Challenges in Diagnosing Nasopharyngeal Tumors
Abstract
Highlights
- ✓
- Even if nasopharyngeal tumors are usually diagnosed at an advanced stage, there are procedures for the effective and rapid diagnosis of nasopharyngeal tumors, which is essential for timely therapeutic interventions and optimizing results.
- ✓
- Patients turn to the doctor most of the time for the appearance of a lump in the neck region or repetitive epistaxis, but the number of cases with an otic onset seems to be increasing recently.
Abstract
Introduction
Materials and Methods
Results
Comparative Analysis of Debut Presentations
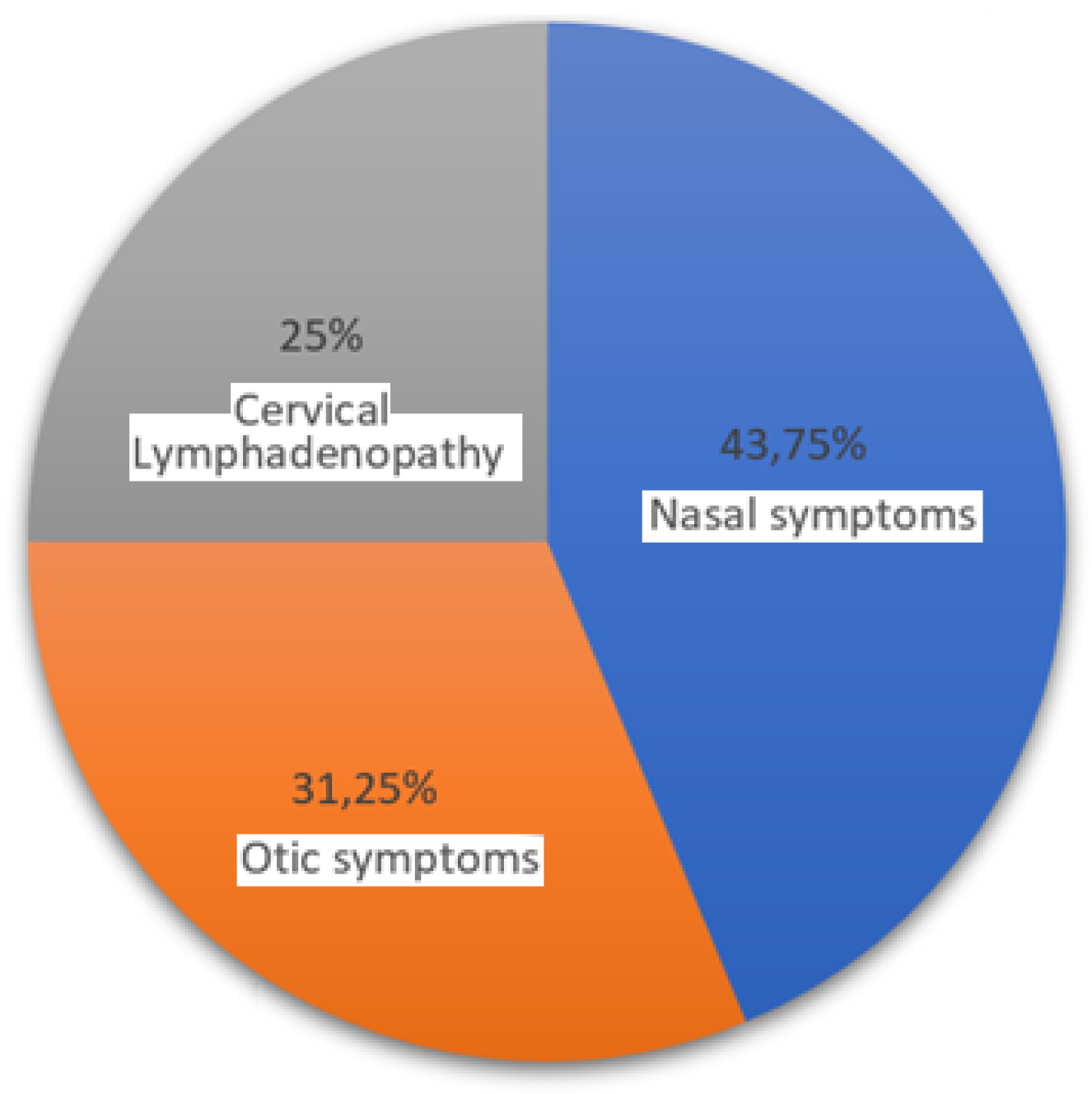
- Relative Frequency of Each Presentation
- o
- Nasal Symptoms. These were the most frequent presentation in your clinic, seen in 43.75% of cases. This suggests that nearly half of the NPC patients initially present with symptoms related to the nasal area, like nosebleeds or nasal obstruction.
