Biological Effect of Modern Bioactive Materials Used in Direct and Indirect Capping; In Vitro Study
Abstract
Introduction
Materials and Methods
Materials
Methods
Statistical Analysis
Results
Discussions
Conclusions
Institutional Review Board Statement
Conflicts of Interest
References
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn Dent Sci Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- European Society of Endodontology (ESE) developed by: Duncan, H.F.; Galler, K.M.; et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int Endod J. 2019, 52, 923–934. [Google Scholar] [CrossRef]
- Ladino, L.G.; Bernal, A.; Calderón, D.; Cortés, D. Bioactive Materials in Restorative Dentistry: A Literature Review. SVOA Dentistry. 2021, 2, 74–81. [Google Scholar]
- Bioactive Restorative Materials. Int Dent J. 2023, 73, 9–10. [CrossRef] [PubMed]
- Walsh, R.M.; He, J.; Schweitzer, J.; Opperman, L.A.; Woodmansey, K.F. Bioactive endodontic materials for everyday use: A review. Gen Dent. 2018, 66, 48–51. [Google Scholar]
- Iliescu, A.A.; Gheorghiu, I.M.; Tănase, M.; Iliescu, A.; Mitran, L.; Mitran, M.; Perlea, P. Reactionary Versus Reparative Dentine in Deep Caries. ARS Medica Tomitana. 2019, 25, 15–21. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Han, G. Research progress of odontogenic extracellular vesicles in regeneration of dental pulp. Oral Dis. 2023, 29, 2565–2577. [Google Scholar] [CrossRef]
- Willershausen, B.; Willershausen, I.; Ross, A.; Velikonja, S.; Kasaj, A.; Blettner, M. Retrospective study on direct pulp capping with calcium hydroxide. Quintessence Int. 2011, 42, 165–171. [Google Scholar]
- Al-Saudi, K.W. A paradigm shift from calcium hydroxide to bioceramics in direct pulp capping: A narrative review. J Conserv Dent Endod. 2024, 27, 2–10. [Google Scholar] [CrossRef]
- Islam, R.; Islam, M.R.R.; Tanaka, T.; Alam, M.K.; Ahmed, H.M.A.; Sano, H. Direct pulp capping procedures—Evidence and practice. Jpn Dent Sci Rev. 2023, 59, 48–61. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Pitt Ford, T.R. Physical and chemical properties of a new root-end filling material. J Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Göhring, K.S.; Lehnert, B.; Zehnder, M. Indications for use of MTA, a review. Part 1: Chemical, physical and biological properties of MTA. Schweiz Monatsschr Zahnmed. 2004, 114, 143–153. [Google Scholar] [PubMed]
- Pinheiro, L.S.; Kopper, P.M.P.; Quintana, R.M.; Scarparo, R.K.; Grecca, F.S. Does MTA provide a more favourable histological response than other materials in the repair of furcal perforations? A systematic review. Int Endod J. 2021, 54, 2195–2218. [Google Scholar] [CrossRef]
- Bachoo, I.K.; Seymour, D.; Brunton, P. A biocompatible and bioactive replacement for dentine: Is this a reality? The properties and uses of a novel calcium-based cement. Br Dent J. 2013, 214, E5. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.; Martens, L.C.; Cauwels, R.G.E.C.; Anthonappa, R.P. Biodentine™ material characteristics and clinical applications: A 3 year literature review and update. Eur Arch Paediatr Dent. 2018, 19, 1–22. [Google Scholar] [CrossRef]
- Ford, T.R.; Torabinejad, M.; Abedi, H.R.; et al. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996, 127, 1491–1494. [Google Scholar] [CrossRef]
- Pérard, M.; Le Clerc, J.; Watrin, T.; et al. Spheroid model study comparing the biocompatibility of Biodentine and MTA. J Mater Sci Mater Med. 2013, 24, 1527–1534. [Google Scholar] [CrossRef]
- Iliescu, A.; Perlea, P.; Tulus, G.; Gheorghiu, I.M.; Iliescu, M.G.; Manolea, H.O. Bioceramics in endodontics. In Bioceramics and Biocomposites: From Research to Clinical Practice; Antoniac, I., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 241–290. [Google Scholar]
- Silano, V.; Paganuzzi-Stammati, A.; Zucco, F. Toxicity Tests with Mammalian Cell Cultures. 2005; https://api.semanticscholar.org/CorpusID:53128858. [Google Scholar]
- ISO 10993-5. Biological evaluation of medical devices. Part 5: Tests for in vitro cytotoxicity. 2009; http://www.iso.org/iso/catalogue_detail.htm?csnumber=36406.
