Telomeres Are Shorter in Portuguese Obese Adults
Abstract
Introduction
Materials and Methods
Sample
DNA extraction
Measurement of relative telomere length
Statistical analysis
Results
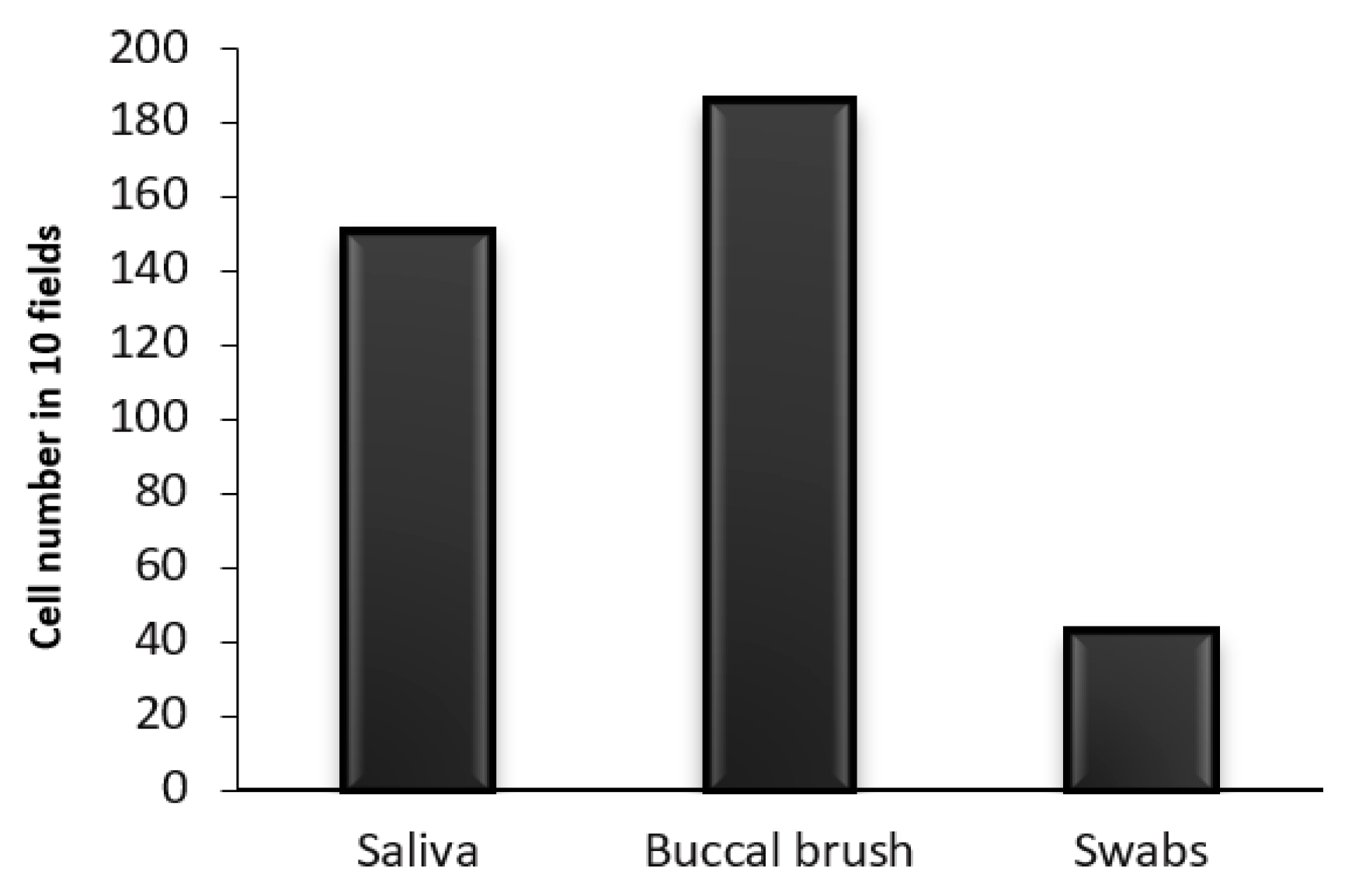
Buccal brush is the best substrate to collect oral epithelium cells
Sample characterization
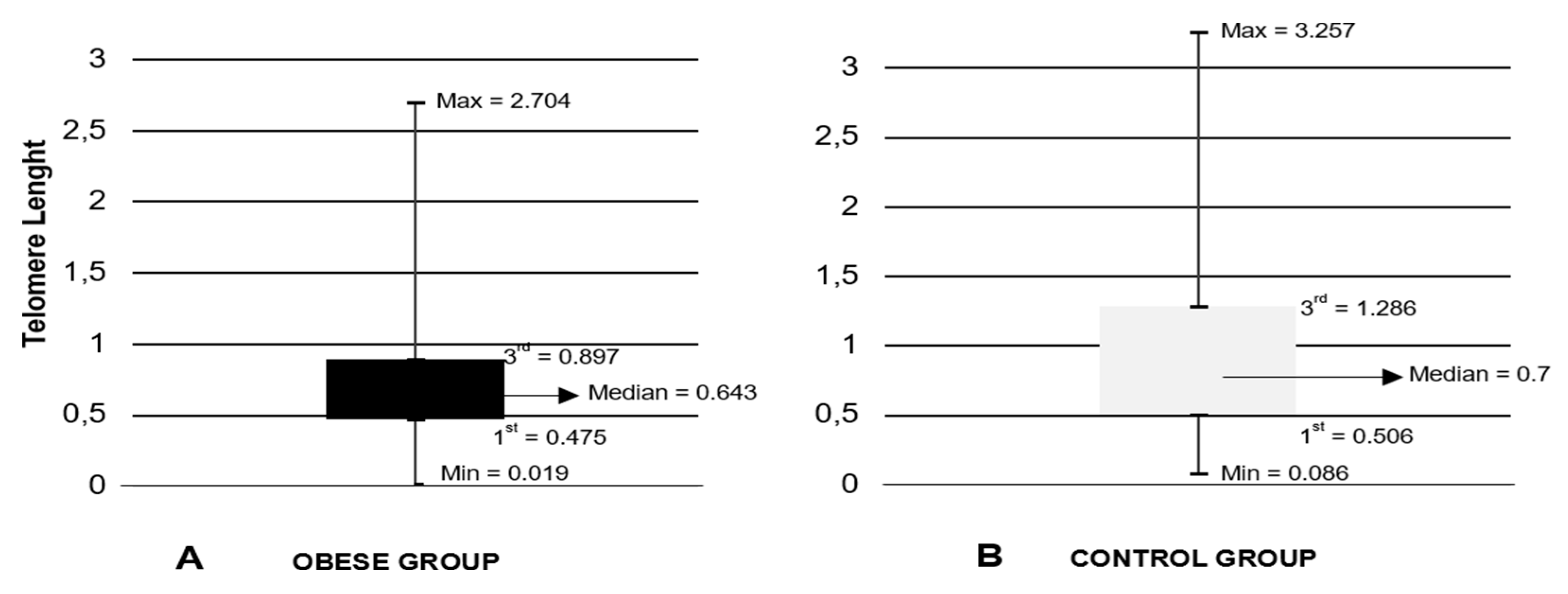