- o
- Otic Symptoms. Otic symptoms, including hearing loss or ear fullness, were the second most common, observed in 31.25% of cases. This indicates that otic presentations are also quite prevalent, making up almost one-third of the cases.
- o
- Cervical Lymphadenopathy. This presentation, characterized by neck masses, was the least frequent, found in 25% of the patients. This is still a significant proportion, emphasizing that a quarter of NPC cases may initially present with neck lymph node.
- 2.
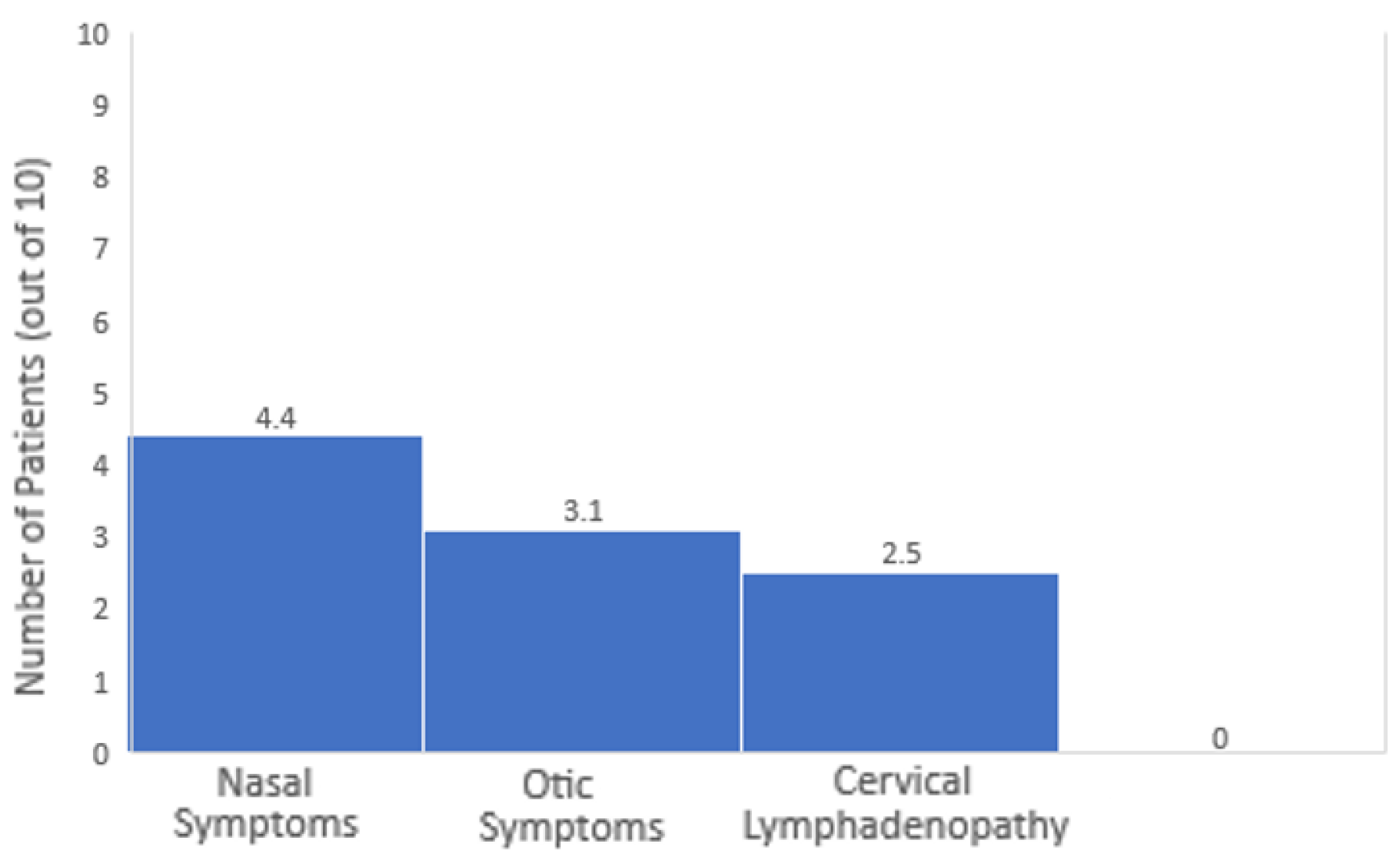
- Proportional Representation
- o
- Nasal vs. Otic Symptoms. Nasal symptoms were more common than otic symptoms. For every 10 patients, approximately 4.4 had nasal symptoms, while about 3.1 had otic symptoms. Thus, nasal symptoms are about 1.4 times more likely to be the presenting feature than otic symptoms.
- o
- Nasal vs. Cervical Lymphadenopathy. Nasal symptoms were significantly more common than cervical lymphadenopathy. For every 10 patients, around 4.4 had nasal symptoms compared to 2.5 with cervical lymphadenopathy. Therefore, nasal symptoms are 1.75 times more likely than cervical lymphadenopathy to be the initial presentation.
