Introduction
Clinical and histopathological data support the notion that periodontal inflammation may contribute to neuronal dysfunction and degeneration [
1,
2]. Additionally, the immunological approach discusses the role of the immune system in the complex interplay between these two types of conditions. Among most studied neurodegenerative condition was: Parkinson’s disease (PD), Alzheimer’s disease and various types of dementia associated with neuronal degeneration) [
1,
2,
3].
Parkinson disease is a neurodegenerative condition, ranking second only to Alzheimer’s disease among progressive neurodegenerative disorders. Its prevalence is around 0.5–1% among individuals aged 65 and older [
1]. The condition predominantly affects dopaminergic neurons in the substantia nigra of the midbrain, leading to the gradual breakdown of neurological connections in the nigrostriatal pathway [
1,
2]. This degeneration results in motor, cognitive, and psychiatric symptoms. The classic manifestations associated with Parkinson’s disease encompass the trio of motor symptoms: tremor, rigidity, and bradykinesia, with postural instability becoming evident as the disease progresses. However, PD is also associated with numerous non-motor symptoms that often appear years or even decades before the onset of motor symptoms [
2,
4]. Multiple mechanisms have been implicated in the pathogenesis of Parkinson’s disease, with the aggregation of α-synuclein playing a central role in the development of the disease.
According to Braak and colleagues [
5,
6,
7], there is a significant prevalence of Lewy Body (LB) pathology associated with alpha-synuclein in the submandibular salivary gland and lower esophagus, followed by the stomach, small intestine, and colon. Lewy Bodies are abnormal proteins that develop inside nerve cells in the brain. They are a characteristic pathological feature observed in several neurodegenerative disorders, including Parkinson’s disease [
2]. Recent reports also suggest the presence of LBs in submandibular biopsy specimens from living individuals diagnosed with Parkinson’s disease (PD) [
7,
8]. Furthermore, the occurrence of LBs is believed to correlate with gastrointestinal dysfunction symptoms that precede the onset of motor symptoms in a considerable number of PD subjects.
Various processes are thought to contribute to PD, and several studies propose that abnormal protein clearance, mitochondrial dysfunction, and neuroinflammation play roles in the initiation and progression of the disease [
2]. While multiple risk factors, such as age, genetic predisposition, environmental toxins, infections, and oxidative stress, have been suggested, research also underscores the significance of the inflammatory response in the disease’s advancement [
9,
10,
11]. However, the interconnection between these pathways remains unclear.
Periodontal disease is an inflammatory condition affecting the supportive structures of teeth. While it involves a variety of microorganisms, gram-negative bacteria have been demonstrated to play a significant pathogenic role. These bacteria release toxins that contribute to both local and systemic inflammation, resulting in an increased production of pro-inflammatory mediators such as IL-1, IL-6, IL-17, TNF-a, and reactive oxygen species (ROS) [
12,
13,
14,
15]. The disturbance in the balance of inflammatory mediators serves as the foundation for the suggested connection between periodontal disease and other systemic conditions, including cardiac disease, diabetes, renal diseases, low birth weight, and neurodegenerative diseases [
16,
17,
18,
19].
Likewise, a plausible association between periodontal disease and the onset and progression of Alzheimer’s and Parkinson’s disease can be postulated, given the common factor of inflammation. Inflammation, a natural defense mechanism safeguarding the body against internal and external threats, plays a crucial role. Microglial cells, the primary immune-defensive cells in the central nervous system, can be triggered by systemic inflammation resulting from infections, initiating a cascade of neurodegeneration associated with various neurodegenerative diseases [
2].
The evolving understanding of neurodegenerative diseases such as Parkinson’s disease introduces the ’two-hit hypothesis,’ suggesting that the condition is complex and arises from multiple inflammatory triggers. The initial ’hit’ involves infectious agents, which sensitize the brain to subsequent ’hits’ that might be benign if the cells were not already in a ’primed’ state. In the aged or diseased brain, the presence of ’primed microglia,’ an atypical condition of microglial cells, can initiate an exaggerated response contributing to the progression of the disease [
9]. This heightened response may lead to microglial activation when exposed to a secondary pro inflammatory stimulus, resulting in the release of free radicals and inflammatory cytokines that elevate neurotoxicity and ultimately contribute to neurodegeneration [
1]. In this context, inflammation is primarily linked to microglial activation, playing a crucial role in the neurodegeneration of dopaminergic neurons in the substantia nigra.