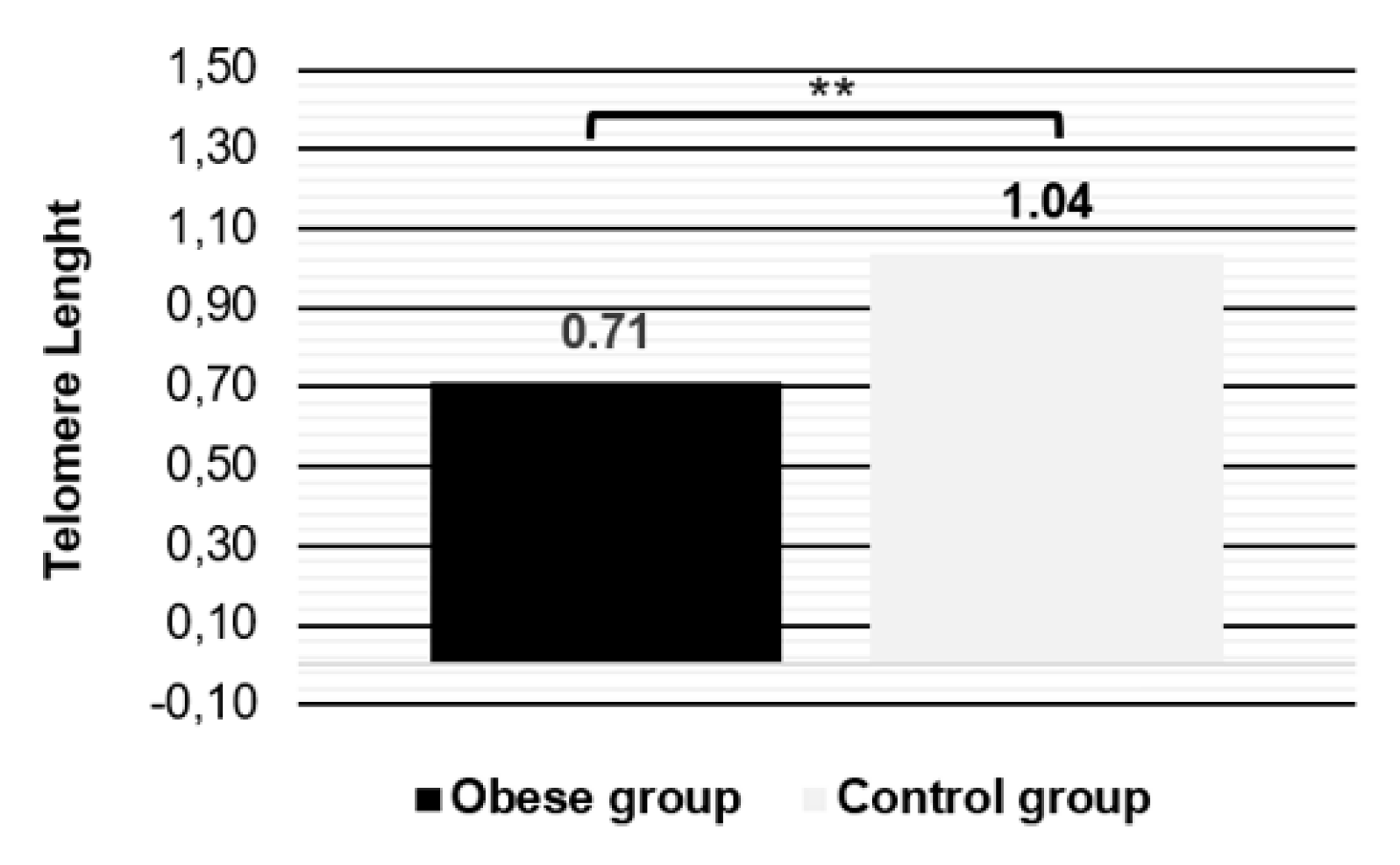
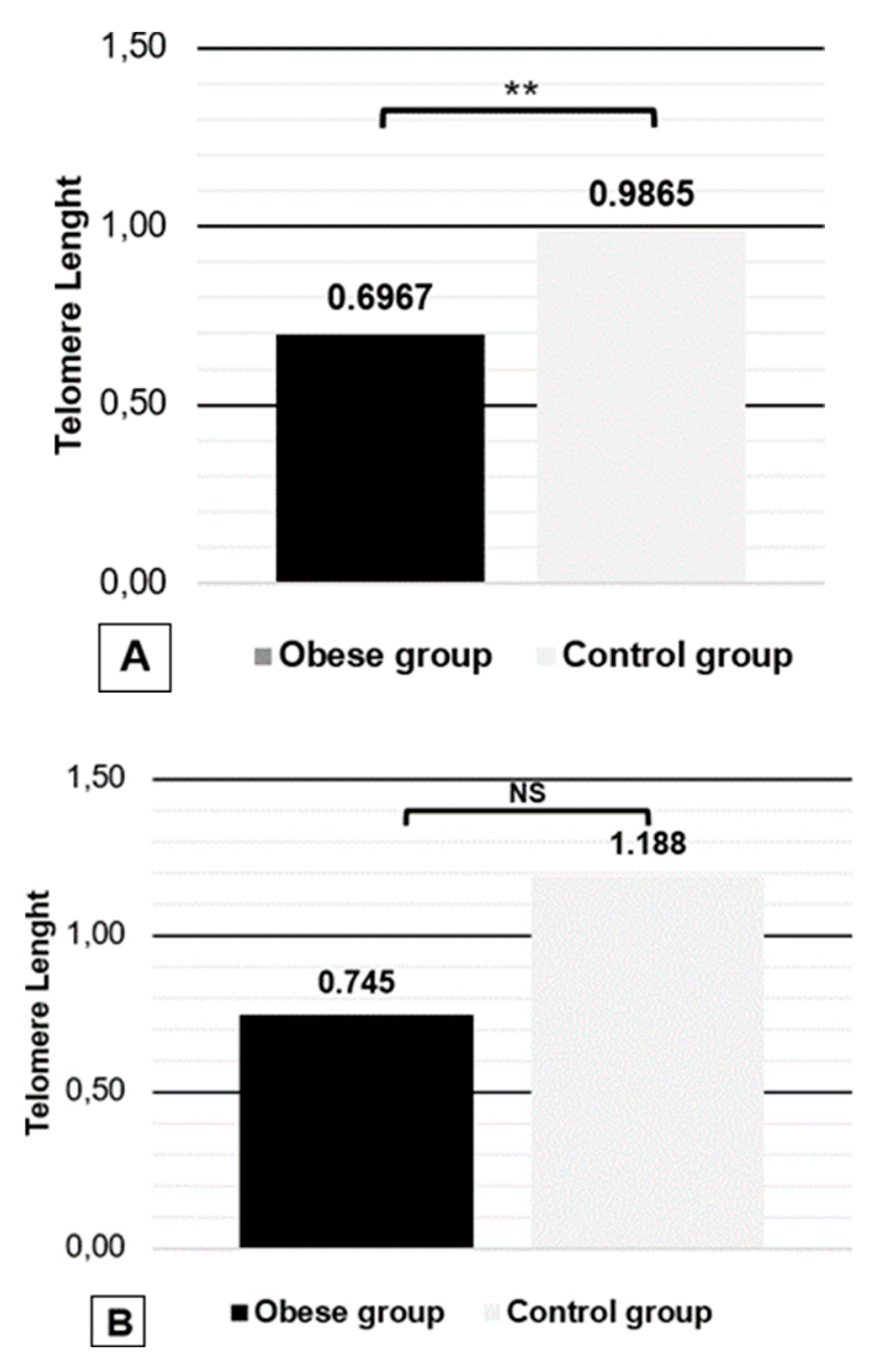
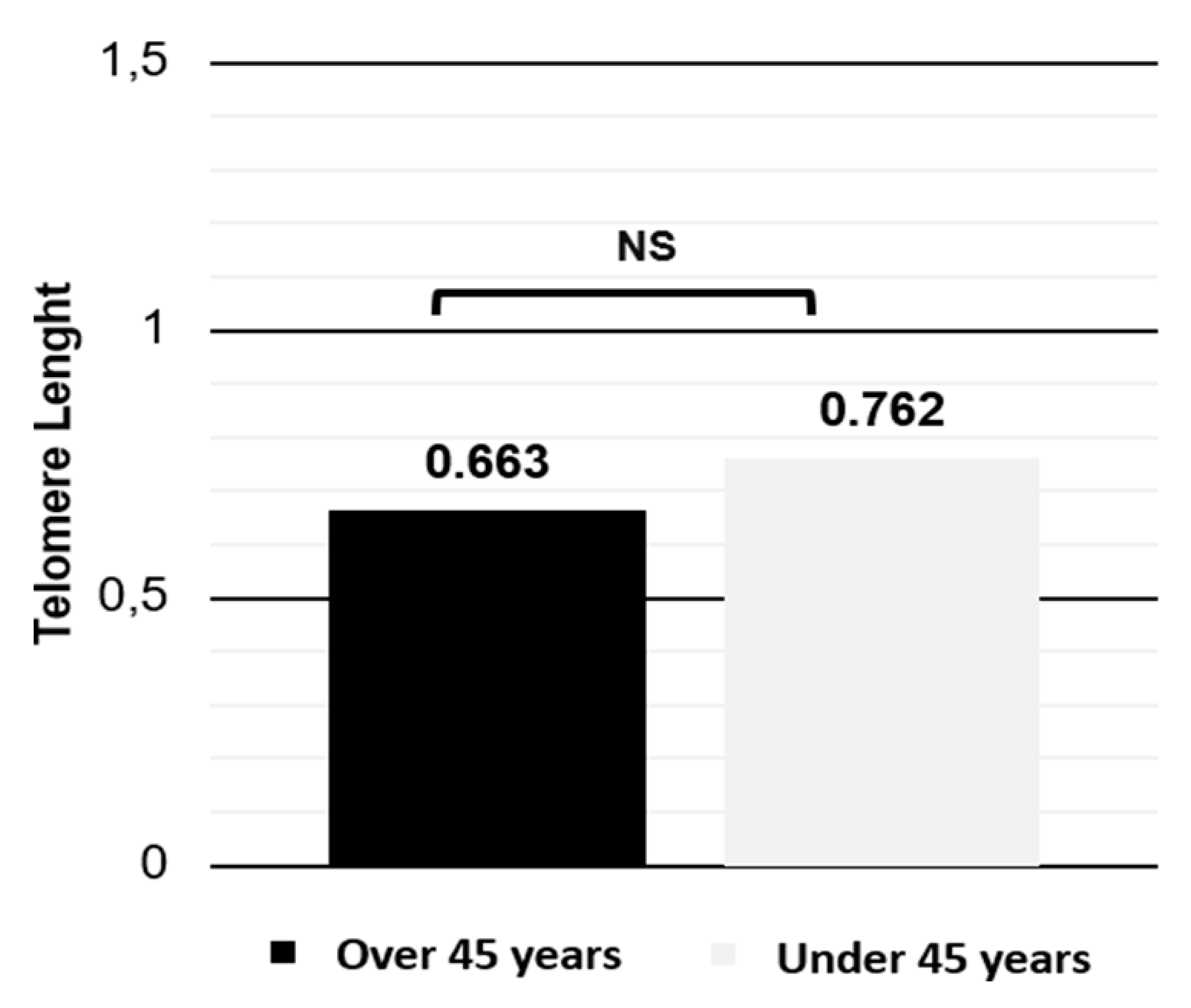
The relative telomere length is shorter in obese females and equal regardless the age
Discussions
Conclusions
Funding and Acknowledgments
Compliance with Ethical Standards
Conflicts of Interest
References
- Obesity and overweight. World Health Organization (2018). https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Obesity. World Health Organization. https://www.who.int/health-topics/obesity#tab=tab_1.