- o
- Otic Symptoms vs. Cervical Lymphadenopathy. Otic symptoms were generally more frequent than cervical lymphadenopathy, being seen in about 3.1 out of every 10 patients compared to 2.5 out of 10. This makes otic symptoms 1.25 times more likely to be the initial presentation than cervical lymphadenopathy.
- 3.
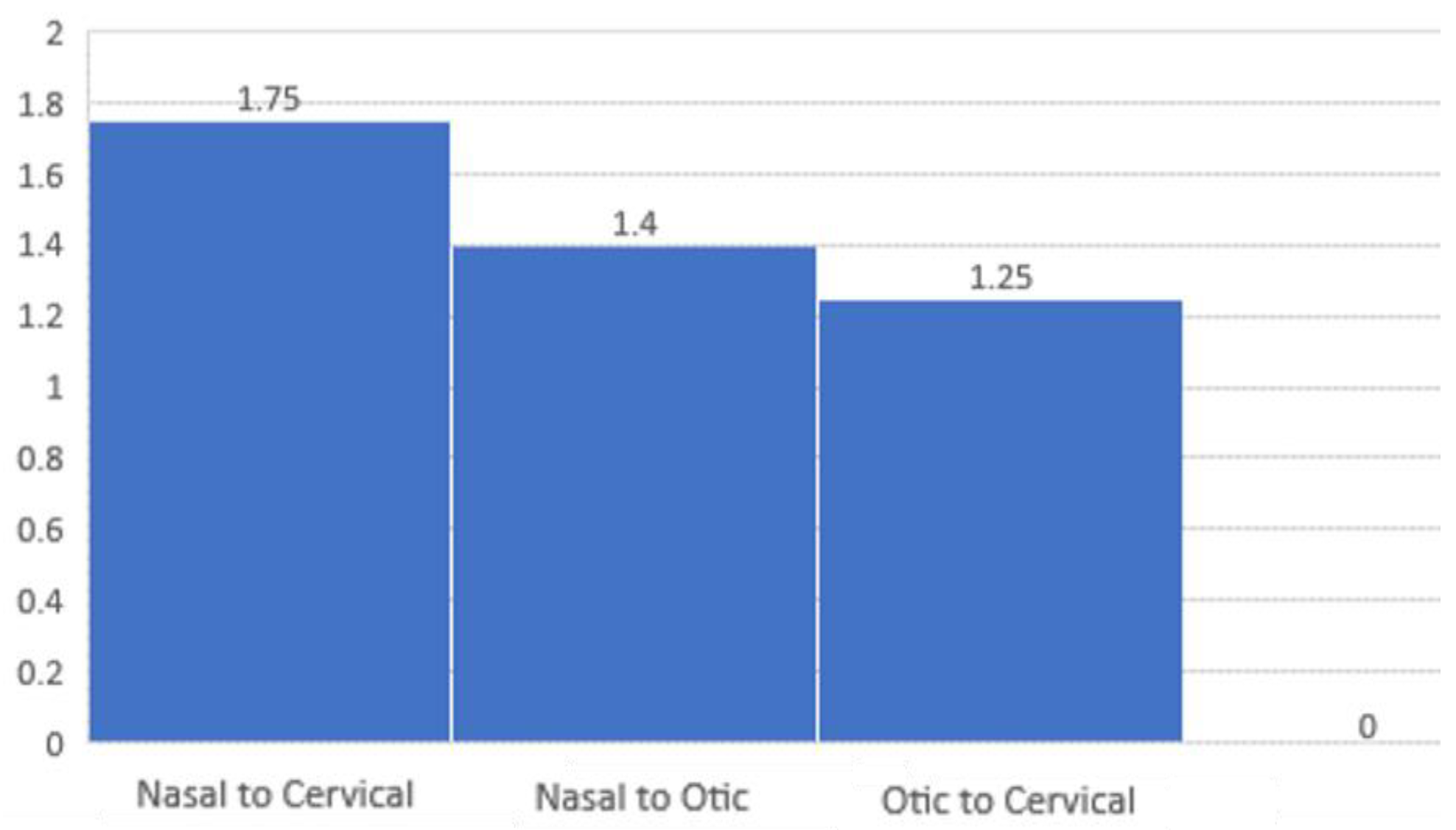
- Patient to Patient Ratios
- o
- Nasal to Otic Symptoms. For every 1 patient presenting with otic symptoms, there were 1.4 patients presenting with nasal symptoms.
- o
- Nasal to Cervical Lymphadenopathy. For every 1 patient with cervical lymphadenopathy, there were 1.75 patients with nasal symptoms.
- o
- Otic Symptoms to Cervical Lymphadenopathy. For every 1 patient with cervical lymphadenopathy, there were 1.25 patients with otic symptoms.
Discussions
- Hidden area. The area is not easily accessible, and the tumor may not be visible during a routine physical examination [17].
- Non-Specific Symptoms. Early-stage nasopharyngeal tumors may not cause specific symptoms, or the symptoms may be nonspecific, such as nasal congestion, a sore throat, or earache. These symptoms can be attributed to various common illnesses, leading to delayed diagnosis [18].
- Similar Symptoms to Other Conditions. Symptoms of nasopharyngeal tumors, such as nosebleeds, ear problems, and neck lumps, can be indicative of various other conditions, making it challenging to attribute them specifically to a nasopharyngeal tumor without further investigation [19].
- Silent Development. In some cases, nasopharyngeal tumors can develop silently without causing noticeable symptoms until they reach an advanced stage. This makes early detection less likely [20].
- Rare Nature of the Tumor. Nasopharyngeal tumors are relatively rare compared to tumors in other head and neck regions. This rarity can contribute to a lack of awareness among healthcare professionals, leading to delayed consideration of nasopharyngeal tumors in the diagnostic and treatment processes [21].
- Asymptomatic Carriers of Epstein-Barr Virus (EBV). Nasopharyngeal carcinoma is strongly associated with Epstein-Barr Virus (EBV) infection. However, many individuals with nasopharyngeal carcinoma may be asymptomatic carriers of EBV, and the presence of the virus alone may not be sufficient for diagnosis [8].
