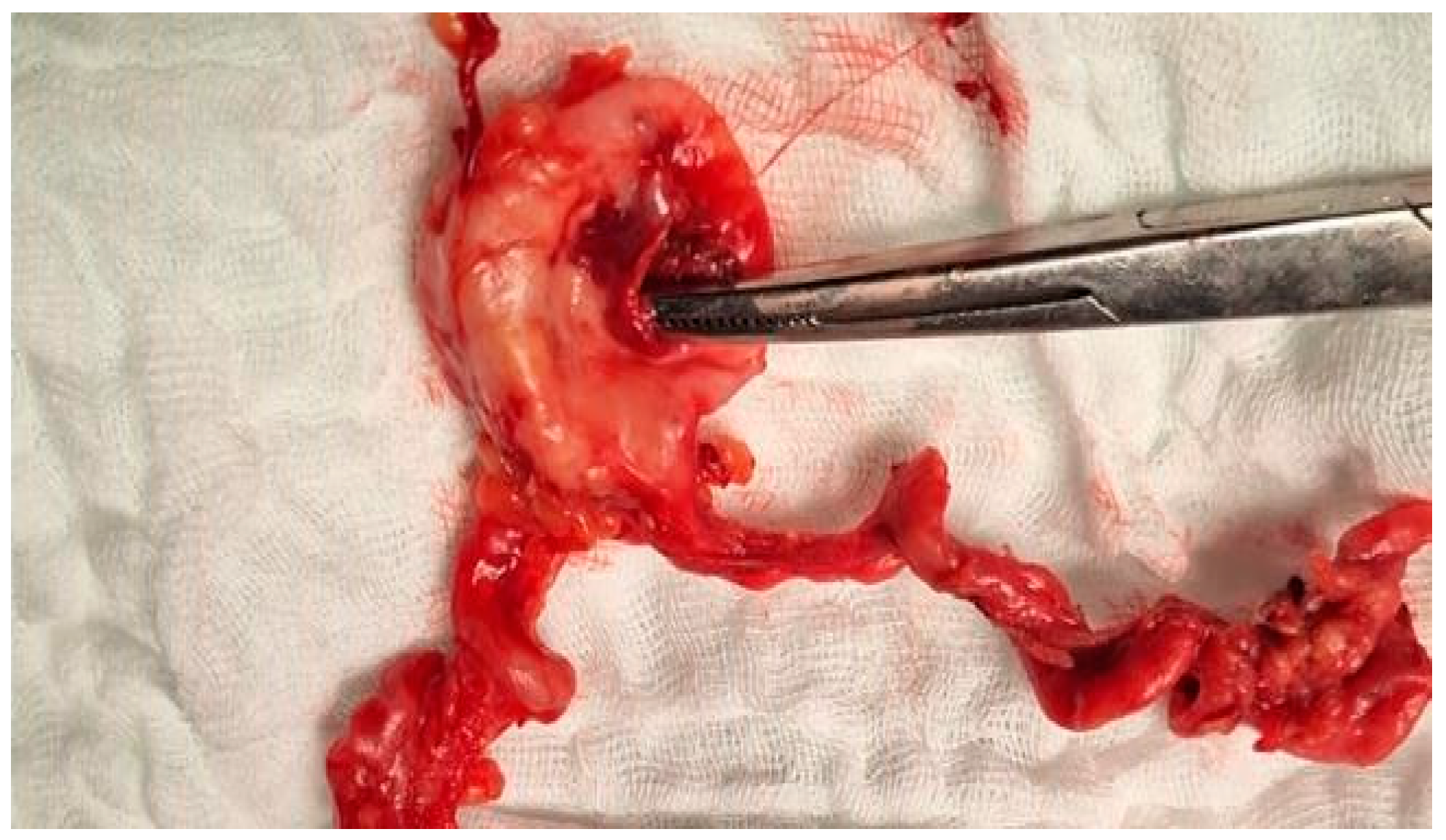
Case Presentation
Discussions
Conclusions
Institutional Review Board Statement
Conflicts of Interest
References
- Panzera F, Inchingolo R, Rizzi M, et al. Giant splenic artery aneurysm presenting with massive upper gastrointestinal bleeding: A case report and review of literature. World J Gastroenterol. 2020, 26, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Orsitto G, Fulvio F, Pinto AG, et al. Geriatric assessment of a giant splenic artery aneurysm accidentally diagnosed. Aging Clin Exp Res. 2011, 23, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Pararas, N.; Rajendiran, S.; Taha, I.; Powar, R.R.; Holguera, C.; Tadros, E. Spontaneous Rupture of a Huge Splenic Artery Aneurysm: A Case Report. Am J Case Rep. 2020, 21, e919956. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.F.; Brioschi, C.; Marone, E.M. Endovascular and Open Surgical Treatment of Ruptured Splenic Artery Aneurysms: A Case Report and a Systematic Literature Review. J Clin Med. 2023, 12, 6085. [Google Scholar] [CrossRef]
- Barrionuevo P, Malas MB, Nejim B, et al. A systematic review and meta-analysis of the management of visceral artery aneurysms. J Vasc Surg. 2019, 70, 1694–1699. [Google Scholar] [CrossRef]
- Künzle, S.; Glenck, M.; Puippe, G.; Schadde, E.; Mayer, D.; Pfammatter, T. Stent-graft repairs of visceral and renal artery aneurysms are effective and result in long-term patency. J Vasc Interv Radiol. 2013, 24, 989–996. [Google Scholar] [CrossRef]
- Yuan, F.; He, L.; Yao, Z.; Long, Y.; Xu, S. Spontaneous rupturing of splenic artery aneurysm: Another reason for fatal syncope and shock (Case report and literature review). Open Med (Wars). 2022, 17, 601–605. [Google Scholar] [CrossRef]
- Yoshikawa C, Yamato I, Nakata Y, et al. Giant splenic artery aneurysm rupture into the stomach that was successfully managed with emergency distal pancreatectomy. Surg Case Rep. 2022, 8, 148. [Google Scholar] [CrossRef]
- Obara, H.; Kentaro, M.; Inoue, M.; Kitagawa, Y. Current management strategies for visceral artery aneurysms: an overview. Surg Today. 2020, 50, 38–49. [Google Scholar] [CrossRef]
- Hosn, M.A.; Xu, J.; Sharafuddin, M.; Corson, J.D. Visceral Artery Aneurysms: Decision Making and Treatment Options in the New Era of Minimally Invasive and Endovascular Surgery. Int J Angiol. 2019, 28, 11–16. [Google Scholar] [CrossRef]