- Cohen, B.I.; Pagnillo, M.K.; Musikant, B.L.; Deutsch, A.S. An in vitro study of the cytotoxicity of two root canal sealers. J Endod. 2000, 26, 228–229. [Google Scholar] [CrossRef]
- Gilbert, D.F.; Mofrad, S.A.; Friedrich, O.; Wiest, J. Proliferation characteristics of cells cultured under periodic versus static conditions. Cytotechnology. 2019, 71, 443–452. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr Pharm Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Miranda, R.B.; Fidel, S.R.; Boller, M.A. L929 cell response to root perforation repair cements: An in vitro cytotoxicity assay. Braz Dent J. 2009, 20, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.T.; McDonald, F.; Pitt Ford, T.R.; Torabinejad, M. Cellular response to Mineral Trioxide Aggregate. J Endod. 1998, 24, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, D.A.; Bement, W.M.; Brumell, J.H. Plasma membrane integrity: Implications for health and disease. BMC Biol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Ha, W.N.; Nicholson, T.; Kahler, B.; Walsh, L.J. Mineral Trioxide Aggregate-A Review of Properties and Testing Methodologies. Materials (Basel). 2017, 10, 1261, Published 2017 Nov 2. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Primus, C.M.; Prati, C. Ion release, porosity, solubility, and bioactivity of MTA Plus tricalcium silicate. J Endod. 2014, 40, 1632–1637. [Google Scholar] [CrossRef]
- Silva, E.J.; Accorsi-Mendonça, T.; Pedrosa, A.C.; Granjeiro, J.M.; Zaia, A.A. Long-Term Cytotoxicity, pH and Dissolution Rate of AH Plus and MTA Fillapex. Braz Dent J. 2016, 27, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Palczewska-Komsa, M.; Kaczor-Wiankowska, K.; Nowicka, A. New