- Case one
- Case two
- Case three
Conclusions
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Tsunoda, A.; Shirakura, S.; Kishimoto, S. Mechanical obstruction of the eustachian tube by the benign tumor of the parapharyngeal space does not cause otitis media with effusion. Otol Neurotol. 2007, 28, 1072–1075. [Google Scholar] [CrossRef]
- Ars, B.; Dirckx, J. Eustachian Tube Function. Otolaryngol Clin North Am. 2016, 49, 1121–1133. [Google Scholar] [CrossRef]
- Komune, N.; Matsuo, S.; Miki, K.; et al. Surgical Anatomy of the Eustachian Tube for Endoscopic Transnasal Skull Base Surgery: A Cadaveric and Radiologic Study. World Neurosurg. 2018, 112, e172–e181. [Google Scholar] [CrossRef]
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017, 372, 20160270. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Shi, J.; Zhu, W.; Wang, X. Association of Plasma Epstein-Barr Virus LMP1 and EBER1 with Circulating Tumor Cells and the Metastasis of Nasopharyngeal Carcinoma. Pathol Oncol Res. 2020, 26, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Mao, Y.P.; Tang, L.L.; Chen, L.; Sun, Y.; Ma, J. The evolution of nasopharyngeal carcinoma staging. Br J Radiol. 2019, 92, 20190244. [Google Scholar] [CrossRef]
- Banko, A.; Miljanovic, D.; Lazarevic, I.; Cirkovic, A. A Systematic Review of Epstein-Barr Virus Latent Membrane Protein 1 (LMP1) Gene Variants in Nasopharyngeal Carcinoma. Pathogens. 2021, 10, 1057. [Google Scholar] [CrossRef]
- Mazilu, L.; Ciufu, N.; Gălan, M.; et al. Postherapeutic follow-up of colorectal cancer patients treated with curative intent. Chirurgia (Bucur). 2012, 107, 55–58. [Google Scholar]
- Haldun, S.; Suceveanu, A.I.; Lupascu, M.; et al. Efficacy of tranexamic acid in nasopharyngeal hemorrhage; single center prospective study and literature review. J Mind Med Sci. 2024, 11, 189–194. [Google Scholar] [CrossRef]
- Herlo, L.F.; Salcudean, A.; Sirli, R.; et al. Gut Microbiota Signatures in Colorectal Cancer as a Potential Diagnostic Biomarker in the Future: A Systematic Review. Int J Mol Sci. 2024, 25, 7937, Published 2024 Jul 20. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.; Smarandache, A.M.; Cristian, D.; Tudor, C.; Duta, L.; Dascalu, A.M. Medical errors and patient safety culture-shifting the healthcare paradigm in Romanian hospitals. Rom J Leg Med. 2020, 28, 195–201. [Google Scholar] [CrossRef]
- Nicoara, A.D.; Alexandrescu, L.; Tofolean, D.E.; et al. The Impact of Cardiac Rehabilitation on Quality of Life in Elderly Heart Failure Patients-Literature Review. Balneo and PRM Research Journal. 2024, 15, 723. [Google Scholar] [CrossRef]
- Lee, A.W.M.; Ng, W.T.; Chan, J.Y.W.; et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev. 2019, 79, 101890. [Google Scholar] [CrossRef]
- Bossi, P.; Chan, A.T.; Licitra, L.; Trama, A.; et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2021, 32, 452–465. [Google Scholar] [CrossRef]
- Lee, H.M.; Okuda, K.S.; González, F.E.; Patel, V. Current Perspectives on Nasopharyngeal Carcinoma. Adv Exp Med Biol. 2019, 1164, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, Z.; Wang, D.; Qin, G. Minimally Invasive Surgery for Early-Stage Nasopharyngeal Carcinoma. J Craniofac Surg. 2022, 33, e834–e837. [Google Scholar] [CrossRef]
- Zeng, C.; Qiao, M.; Chen, Y.; Xie, H. EBV-positive glycoproteins associated with nasopharyngeal carcinoma. Pathol Res Pract. 2024, 260, 155427. [Google Scholar] [CrossRef]