Materials and Methods
This systematic review was conducted using the PICO (Population, Intervention, Comparison, Outcome) framework to investigate the following clinical question: “In adults with periodontal disease (P), does receiving assessment and appropriate treatment for the disease (I), compared to a control group receiving either no treatment or minimal treatment (C) impact the risk and severity of neurodegenerative conditions, such as Parkinson’s disease (O)? “
An exhaustive search was performed in databases including PubMed, Scopus and Web of Science, and targeting articles published between 2006 and 2023 that explore the link between both conditions. Keywords comprised “periodontal disease”, “Parkinson’s disease “, “mechanisms,”, “periodontal disease”, “clinical studies”, “microbiological studies,” “genetic studies,” and “cognitive deficit”. The initial search yielded a substantial number of articles.
Inclusion Criteria
Inclusion criteria were defined to select studies that met the focus and quality standards of this review. The selected studies encompassed various methodologies, including clinical trials, observational studies, experimental studies, and in-vitro research. To be included, studies had to directly examine or discuss the potential links between periodontal disease and Parkinson’s disease. Longitudinal studies and investigations discussing mechanisms, clinical associations, microbiological findings, genetic correlations, and cognitive impact were considered.
Patients diagnosed with periodontal disease according to criteria established by oral health and periodontal organizations.
Patients receiving dental assessment and treatment for periodontal disease.
Patients assessed for the presence and severity of neurodegenerative conditions (Parkinson’s disease diagnosis).
Age range (18-80 years).
Patients who can perform oral hygiene adequately according to the provided instructions (or their caregivers).
Exclusion criteria
Exclusion criteria were applied to eliminate studies that did not contribute to the understanding of the connection between the two conditions. Studies not relevant to the research focus, without available full-text, or with insufficient or inconclusive data also were excluded.
Patients under 18 years old.
Patients with acute forms of periodontal illness (abscess, pericoronitis, herpetic gingivostomatitis, aphthous stomatitis).
Patients with severe motor deficits that hinder oral hygiene practices and those with systemic conditions such as diabetes, cardiovascular diseases, immunodeficiency.
Pregnant woman.
Patients with known antibiotic allergies.