Bioactive Calcium Silicate Cement Mineral Trioxide Aggregate Repair High Plasticity (MTA HP)-A Systematic Review. Materials (Basel). 2021, 14, 4573. [Google Scholar] [CrossRef]
- Guimarães, B.M.; Prati, C.; Duarte, M.A.H.; Bramante, C.M.; Gandolfi, M.G. Physicochemical properties of calcium silicate-based formulations MTA Repair HP and MTA Vitalcem. J Appl Oral Sci. 2018, 26, e2017115, Published 2018 Apr 5. [Google Scholar] [CrossRef]
- Silva, E.J.; Carvalho, N.K.; Zanon, M.; Senna, P.M.; et al. Push-out bond strength of MTA HP, a new high-plasticity calcium silicate-based cement. Braz Oral Res. 2016, 30, S1806–83242016000100269. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; et al. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int Endod J 2017, 50 Suppl 2, e63–e72. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.D.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. MTA HP Repair stimulates in vitro an homogeneous calcium phosphate phase coating deposition. J Clin Exp Dent. 2019, 11, e322–e326, Published 2019 Apr 1. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. Biodentine(TM) induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Abuarqoub, D.; Aslam, N.; Zaza, R.; et al. The Immunomodulatory and Regenerative Effect of Biodentine™ on Human THP-1 Cells and Dental Pulp Stem Cells: In Vitro Study. Biomed Res Int. 2022, 2022, 2656784. [Google Scholar] [CrossRef]
- Birant, S.; Gokalp, M.; Duran, Y.; Koruyucu, M.; Akkoc, T.; Seymen, F. Cytotoxicity of NeoMTA Plus, ProRoot MTA and Biodentine on human dental pulp stem cells. J Dent Sci. 2021, 16, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.E.; Lee, B.N.; Koh, J.T.; et al. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor Dent Endod. 2014, 39, 89–94. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; et al. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
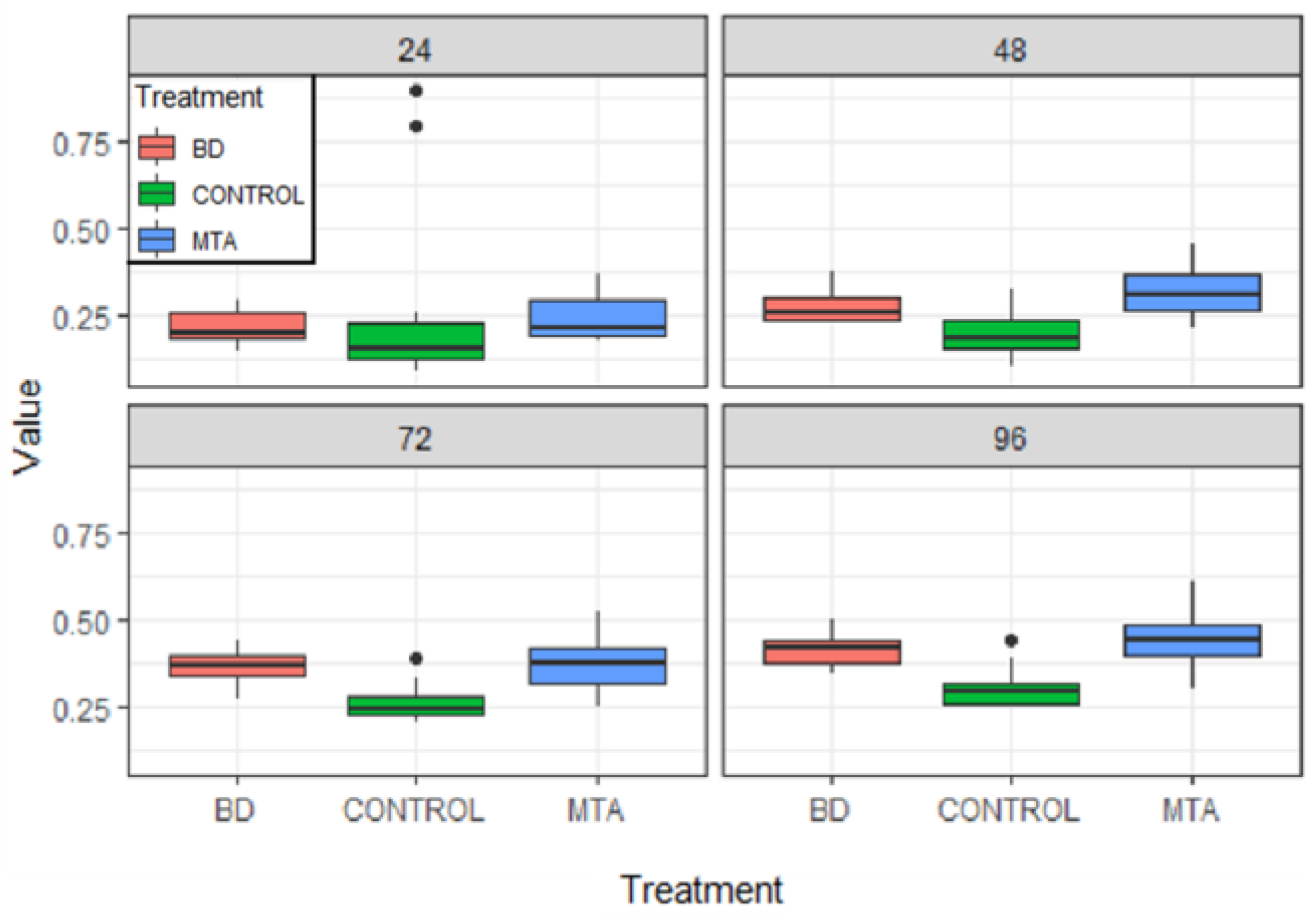
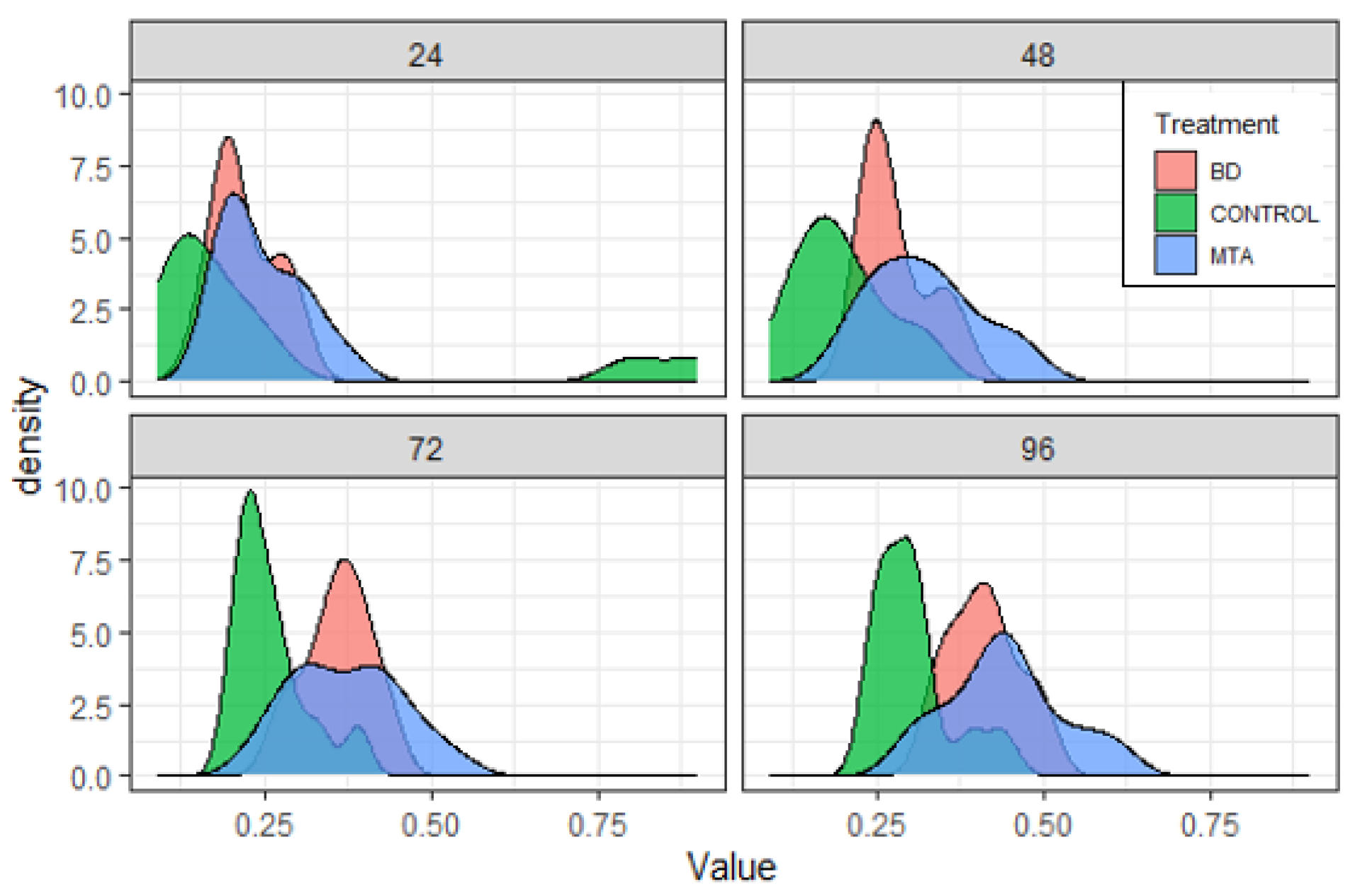
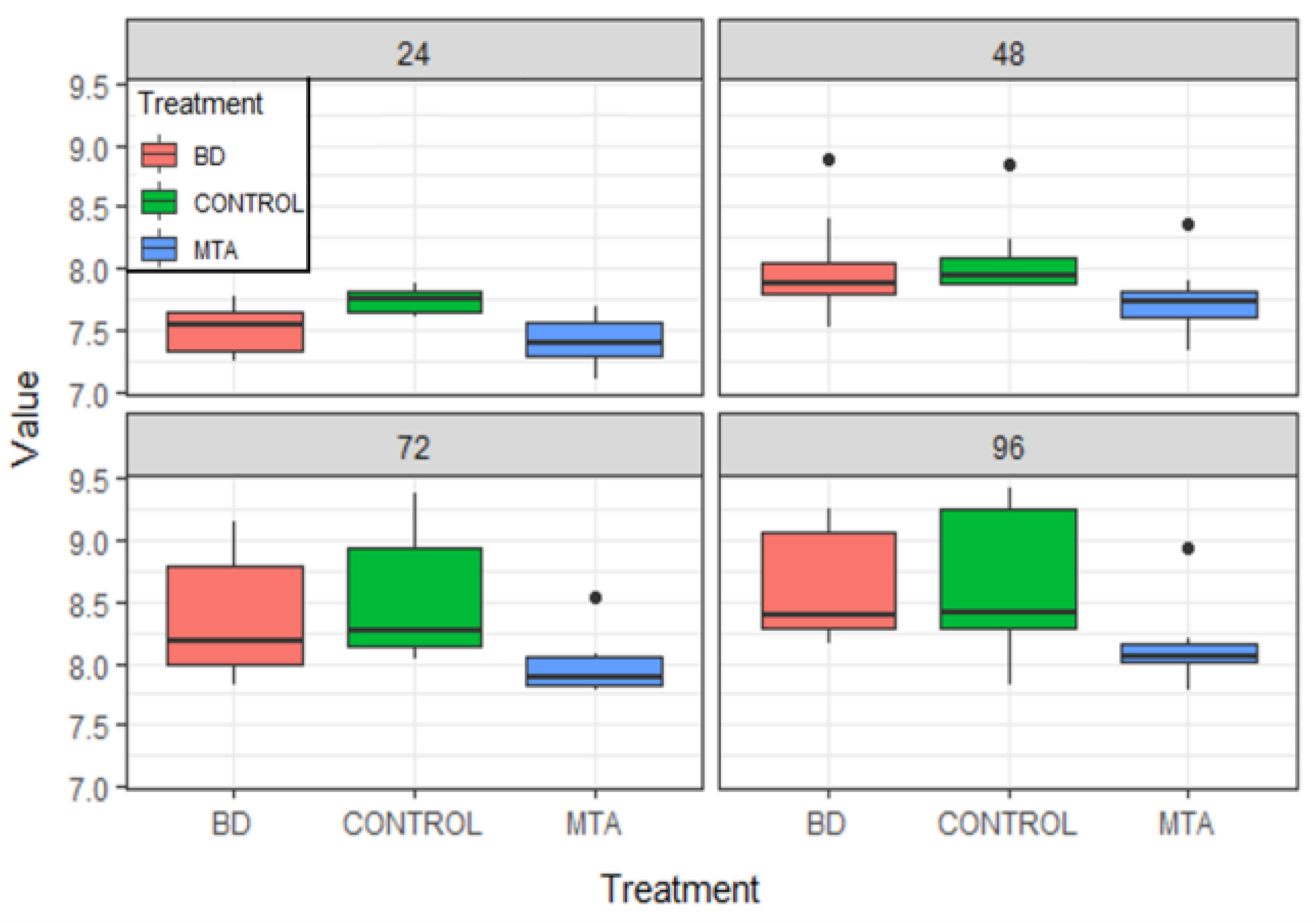
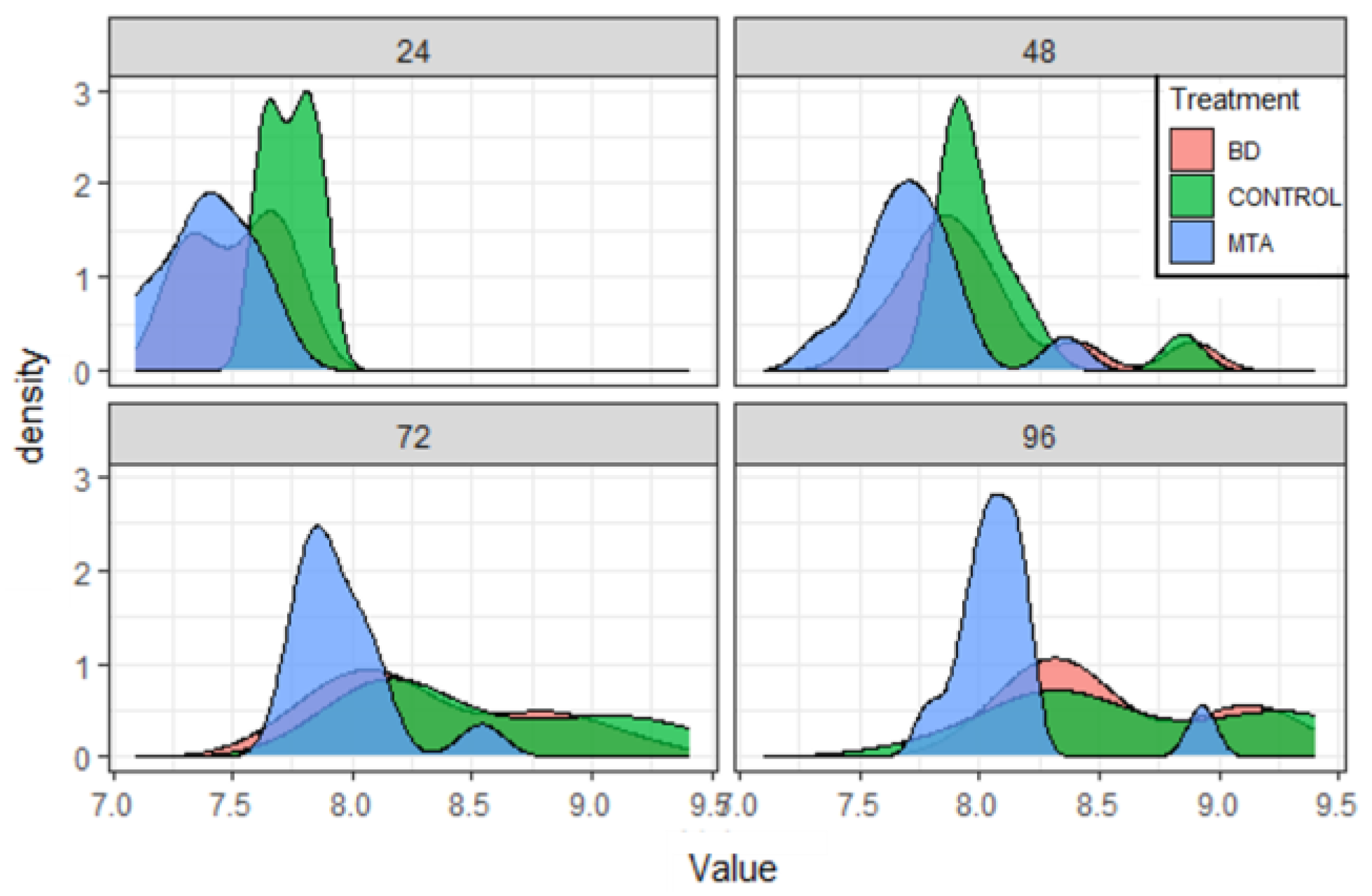
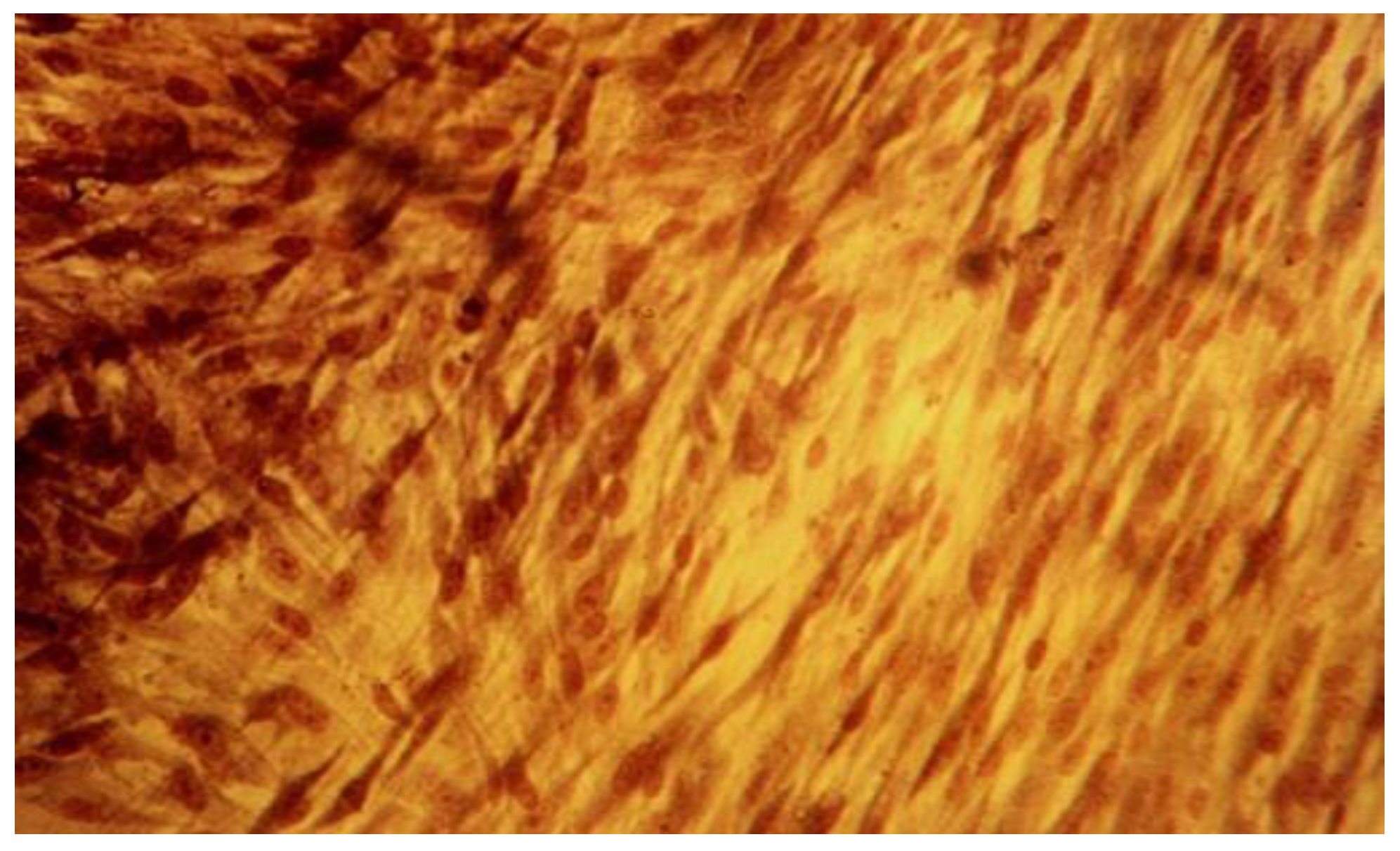
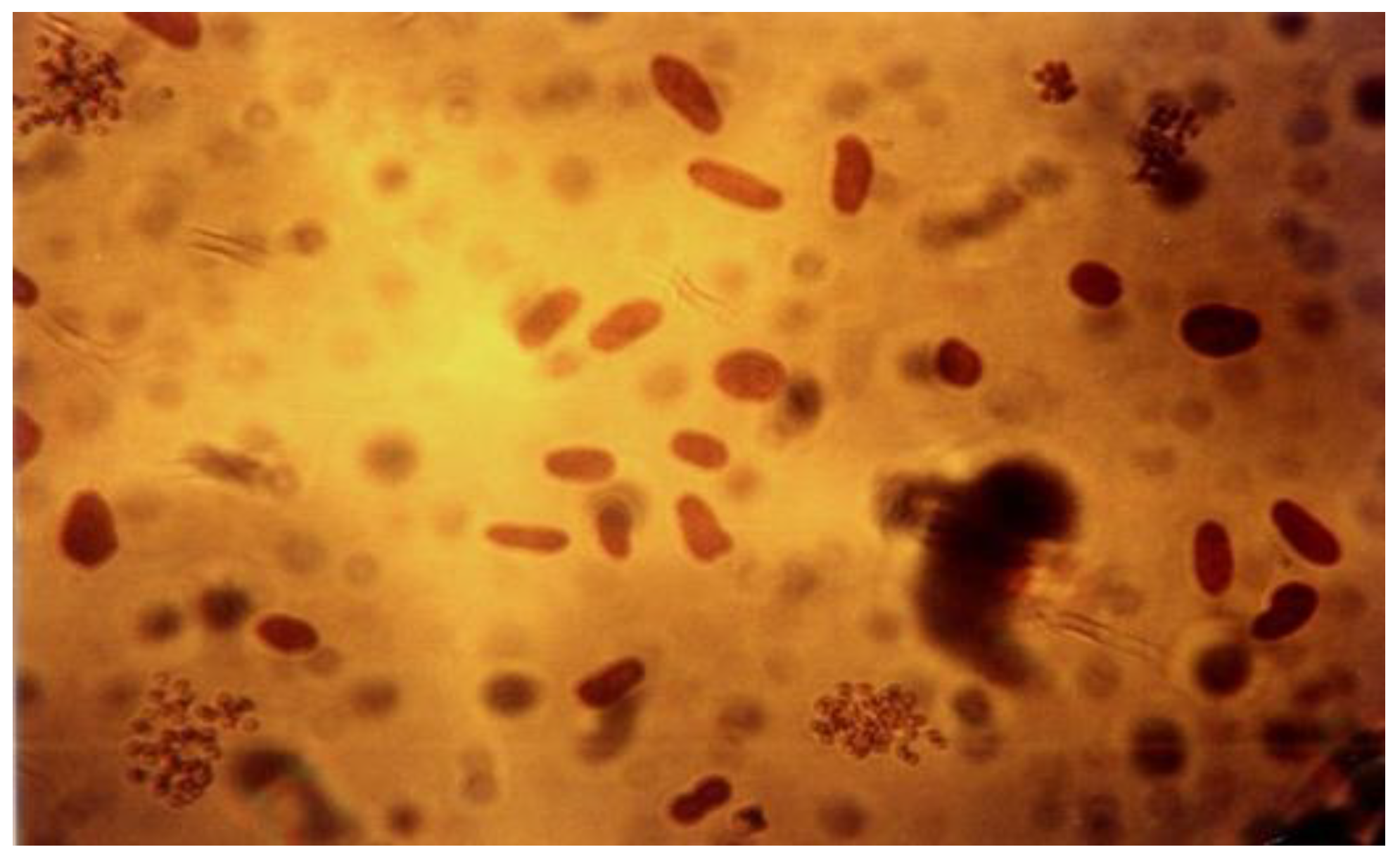

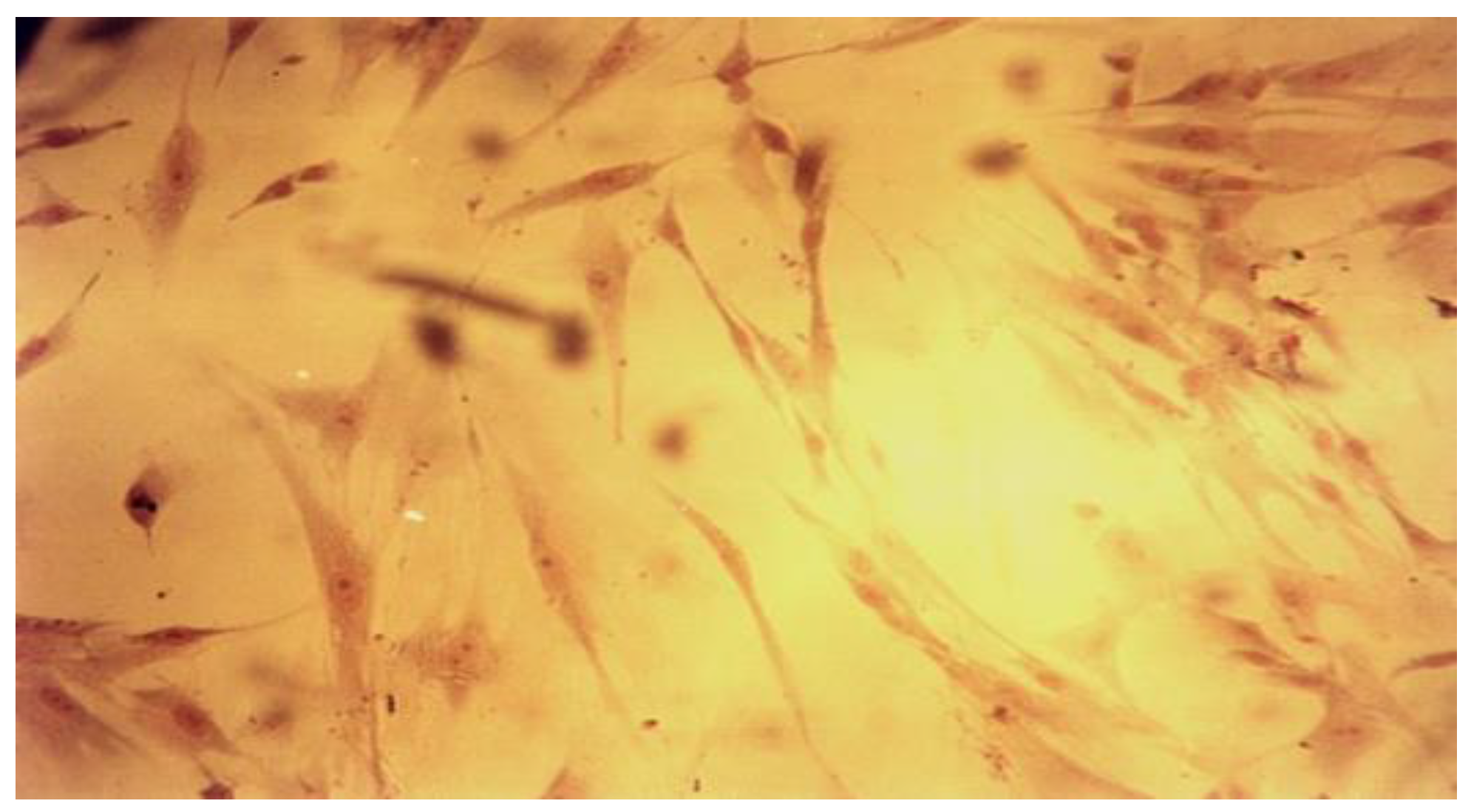
| Product | Dental Material Category | Presentation/Composition | Properties | Field of Applications |
|---|---|---|---|---|
| MTA Repair HP | Bioactive, reparative calcium silicate-based cement | Powder/liquid capsulesPowder: Tricalcium Silicate, Dicalcium Silicate, Tricalcium Aluminate, Calcium Oxide, Calcium TungstateLiquid: Water and plasticizer |
|
|