- Association of nutrition. APN: Obesity. http://www.apn.org.pt/v0E0J/nutrition-fact-sheets.
- Vidacek, N.Š.; Nanic, L.; Ravlic, S.; et al. Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J Gerontol A Biol Sci Med Sci. 2017, 73, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Wulaningsih, W.; Watkins, J.; Matsuguchi, T.; Hardy, R. Investigating the associations between adiposity, life course overweight trajectories, and telomere length. Aging 2016, 8, 2689–2701. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.H.; Iles, M.M.; Dunning, A.M.; Pooley, K.A. Telomere length and common disease: study design and analytical challenges. Hum Genet. 2015, 134, 679–689. [Google Scholar] [CrossRef]
- Blackburn, E.H. Telomeres and telomerase: the means to the end (Nobel lecture). Angew Chem Int Ed Engl. 2010, 49, 7405–7421. [Google Scholar] [CrossRef]
- Pfeiffer, V.; Lingner, J. Replication of telomeres and the regulation of telomerase. Cold Spring Harb Perspect Biol. 2013, 5, a010405. [Google Scholar] [CrossRef] [PubMed]
- Mathon, N.F.; Lloyd, A.C. Cell senescence and cancer. Nat Rev Cancer. 2001, 1, 203–213. [Google Scholar] [CrossRef]
- Yeh, J.K.; Wang, C.Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef]
- Iglesias Molli, A.E.; Panero, J.; Dos Santos, P.C.; et al. Metabolically healthy obese women have longer telomere length than obese women with metabolic syndrome. PLoS ONE 2017, 12, e0174945. [Google Scholar] [CrossRef]
- Mundstock, E.; Sarria, E.E.; Zatti, H.; et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity (Silver Spring) 2015, 23, 2165–2174. [Google Scholar] [CrossRef]
- Lin, J.; Smith, D.L.; Esteves, K.; Drury, S. Telomere length measurement by qPCR - Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology. [CrossRef]
- Dagnall, C.L.; Hicks, B.; Teshome, K.; et al. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLoS ONE 2017, 12, e0184098. [Google Scholar] [CrossRef]
- Finnicum, C.T.; Dolan, C.V.; Willemsen, G.; et al. Relative Telomere Repeat Mass in Buccal and Leukocyte-Derived DNA. PLoS ONE 2017, 12, e0170765. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Camacho, P.; Ribeiro, E.; et al. DNA methyltransferase expression (DNMT1, DNMT3a and DNMT3b) as a potential biomarker for anti-VEGF diabetic macular edema response. Eur J Ophthalmol. 2023, 33, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Thermo Fisher Scientific, editor. Qubit Fluorometric Quantification. https://www.thermofisher.com/pt/en/home/industrial/spectroscopy-elemental-isotope-analysis/molecular-spectroscopy/fluorometers/qubit.html?ef_id=a4a13beb609f178a16f6 5fe8c4fe9885:G:s&s_kwcid=AL!3652!10!77034693349276!77034707720811&cid=bid_pca_aqb_r01_co_cp1359_pjt0000_bid00000_0s e_bng_bt_pur_con&msclkid=a4a13beb609f178a16f65fe8c4fe9885.
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013, 3, 71–85. [Google Scholar] [PubMed]
- El Maï, M.; Bird, M.; Allouche, A.; et al. Gut-specific telomerase expression counteracts systemic aging in telomerase-deficient zebrafish. Nat Aging. 2023, 3, 567–584. [Google Scholar] [CrossRef]
- Bio-Rad Laboratories. Real-Time PCR Applications Guide 2006. https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_5279.pdf.
- Michael, W. Pfaffl. Relative quantification. 2004. https://www.gene- quantification.de/pfaffl-rel-quan-book-ch3.pdf. Published by International University Line (Editor: T. Dorak), pp. 63–82.