Study selection
Following the application of inclusion and exclusion criteria, a refined selection of articles was identified for detailed analysis. Full-text versions of the selected articles were retrieved for thorough review. Data extraction was carried out systematically to capture key information from each study. Extracted data included study title, publication year, study type (e.g., clinical trial, observational study, experimental study), main focus (sample, mechanisms, clinical associations, microbiological findings, genetic correlations, cognitive impact), and key findings (results or conclusion). Additionally, data regarding study participants, methodology, interventions, assessment tools, and statistical analyses were extracted as applicable.
Results
According to the study criteria, the research was performed using databases such as PubMed, Scopus, and Web of Science. Articles selected for inclusion were published between 2006 and 2023, focusing on the relationship between periodontal disease and Parkinson’s disease.
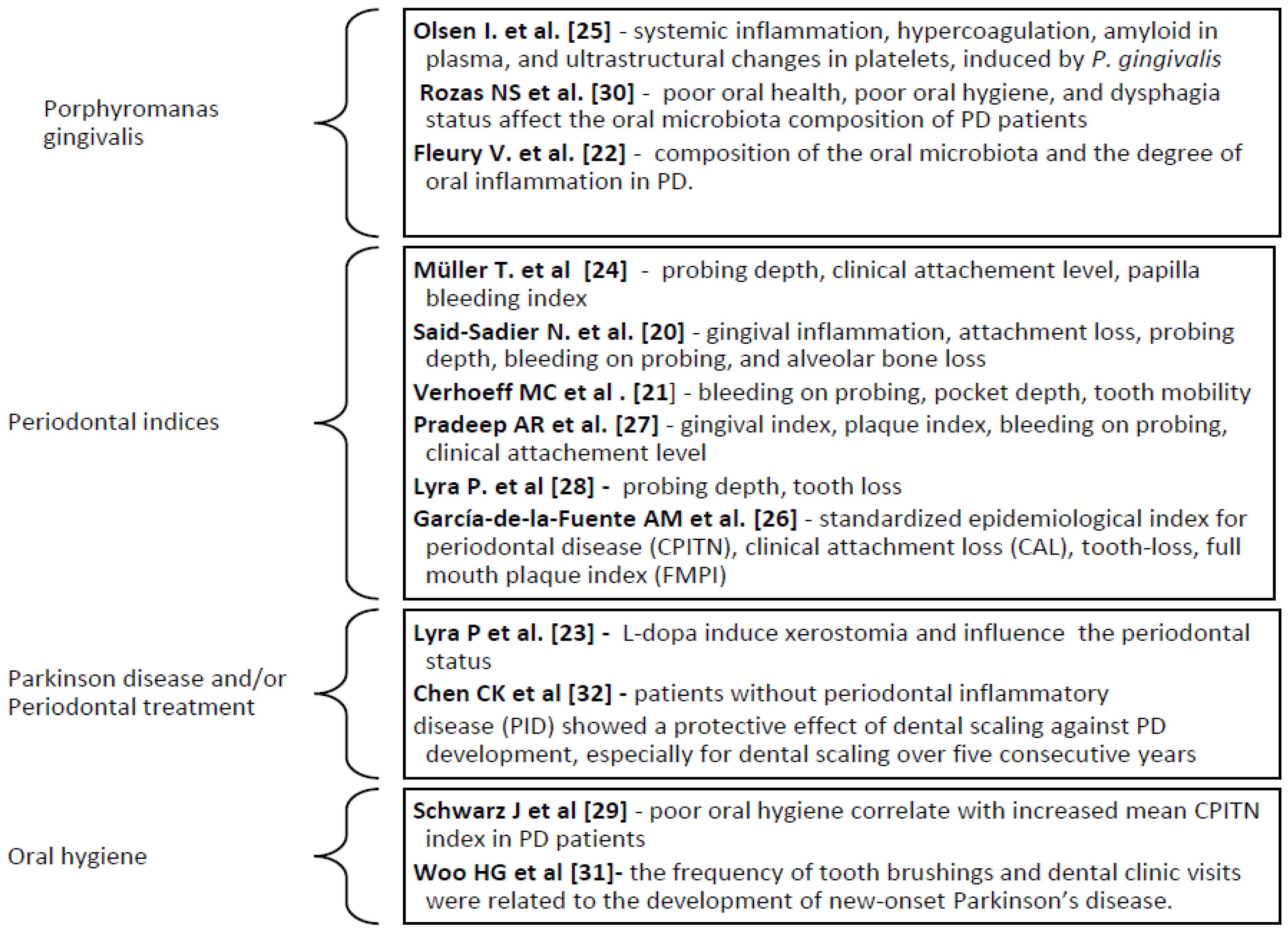
Following the analysis of the titles, abstracts and protocols of 46 studies, 18 articles were selected for detailed evaluation. After the evaluation in their entirety, 5 studies were excluded. Among the selected studies (Table 1) in the research, 4 are review studies [
20,
21,
22,
23,
24,
25] and 9 are randomized controlled trials [
26,
27,
28,
29,
30,
31]. The main indicators we followed in the research relate to: clinical signs of gingival inflammation, bleeding on probing (BoP), bone loss (BL), probing depth (PD), and clinical attachment loss (CAL) [
20,
21,
22,
23,
24,
27,
28]. Additionally, there was studies that monitored the levels of inflammatory markers such as epidermal growth factor (EGF), interleukin- 8 (IL-8), interleukin-17 (IL-17), interferon γ-induced protein 10 (IP-10), and monocyte chemoattractant protein-1 (MCP-1) [
21,
22]. The presence in the microbiota of patients diagnosed with Parkinson’s disease of one of the main periodontal pathogens was investigated in two studies (P. gingivalis) [
22,
25,
30] (
Figure 1).
The findings from the selected studies underscore the potential impact of coexisting periodontal disease on the severity of cognitive decline and motor dysfunction in patients diagnosed with Parkinson’s disease [
30,
31,
32]. The underlying mechanisms may involve the synergy of inflammatory responses triggered by both conditions, leading to an exacerbated neuroinflammatory cascade and neurodegeneration in individuals with Parkinson’s disease and periodontal disease.