- Zhu C, Zhao J, Yuan D, et al. Endovascular and Surgical Management of Intact Splenic Artery Aneurysm. Ann Vasc Surg. 2019, 57, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Wang, T.H.; Yuan, D.; Zhao, J.C. Multiple Splenic Artery Aneurysms: A Case Report and Review of the Literature. Front Surg, 7638. [Google Scholar] [CrossRef]
- Tekola, B.D.; Arner, D.M.; Behm, B.W. Coil migration after transarterial coil embolization of a splenic artery pseudoaneurysm. Case Rep Gastroenterol. 2013, 7, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Pokala, B.; Vargas, L.M.; Oleynikov, D.; Kothari, V. Laparoscopic endoscopic combined surgery for removal of migrated coil after embolization of ruptured splenic artery aneurysm. Journal of Surgical Case Reports. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Tiberio, G.A.; Bonardelli, S.; Gheza, F.; Arru, L.; Cervi, E.; Giulini, S.M. Prospective randomized comparison of open versus laparoscopic management of splenic artery aneurysms: a 10-year study. Surg Endosc. [CrossRef]
- Akbulut, S.; Otan, E. Management of Giant Splenic Artery Aneurysm: Comprehensive Literature Review. Medicine (Baltimore). 2015, 94, e1016. [Google Scholar] [CrossRef]
- Teng, T.Z.J.; Thong, X.R.; Lau, K.Y.; Balasubramaniam, S.; Shelat, V.G. Acute appendicitis-advances and controversies. World J Gastrointest Surg. 2021, 13, 1293–1314. [Google Scholar] [CrossRef]
- Skjold-Ødegaard, B.; Søreide, K. The Diagnostic Differentiation Challenge in Acute Appendicitis: How to Distinguish between Uncomplicated and Complicated Appendicitis in Adults. Diagnostics (Basel). 2022, 12, 1724. [Google Scholar] [CrossRef]
- Germain, D.P. Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis. 2007, 2, 32. [Google Scholar] [CrossRef]
- Cheng, K.S.; Chou, J.W. A Symptomatic Calcified Splenic Artery Aneurysm. Clin Gastroenterol Hepatol. 2020, 18, e58. [Google Scholar] [CrossRef]
- de Mathelin P, Hericher F, Addeo P. Rupture of a Splenic Artery Aneurysm During Pregnancy. J Gastrointest Surg. 2023, 27, 2694–2695. [Google Scholar] [CrossRef]
- Varnavas, G.; Dolapsakis, C. A giant splenic artery aneurysm. CMAJ. 2020, 192, E608. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, J.; Liu, F.; Guo, W. Splenic Arteriovenous Fistula Accompanied by Splenic Artery Aneurysm Associated with Acute-onset Portal Hypertension and Gastrointestinal Bleeding: Case Report and Literature Review. Ann Vasc Surg. 2022, 78, e17–e378. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.R.; Wicks, J.; Sharma, A.K. Navigating tortuous collaterals during splenic artery aneurysm embolization. J Vasc Surg Venous Lymphat Disord. 2023, 11, 1285. [Google Scholar] [CrossRef] [PubMed]