| BIODENTINE | Bioactive new calcium silicate-based cement, generally known as “dentine substitute” | Capsules/single–dose containersPowder: Tricalcium Silicate, Dicalcium Silicate, Calcium carbonate and oxide filler, iron oxide, zirconium oxideLiquid: hydro-soluble polymer, calcium chloride |
|
|
| Dental Product | Biological Properties |
|---|---|
| MTA Repair HP |
|
| BIODENTINE |
|
© 2024 by the author. 2024 Irina Maria Gheorghiu, Alexandru Andrei Iliescu, George Alexandru Denis Popescu, Stana Paunica, Anca Silvia Dumitriu.
Share and Cite
Gheorghiu, I.M.; Iliescu, A.A.; Popescu, G.A.D.; Paunica, S.; Dumitriu, A.S. Biological Effect of Modern Bioactive Materials Used in Direct and Indirect Capping; In Vitro Study. J. Mind Med. Sci. 2024, 11, 444-451. https://doi.org/10.22543/2392-7674.1544
Gheorghiu IM, Iliescu AA, Popescu GAD, Paunica S, Dumitriu AS. Biological Effect of Modern Bioactive Materials Used in Direct and Indirect Capping; In Vitro Study. Journal of Mind and Medical Sciences. 2024; 11(2):444-451. https://doi.org/10.22543/2392-7674.1544
Chicago/Turabian StyleGheorghiu, Irina Maria, Alexandru Andrei Iliescu, George Alexandru Denis Popescu, Stana Paunica, and Anca Silvia Dumitriu. 2024. "Biological Effect of Modern Bioactive Materials Used in Direct and Indirect Capping; In Vitro Study" Journal of Mind and Medical Sciences 11, no. 2: 444-451. https://doi.org/10.22543/2392-7674.1544
APA StyleGheorghiu, I. M., Iliescu, A. A., Popescu, G. A. D., Paunica, S., & Dumitriu, A. S. (2024). Biological Effect of Modern Bioactive Materials Used in Direct and Indirect Capping; In Vitro Study. Journal of Mind and Medical Sciences, 11(2), 444-451. https://doi.org/10.22543/2392-7674.1544