The studies indicate a notably elevated prevalence of periodontal diseases among individuals with Parkinson’s disease, ranging between 20% to 94%, compared to those without Parkinson’s [
21]. From elevated bleeding indices to more pronounced issues like gingivitis, periodontitis, and tooth mobility, the oral cavity reflects the systemic challenges faced by PD patients. In terms of immunological aspects, study of Said-Sadier et al. identified that indicators, including gingival inflammation, attachment loss, probing depth, bleeding on probing, and alveolar bone loss, have correlations with cognitive impairment [
20]. Patients experiencing cognitive decline alongside preexisting severe periodontitis exhibit a reduction in epidermal growth factor (EGF), interleukin 8 (IL-8), interferon γ-induced protein 10 (IP-10), and monocyte chemo-attractant protein-1 (MCP-1), along with an overexpression of interleukin 1-β (IL-1β) (significant associations). Regarding on to the prevalence of periodontal diseases and the changes in the oral microbiome, a study revealed that Parkinson’s disease patients exhibit a higher prevalence compared to control subjects, pointing towards a potential connection between the two conditions [
21,
22,
23].
Also, Fleury et al. in a study [
22] delved into the microbiome changes in early and mid-stage Parkinson’s disease, noting differences in both saliva and subgingival dental plaque microbiota between patients and controls. Results indicate that the oral microbiome undergoes changes in early and mid-stage Parkinson’s disease (PD). Despite PD patients displaying good dental and periodontal health, local inflammation was evident in the oral cavity. The levels of IL-1 and IL-1RA were significantly elevated in PD patients compared to controls, with a tendency for an increased level of TNF-alpha in patients. Both saliva and subgingival dental plaque microbiota exhibited differences between patients and controls. Streptococcus mutans, Kingella oralis, Actinomyces, Veillonella, Scardovia, Lactobacillaceae, Negativicutes, and Firmicutes were more abundant in patients with PD, while Treponema, Lachnospiraceae were less abundant.
The oral health issues highlighted by Müller et al. that patients with Parkinson’s disease exhibited a less favorable oral health in comparison to the control subjects [
24]. Lower frequencies of toothbrushing and dental visits were noted, along with compromised oral self-cleaning mechanisms, evident through reduced salivary flow in PD subjects. Additionally, more pronounced gingival recession and tooth mobility were observed in individuals with PD when compared to the control subjects.
Study of Olsen et al. provided support for the hypothesis implicating P. gingivalis in the development of PD, identifying specific components of this periodontal pathogen in the systemic blood circulation of individuals with PD [
25]. Contrastingly, a study highlights the notion of a higher prevalence of periodontitis or tooth loss in PD patients [
26]. Instead, it emphasized an increased clinical attachment level associated with challenges in controlling dental biofilm due to progressive neurological changes accompanying the disease.
Study of Pradeep et al.l [
27] unveiled significant statistical variances in periodontal parameters among subjects with Parkinson’s disease establishing a direct link between the severity of PD and the escalation of periodontal indices. Significant statistical differences were observed in periodontal parameters between subjects with Parkinson’s disease and controls. As the severity of PD increased, all evaluated periodontal clinical parameters and indices demonstrated a deterioration. The mean probing depth value increased from 2.75 mm to 6.17 mm, and the mean clinical attachment level (CAL) value increased from 3.14 mm to 6.74 mm. The mean gingival index (GI), periodontal indices (PI), and %BoP (bleeding on probing) values rose from 0.55, 1.35, and 20.37 to 2.66, 3.80, and 70.86, respectively, with increasing severity of PD.
In the investigation of tooth loss and edentulism, a study disclosed proportions of severe tooth loss and complete edentulism in PD patients [
28]. Intriguingly, there was no discernible evidence indicating a connection between the number of missing teeth and Hba1c levels. However, blood pressure levels in PD individuals exhibited a correlation with the number of missing teeth. The mean Community Periodontal Index of Treatment Needs (CPITN) indices depicted a divergence between patients and controls, as documented in Schwarz study [
29], with gender disparities observed within the control group. Additionally, it appears that females in the control group exhibited lower CPITN indices in all sextants compared to male controls. However, this gender difference was reversed among individuals with Parkinson’s disease. It is believed that challenges in oral hygiene play a role in the heightened periodontal pathology seen in Parkinson’s patients, potentially further impacting their overall quality of life.
Rozas et al. delved into distinctions within the oral microbiota of PD individuals and controls, attributing potential influences to factors such as dysphagia, drooling, and salivary pH [
30]. Emphasizing tooth loss as a potential harbinger for PD development, one study brought to light a correlation between the number of lost teeth and an augmented risk of new-onset PD [
31]. Also, Chen et al. study elucidated the protective impact of dental scaling, suggesting a more pronounced effect in PD individuals compared to their counterparts without PD, irrespective of continuous application over a five-year period [
32].