- Grun, L.K.; Teixeira, N.D.R., Jr.; Mengden, L.V.; et al. TRF1 as a major contributor for telomeres’ shortening in the context of obesity. Free Radic Biol Med. 2018, 129, 286–295. [Google Scholar] [CrossRef]
- Theda, C.; Hwang, S.H.; Czajko, A.; Loke, Y.J.; Leong, P.; Craig, J.M. Quantitation of the cellular content of saliva and buccal swab samples. Sci Rep. 2018, 8, 6944. [Google Scholar] [CrossRef]
- Nordfjäll, K.; Eliasson, M.; Stegmayr, B.; Melander, O.; Nilsson, P.; Roos, G. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008, 16, 2682–2689. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Rode, L.; Nordestgaard, B.G.; Weischer, M.; Bojesen, S.E. Increased body mass index, elevated C-reactive protein, and short telomere length. J Clin Endocrinol Metab. 2014, 99, E1671–E1675. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wen, J.; Qu, Q.; et al. Association of weight range with telomere length: A retrospective cohort study. Front Endocrinol 2023, 14, 1106283. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Hageman, G.J.; Antoniou, E.E.; et al. Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. Am J Clin Nutr. 2018, 108, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Renner, W.; Almer, G.; et al. Subcutaneous adipose tissue distribution and telomere length. Clin Chem Lab Med. 2019, 57, 1358–1363. [Google Scholar] [CrossRef]
- Toupance, S.; Karampatsou, S.I.; Labat, C.; et al. Longitudinal Association of Telomere Dynamics with Obesity and Metabolic Disorders in Young Children. Nutrients. 2022, 14, 5191. [Google Scholar] [CrossRef]
- Raftopoulou, C.; Paltoglou, G.; Charmandari, E. Association between Telomere Length and Pediatric Obesity: A Systematic Review. Nutrients. 2022, 14, 1244. [Google Scholar] [CrossRef]
- Lejawa, M.; Osadnik, K.; Osadnik, T.; Pawlas, N. Association of Metabolically Healthy and Unhealthy Obesity Phenotypes with Oxidative Stress Parameters and Telomere Length in Healthy Young Adult Men. Analysis of the MAGNETIC Study. Antioxidants 2021, 10, 93. [Google Scholar] [CrossRef]
| Obese group | Control group | |
|---|---|---|
| Age (mean ± s.d.) | 44.36 ± 11.09 | 25.46 ± 6.43 |
| BMI (mean ± s.d.) | 44.5 ± 7.99 | 21.7 ± 1.73 |
| Class I | 11% | N.A. |
| Class II | 20% | N.A. |
| Class III | 69% | N.A. |
| Total | 72 | 74 |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, C.; Braz, M.T.; Marques-Ramos, A.; Silva-Nunes, J.; Veiga, L.; Brito, M. Telomeres Are Shorter in Portuguese Obese Adults. J. Mind Med. Sci. 2024, 11, 381-387. https://doi.org/10.22543/2392-7674.1483
Duarte C, Braz MT, Marques-Ramos A, Silva-Nunes J, Veiga L, Brito M. Telomeres Are Shorter in Portuguese Obese Adults. Journal of Mind and Medical Sciences. 2024; 11(2):381-387. https://doi.org/10.22543/2392-7674.1483
Chicago/Turabian StyleDuarte, Catarina, Maria Teresa Braz, Ana Marques-Ramos, José Silva-Nunes, Luisa Veiga, and Miguel Brito. 2024. "Telomeres Are Shorter in Portuguese Obese Adults" Journal of Mind and Medical Sciences 11, no. 2: 381-387. https://doi.org/10.22543/2392-7674.1483
APA StyleDuarte, C., Braz, M. T., Marques-Ramos, A., Silva-Nunes, J., Veiga, L., & Brito, M. (2024). Telomeres Are Shorter in Portuguese Obese Adults. Journal of Mind and Medical Sciences, 11(2), 381-387. https://doi.org/10.22543/2392-7674.1483