- Trochimczuk, M.; Gewartowska, M.; Stańczyk, M. Endovascular treatment of a giant splenic artery aneurysm. Pol Arch Intern Med. 2022, 132, 16180. [Google Scholar] [CrossRef] [PubMed]
- Atanasijevic, I.; Babic, S.; Tanaskovic, S.; Gajin, P.; Ilijevski, N. Giant splenic artery aneurysm treated surgically with spleen and pancreas preservation. Ann Saudi Med. 2021, 41, 253–256. [Google Scholar] [CrossRef]
- Leow KS, Lohan R, Ooi DGS, Leong CR, Babu SB. Embolization of a Giant Splenic Artery Aneurysm. J Vasc Interv Radiol. 2021, 32, 472. [Google Scholar] [CrossRef]
- Agrawal, A.; Whitehouse, R.; Johnson, R.W.; Augustine, T. Giant splenic artery aneurysm associated with arteriovenous malformation. J Vasc Surg. 2006, 44, 1345–1349. [Google Scholar] [CrossRef]
- Anwar, N.; Reynolds, A.; Naz, N. Splenic artery aneurysm: a rare complication of autosomal dominant polycystic kidney disease. BMJ Case Rep. 2024, 17, e258601. [Google Scholar] [CrossRef]
- Ossola, P.; Mascioli, F.; Coletta, D. Laparoscopic and Robotic Surgery for Splenic Artery Aneurysm: A Systematic Review. Ann Vasc Surg. 2020, 68, 527–535. [Google Scholar] [CrossRef]
- Mulpuri, V.B.; Samanta, J.; Gupta, P.; Gupta, V. En bloc resection in giant bilobed splenic artery aneurysm. BMJ Case Rep. 2021, 14, e244319. [Google Scholar] [CrossRef]

© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, V.D.; Motofei, I.; Stefanescu, G.V.; Silaghi, A. Spontaneous Rupture of a Splenic Artery Aneurysm with Hemoperitoneum—Case Presentation. J. Mind Med. Sci. 2024, 11, 536-540. https://doi.org/10.22543/2392-7674.1561
Constantin VD, Motofei I, Stefanescu GV, Silaghi A. Spontaneous Rupture of a Splenic Artery Aneurysm with Hemoperitoneum—Case Presentation. Journal of Mind and Medical Sciences. 2024; 11(2):536-540. https://doi.org/10.22543/2392-7674.1561
Chicago/Turabian StyleConstantin, Vlad Denis, Ion Motofei, Gelu Valentin Stefanescu, and Adrian Silaghi. 2024. "Spontaneous Rupture of a Splenic Artery Aneurysm with Hemoperitoneum—Case Presentation" Journal of Mind and Medical Sciences 11, no. 2: 536-540. https://doi.org/10.22543/2392-7674.1561
APA StyleConstantin, V. D., Motofei, I., Stefanescu, G. V., & Silaghi, A. (2024). Spontaneous Rupture of a Splenic Artery Aneurysm with Hemoperitoneum—Case Presentation. Journal of Mind and Medical Sciences, 11(2), 536-540. https://doi.org/10.22543/2392-7674.1561