Discussions
The link between these oral manifestations and the neurological aspects of PD raises intriguing questions about the bidirectional relationship between the two. From the studies that formed the basis of this research, three conclusions were noted, as follows.
Microbial Dynamics and PD Development: The oral microbiome, a diverse ecosystem of microorganisms inhabiting the mouth, has emerged as a key player in the periodontal disease patients. Changes in the oral microbiome during early and mid-stage periodontal disease, coupled with the identification of specific components of the periodontal pathogen (P. gingivalis in the systemic circulation) suggest a microbial dimension to Parkinson’s disease development [
24,
25].
Tooth Loss, Blood Pressure, and PD Risk: Tooth loss, often considered a consequence of local factors, takes on a broader significance in the context of periodontal disease. The association between tooth loss and increased risk of new-onset Parkinson’s disease, coupled with correlations to blood pressure levels, emphasizes the systemic repercussions of oral health. These findings imply that the consequences of oral health extend beyond the oral cavity, influencing conditions with far-reaching impacts [
26,
28,
31].
Periodontal disease treatment. The protective effect of dental scaling as part to periodontal treatment, particularly in individuals with Parkinson’s disease, adds a preventive dimension to the discussion. Consistent scaling over five years is associated with a significantly lower risk of developing periodontal disease. This highlights the potential for proactive oral health measures to serve as a shield against the onset of neurological conditions, urging a reconsideration of the traditional boundaries between oral and systemic health [
30,
31,
32].
The studies from the research emphasize the critical role to maintain optimal oral hygiene in patients with both periodontal disease and Parkinson’s disease [
21,
22,
23,
24,
25,
26,
27]. Poor oral hygiene is commonly observed in patients with Parkinson’s disease. The motor symptoms of Parkinson’s, such as tremors and muscle rigidity, can make it challenging to perform routine oral hygiene effectively. Brushing and flossing, crucial for maintaining oral health, may become difficult, leading to an increased risk of dental issues including periodontal disease.
Additionally, the side effects of medications commonly used to manage Parkinson’s symptoms, such as dry mouth (xerostomia), further contribute to oral hygiene challenges.
The medication used for patients in the current studies with Parkinson’s disease are dopaminergic medication and L-dopa equivalent (Levodopa, Carbidopa, COMT inhibitor, MAO inhibitor) [
20,
21,
22,
23,
30].
Reduced saliva flow can create an environment conducive to bacterial growth, leading to an elevated risk of cavities, gum disease, and other oral health problems. This underscores the importance of proactive measures to manage dry mouth symptoms and prevent related oral health complications. It becomes evident through the literature that collaborative efforts between dental and medical professionals are imperative. Such collaboration is crucial for providing comprehensive care that addresses the specific challenges faced by individuals dealing with both Parkinson’s disease and periodontal issues, as outlined in the existing body of research.
Regular dental and neurological follow-up are crucial for preventing periodontal disease and maintaining the results of periodontal treatment in the context of Parkinson’s disease. These routine consultations with both a dentist and a neurologist play a key role in addressing the specific oral health needs of individuals with Parkinson’s, ensuring early detection of any dental issues, and optimizing the overall management of the disease. Proactive preventive measures and consistent follow-up care contribute significantly to the oral and overall well-being of patients with Parkinson’s disease
The study of Leira et al. from 2024 concludes that patients with periodontitis have a 1.7 times greater risk of developing neurodegenerative disease compared to those without periodontal disease [
33]. Patients with periodontitis exhibit poorer performance in various neuropsychological assessments of cognitive function. Also, the study suggests that periodontitis induces recurrent episodes of bacteremia and endotoxemia, contributing to a state of low-grade chronic inflammation that significantly contributes to the development of neurodegenerative processes associated with cognitive dysfunction [
34]. However, limited interventional clinical trial has been published on the effect of periodontal treatment in the primary and secondary prevention. The study of Schwahn et al. indicate a significant decrease in dementia risk associated with various oral health interventions, including periodic visits to the dentist, and a beneficial effect of periodontal treatment on brain atrophy [
35].