- Mints, M.; Tirosh, I. Nasopharyngeal carcinoma joins the single-cell party. Cancer Commun (Lond). 2020, 40, 453–455. [Google Scholar] [CrossRef]
- De Felice, F.; Marchetti, C.; Serpone, M.; et al. Upper-neck irradiation versus standard whole-neck irradiation in nasopharyngeal carcinoma: A systematic review and meta-analysis. Tumori. 2023, 109, 529–536. [Google Scholar] [CrossRef]
- Yin, L.; Li, Z.; Xue, G.; Lu, L. Nasopharyngeal carcinoma misdiagnosed as pituitary tumor with multiple cranial neuropathies. J Cancer Res Ther. 2021, 17, 1748–1750. [Google Scholar] [CrossRef]
- Zhang, W.G.; Liu, Z.Y.; Pang, S.W. Separation of nasopharyngeal epithelial cells from carcinoma cells on 3D scaffold platforms. Biotechnol Bioeng. 2021, 118, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; He, W.; Ren, C.; et al. Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct Target Ther. 2020, 5, 245. [Google Scholar] [CrossRef]
- Trevisiol, C.; Gion, M.; Vaona, A.; et al. The appropriate use of circulating EBV-DNA in nasopharyngeal carcinoma: Comprehensive clinical practice guidelines evaluation. Oral Oncol. 2021, 114, 105128. [Google Scholar] [CrossRef]
- Lee, C.Y.; Wang, T.H.; Kao, Y.S. Chemotherapy, Radiation Therapy, and Nasopharyngeal Carcinoma. JAMA Oncol. 2024, 10, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Petersson, F. Non-Keratinizing Nasopharyngeal Carcinoma with Adenomatous Differentiation. Head Neck Pathol. 2020, 14, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Ganeson, S.K.; Saniasiaya, J.; Mohamad, I.; Abdul Gani, N. Basaloid Nasopharyngeal Carcinoma: An Entity That Remains Oblivious. Gulf J Oncolog. 2020, 1, 83–86. [Google Scholar]
- Chua, M.L.K.; Lua, J.Y.H.; Ng, W.T.; Lee, A.W.M. Management of the neck in nasopharyngeal carcinoma-time for a radical change? Chin Clin Oncol. 2023, 12, 8. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, H.; Ma, F.; Shen, H.; Lu, H. Acrometastasis of nasopharyngeal carcinoma: A case report. J Int Med Res. 2020, 48, 300060520924519. [Google Scholar] [CrossRef]
- Savlovschi, C.; Brănescu, C.; Serban, D.; et al. Amyand’s hernia--a clinical case. Chirurgia (Bucur). 2010, 105, 409–414. [Google Scholar]
- Liu, H.; Audino, A.N. Metastatic nasopharyngeal carcinoma mimicking nodular sclerosis Hodgkin lymphoma. Blood. 2020, 136, 2596. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Fang, Q.; Li, P.; Wu, J.; Zhang, X. Secondary Squamous Cell Carcinoma of the Oral Cavity after Nasopharyngeal Carcinoma. Cancer Res Treat. 2020, 52, 109–116. [Google Scholar] [CrossRef]
- Yang, J.H.; Lin, L.K.; Zhang, S. Epigenetic silencing of microRNA-335 contributes to nasopharyngeal carcinoma metastasis. Am J Otolaryngol. 2020, 41, 102302. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Sun, X.; et al. Outcomes of recurrent nasopharyngeal carcinoma patients treated with endoscopic nasopharyngectomy: A meta-analysis. Int Forum Allergy Rhinol. 2020, 10, 1001–1011. [Google Scholar] [CrossRef]
- Batawi, H.; Micieli, J.A. Nasopharyngeal carcinoma presenting as a sixth nerve palsy and Horner’s syndrome. BMJ Case Rep. 2019, 12, e232291, Published 2019 Oct 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, P.; Li, Q.; Fan, H. A case of nasopharyngeal carcinoma presenting as Susac syndrome. Br J Hosp Med (Lond). 2021, 82, 1–3. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, S.; Tie, X.; Huang, X.; Cui, L. Growth Inhibition of Nasopharyngeal Carcinoma Cells Mediated by p53 Gene-Containing Nanolipid Composites. J Nanosci Nanotechnol. 2020, 20, 6026–6032. [Google Scholar] [CrossRef] [PubMed]