This is in line with the specialized literature, which specifies that periodontal pathogens have the capacity to stimulate the production of proinflammatory cytokines. [
3,
11]. While these cytokines are typically degraded locally, they often enter the systemic circulation. In advanced stages, periodontal disease gives rise to systemic inflammation, evidenced by increased levels of C-reactive protein in the blood of individuals with periodontitis compared to control groups [
11,
12]. Additionally, current data suggest that lipopolysaccharide (LPS) from the structure of gram-negative bacterial walls plays a crucial role in contributing to the development and progression of neurodegenerative diseases [
13,
14,
15]. Consequently, the bacterial structure and the generated inflammatory mediators have the potential to breach the blood-brain barrier, potentially fostering the progression of conditions such as Parkinson’s disease.
The second significant mechanism involves the neuronal pathway, where in inflammation is transmitted via the autonomic nervous system (ANS). Through this pathway, peripheral signals can rapidly elevate proinflammatory cytokine levels in the brain. Studies propose that patients with periodontal disease may experience the transmission of inflammatory signals from the oral cavity through a neuronal mechanism, potentially exacerbating symptoms of neurodegenerative diseases [
3,
4,
5].
Porphyromonas gingivalis, the primary gram-negative bacteria responsible for periodontal disease, alters microbial flora, promoting a state of dysbiosis [
16]. During routine activities like brushing, flossing, chewing, and dental treatments, P. gingivalis enters into the vascular system through the damaged periodontium. This entry has been shown to increase the permeability of the blood-brain barrier, facilitating bacterial access to the brain [
16,
17,
18,
19]. Out of the 13 studies that serves as the basis of the current research, 4 studies specifically investigated the significance and mechanism of Porphyromonas gingivalis as a linking factor between periodontal disease and Parkinson’s disease [
20,
24,
25,
31]. These 4 studies align with the specialized literature regarding the understanding of the mechanisms of periodontal disease.
These gram-negative bacteria exhibit direct mechanisms, contributing to the deterioration of periodontal tissue and indirect mechanisms that amplify inflammation and pathology associated with periodontal disease. Direct mechanisms of bacterial factors are related to bacterial structure, as well as the release of endotoxins, exotoxins, and enzymes. The indirect mechanism refers to immune response reactions along with pro-inflammatory factors that occur [
34]. Porphyromonas gingivalis secretes proteases, such as gingipains, contributing to the degradation of connective tissue [
2]. Bacterial fimbriae facilitate the adherence of P. gingivalis to dental surfaces and host tissues, thereby facilitating invasion and colonization. Endotoxins, such as lipopolysaccharide, when circulating in the systemic vascular system, can lead to various conditions, including Parkinson’s disease. Moreover, it activates inflammatory pathways by stimulating the release of pro-inflammatory cytokines [
19]. The bacteria interfere with the host’s immune response by inhibiting neutrophil activity and modifying the gene expression of cytokines, exacerbating the inflammatory process. Also, P. gingivalis participates in biofilm formation, facilitating the adherence of other periodontal pathogens and protecting them against the immune system. The relationship between gingival fibroblasts, the lipopolysaccharide produced by P. gingivalis, and Parkinson’s disease is a subject of ongoing research. However, preliminary studies suggest the potential for an interaction between chronic gingival inflammation and the development of Parkinson’s disease. This bacterium produces lipopolysaccharide (LPS), a component known for its inflammation-inducing capability. While the precise factors triggering Parkinson’s disease are not fully understood, chronic inflammation and oxidative processes have been implicated in its pathogenesis [
18,
19].
Additionally, certain studies demonstrate that P. gingivalis can evade immune surveillance by utilizing autophagy, allowing its survival within dendritic cells (DCs) [
14]. Yet, recent research indicates that P. gingivalis and LPS enhance autophagic activity, suggesting that periodontal pathogens influence autolysosome formation, leading to the accumulation of autophagosomes that provide nutrients for their survival [
16]. The research conducted by Olsen et al. [
25] offered backing for the hypothesis linking P. gingivalis to the progression of PD. They identified distinct components of this periodontal pathogen circulating in the systemic blood of individuals with PD.
The AKT-mTOR known as phosphatidylinositol-3-kinase (Akt) and the mammalian target of rapamycin (mTOR) signaling pathway plays a role in regulating various cellular processes, including cell survival, growth, and proliferation. Overactivation of the AKT-mTOR pathway may contribute to the inflammation, oxidative stress, and neuronal dysfunction observed in patients with Parkinson’s disease [
16].
Also, activation of the Akt/mTOR signaling axis suppresses antimicrobial autophagic mechanisms, resulting in intracellular survival of P. gingivalis. On the other hand, autophagy also induces a type of cell death against infection with periodontal bacteria [
15]. In the context of periodontal disease, AKT-mTOR may contribute to the development of gingival inflammation and the destruction of periodontal tissues. Typically, AKT and mTOR are signaling molecules involved in cell growth and survival. However, during periodontal inflammation, these mechanisms can be disrupted, leading to an excessive release of enzymes and proinflammatory cytokines, ultimately resulting in the destruction of periodontal tissues and tooth loss [
10,
11].
The AKT-mTOR mechanism also can influence the expression of genes involved in dopamine synthesis and secretion. Additionally, it plays a role in regulating autophagy, the process of eliminating abnormal proteins from cells. Dysregulation of the AKT-mTOR mechanism can impact the autophagy process, contributing to the accumulation of abnormal proteins and the destruction of neurons [
16]. Also, it has been demonstrated that lipopolysaccharide (LPS) induces autophagy in human gingival fibroblasts (HGFs) by inhibiting the Akt/mTOR signaling pathway [
9]. Moreover, autophagy plays a role in restricting the release of proinflammatory cytokines, providing insight into the relationship between autophagy and inflammation. Autophagy has a dual role in responding to periodontal pathogens. Firstly, it enhances the survival of periodontal pathogens, as host-adapted pathogens exploit host autophagy for survival and persistence within the host [
15]. Some periodontal bacteria have evolved mechanisms to utilize the host’s autophagic response, providing a means for bacteria to evade the host’s immune defenses. On the other hand, apoptosis and autophagy represents crucial regulatory cellular processes which are presumed from studies in the specialized literature to be involved in the connection between Parkinson’s disease and periodontal disease. Apoptosis is a programmed cell death mechanism, and its excessive activation can play a role in the neuronal loss seen in Parkinson’s disease. Simultaneously, autophagy serves as a cellular process for recycling and eliminating damaged cellular components, and dysregulation in autophagy may be linked to the accumulation of proteins and toxic substances in neuronal cells, phenomena associated with Parkinson’s disease [
16].
Regarding the IL-17 concentration, elevated levels have been reported in periodontal tissues in inflammation [
19]. IL-17 can contribute to inflammation and tissue damage by stimulating the production of other inflammatory cytokines and enzymes. In this context, IL-17 and inflammation can play a role in triggering and progressing Parkinson’s disease by promoting inflammation and oxidative stress in the central nervous system [
19].
In summary, potential connections and interactions exist between periodontal disease, IL-17, apoptosis, autophagy, and the AKT-mTOR pathway. However, it is crucial to acknowledge that these associations are not fully understood and necessitate further research to establish a clear relationship between these conditions. Therefore, the main conclusions from the specialized literature regarding the identified pathophysiological mechanisms between neurodegenerative diseases and periodontal disease are as follows.
Inflammation. Both diseases are associated with chronic inflammatory processes. In periodontal disease, inflammation is caused by bacteria present in dental plaque. This chronic inflammation can directly affect the supporting structures of the teeth and contribute to the destruction of periodontal tissues. In Parkinson’s disease, there is evidence that chronic inflammation and an exaggerated immune response may play a role in the destruction of dopaminergic neurons in the brain [
20,
21,
22,
23].
Immune response. An exaggerated immune response can play a role in both conditions. In periodontal disease, the host’s immune response can lead to the destruction of periodontal tissues in an attempt to eliminate bacteria in affected areas. In Parkinson’s disease, there are theories suggesting that an abnormal immune response may contribute to the destruction of dopaminergic neurons [
11].
Oral microbiome. The oral microbiome represents the complex community of bacteria living in the oral cavity. There are studies suggesting that certain types of oral bacteria associated with periodontal disease can migrate to the brain and trigger an inflammatory reaction, which may contribute to the damage of dopaminergic neurons in Parkinson’s disease [
24,
25,
30].
Oxidative stress. Both Parkinson’s disease and periodontal disease are associated with an imbalance in the body’s antioxidant system, known as oxidative stress. This disorder can lead to cell and tissue damage, including damage to brain neurons and periodontal tissues [
20].