- Challapalli, S.D.; Simpson, M.C.; Adjei Boakye, E.; et al. Survival differences in nasopharyngeal carcinoma among racial and ethnic minority groups in the United States: A retrospective cohort study. Clin Otolaryngol. 2019, 44, 14–20. [Google Scholar] [CrossRef]
- Alius, R.O.; Zainea, V.; Voiosu, C.; Ionita, I.G.; Rusescu, A.; Balalau, O.D.; Alius, C.; Pulpa, R.O.; Hainarosie, R. Nasal surgery versus pharyngeal surgery in the treatment of obstructive sleep apnea. J Mind Med Sci. 2022, 9, 318–323. [Google Scholar] [CrossRef]
- Argirion, I.; Zarins, K.R.; Suwanrungruang, K.; et al. Subtype Specific Nasopharyngeal Carcinoma Incidence and Survival Trends: Differences between Endemic and Non-Endemic Populations. Asian Pac J Cancer Prev. 2020, 21, 3291–3299. [Google Scholar] [CrossRef]
- Dumitru, E.; Dumitru, I.M.; Popescu, R.; Resul, G.; Bulbuc, I.; Rugina, S. Simultaneous occurrence of two rare diseases: Actinomycosis and melanoma of the rectum. J Gastrointestin Liver Dis. 2014, 23, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.J.; Luu, M.; David, J.M.; et al. Facility Volume and Survival in Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2018, 100, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, P.; Zhang, H.; Wu, L.; Min, K. New T staging recommendations for recurrent nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2024, 150, 298, Published 2024 Jun 8. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Cao, D.; Dong, A.; et al. Systematic construction and external validation of an immune-related prognostic model for nasopharyngeal carcinoma. Head Neck. 2022, 44, 1086–1098. [Google Scholar] [CrossRef]
© 2024 by the author. 2024 Alexandru Aristide Alexe, Mihai Victor Lupascu, Haldun Septar, Anca Pantea Stoian, Andra Iulia Suceveanu, Viorel Gherghina, Iuliana Cindea, Alina Doina Nicoara, Laura Mazilu, Razvan Hainarosie, Felix Voinea, Adrian Paul Suceveanu.
Share and Cite
Alexe, A.A.; Lupascu, M.V.; Septar, H.; Pantea Stoian, A.; Suceveanu, A.I.; Gherghina, V.; Cindea, I.; Nicoara, A.D.; Mazilu, L.; Hainarosie, R.; et al. Challenges in Diagnosing Nasopharyngeal Tumors. J. Mind Med. Sci. 2024, 11, 437-443. https://doi.org/10.22543/2392-7674.1548
Alexe AA, Lupascu MV, Septar H, Pantea Stoian A, Suceveanu AI, Gherghina V, Cindea I, Nicoara AD, Mazilu L, Hainarosie R, et al. Challenges in Diagnosing Nasopharyngeal Tumors. Journal of Mind and Medical Sciences. 2024; 11(2):437-443. https://doi.org/10.22543/2392-7674.1548
Chicago/Turabian StyleAlexe, Alexandru Aristide, Mihai Victor Lupascu, Haldun Septar, Anca Pantea Stoian, Andra Iulia Suceveanu, Viorel Gherghina, Iuliana Cindea, Alina Doina Nicoara, Laura Mazilu, Razvan Hainarosie, and et al. 2024. "Challenges in Diagnosing Nasopharyngeal Tumors" Journal of Mind and Medical Sciences 11, no. 2: 437-443. https://doi.org/10.22543/2392-7674.1548
APA StyleAlexe, A. A., Lupascu, M. V., Septar, H., Pantea Stoian, A., Suceveanu, A. I., Gherghina, V., Cindea, I., Nicoara, A. D., Mazilu, L., Hainarosie, R., Voinea, F., & Suceveanu, A. P. (2024). Challenges in Diagnosing Nasopharyngeal Tumors. Journal of Mind and Medical Sciences, 11(2), 437-443. https://doi.org/10.22543/2392-7674.1548


