Abstract
Background. The objective of this study was to describe the histological and immunohistochemical aspects of vomeronasal organ in two groups of (normal and pathological) embryos and fetuses, with chromosomal morphological abnormalities. Methods. The research was based on a retrospective, descriptive analysis, carried out over a period of 5 years. The study included 46 biopsy fragments taken from aborted embryos and fetuses aged between 9 and 23 weeks. We compared the microscopic structure of the vomeronasal organ using different histologic and immunohistochemical staining procedures. Results. Our results showed that in both groups of embryos and fetuses there are three major stages of histological development of the vomeronasal organ, depending by intrauterine age. We also observed that in the group of abnormal embryos and fetuses, although the morphological and immunohistochemical characteristics of vomeronasal organ were similar to the control group, the specific structures of the vomeronasal organ persisted beyond 22 weeks. In the control group, the vomeronasal organ was no longer visible at this age. The results of the immunohistochemical study indicated that the positive surface for cytokines in the control group decreased with age, and in the group with morphological anomalies, the number of positive cells was low. On the other hand, in fetuses with chromosomal anomalies, the positivity, although a slight downward trend was recorded, it remained high. Conclusions. This study is still ongoing in order to identify the increased frequency of vomeronasal organ in fetuses. Moreover, we anticipate that our study can be a starting point for other researchers in order to identify a relationship between the behavioral pattern of the fetuses with trisomy 21 and the persistence of the VNO.
Introduction
The vomeronasal organ (VNO) belongs to the secondary olfactory pathway(s) and it is a bilateral chemosensory structure located in the mucous membrane of the nasal septum of most mammals. This structure is extremely variable in terms of anatomical development and functionality and humans possess a non-chemosensory homologue of this, whose function is still a subject of debate.
The VNO is an epithelial, tubular-shaped invagination of the nasal mucosa which is located in the anterior-inferior part of the nasal septum and is responsible for the detection of different chemical structures, such as pheromones and kairomones [1,2]. The VNO has a specific and unique morphology which is described as a yellowish mucosa, best visualized in pit-shaped structures, with an active secretion of mucus, that sometimes could lead to the formation of a mucus bubble on the opening of the organ [3,4]. The vomeronasal cavities can be observed by endoscopy in some human adults, but they lack sensory neurons and nerve fibers [1,2,3]. A small nerve fiber located medially to the VNO and connected with this, was described. It runs longitudinally to the nasal septum and it is considered by some investigators to be a distant process of the Cranial Nerve 0 or terminal nerve. Even though the presence of the VNO is variable in human adults, a wide range of cohort studies suggested the presence of the VNO’s structure in all the examined fetuses [4,5].
In human embryos, the nerve fibers which connect VNO with the hypothalamus represent the pathway that allows the migration of GnRH-secreting cells from the olfactory placode to the hypothalamus. After this essential step of neural migration, the fetal VNO regresses and the neural connections disappear [5,6]. If we look for the nerve connections between VNO and hypothalamus (by accessory olfactory bulbs) at the human adults, we can observe that there are no nervous pathways, nor an accessory olfactory bulb to link to the VNO’s receptor cells, raising thus numerous questions in terms of their functionality. In other species, the VNO is innervated by a nerve’s projection of the accessory olfactory bulb, connected further to the hypothalamus and amygdala.
Some studies have hypothesized that the vomeronasal nerve is a distant projection of the terminal nerve, also called cranial nerve 0, which is an unmyelinated nerve and its neuronal cells contain gonadotropin-releasing hormone (GnRH) [7]. Several researchers have concluded that the VNO undergoes cyclic changes and becomes hypertrophic in certain period such as ovulation and spring [6,7].
Evolutionary Stages of the VNO Development
Primary neurogenesis consists of the formation of the tubular VNO between weeks 7-10 through the invagination of the olfactory placode, that forms the olfactory groove. The olfactory tract is divided into an antero-medial sensory epithelium and posterolateral respiratory epithelium. Until E11.5, the VNO thickens on the medial walls of the olfactory groove which continues to invaginate towards the mesenchyme, in order to form the vomeronasal groove [1]. During this stage, two populations of neural precursors were identified: slow-proliferating precursor cells, self-renewing, that express Meis 1/2 at high levels and rapid-proliferating precursor cells that express high levels of Sox2 and Asc118. The epithelium of the developing organ contains three primary types of cells: Hes-1 + -towards the lumen, Asc11+ -basement membrane and sensitive vomeronasal neurons – intermediate zone [2]. After E13.5, the length and size of the VNO start to increase and its shape resembles a kidney with a crescent shaped lumen that separates the sensory and non-sensory epithelia [2,3,4].
Early neurogenesis is characterized by migratory neurons that were formed during the primary neurogenesis phase and include immunoreactive cells for gonadotropin-releasing hormone 1 (GnRH-1), gamma amino butyric acid (GABA) and tyrosine hydroxylase [2]. It is considered that disorders in the formation and migration of GnRH-1 neurons negatively affect the sexual development and fertility of mammals. Certain studies showed that GnRH-1 neurons migrate along the axons of the terminal nerve to the brain, which is considered a transient caudal branch of the vomeronasal nerve [8,9]. During the established neurogenesis, the VNO is differentiated into medial sensory and lateral non-sensory epithelium, both separated by the lumen [10]. As age increases, the neurons formed on the non-sensory side tend to undergo cell death [2]. This period is characterized by the pseudostratified type of epithelium and contains three types of cells: clear, dark and basal cells. The VNO increases in volume with age due to hypertrophy and it represents the final prenatal stage of the human VNO development. Vomeronasal nerves can be identified up to E18 and after this period they can degenerate [11].
Materials and Methods
The objective of this study was to describe the histological and immunohistochemical aspects of VNO in two cohorts of normal and pathological embryos and fetuses. We also followed the chronological development of the VNO during embryogenesis (reported by weeks) and we realized a comparative evaluation of these stages in the two study groups. Another objective of this study was the application of immunohistochemical techniques in order to detect receptor proteins involved in olfaction, secretory epithelial cells and axonal proteins.
The research was conducted into the Clinical Hospital of Obstetrics and Gynecology “Dr. I.A. Sbarcea” Brasov and in the University of Transilvania, Faculty of Medicine, Brasov. The research was based on a retrospective, descriptive analysis, carried out over a period of 5 years (2017-2022). The study included 46 biopsy fragments taken from aborted embryos and fetuses aged between 9 and 23 weeks, which were sent to the Department of Pathological Anatomy of the Clinical Hospital of Obstetrics and Gynecology Brasov, after the informed consent of the parents or legal representatives was given.
The study was divided into two main groups: the control group comprising 18 normal embryos and fetuses spontaneously aborted, as a result of maternal pathologies, without any chromosomal or anatomical abnormalities and the study group which consisted of 22 aborted embryos and fetuses, of which 10 were diagnosed with chromosomal abnormalities – trisomy 21, 6 were diagnosed with complex anatomical anomalies incompatible with life and 6 were excluded due to the impossibility of detecting the nasal septum because of the process of accentuated lysis or because its absence. The diagnoses of chromosomal abnormalities were made through ultrasound and genetic screening tests at the beginning of the pregnancy, and the therapeutic interruption of the pregnancy course was recommended. The exclusion criteria were the following: macroscopic absence of the nasal septum or the upper wall of the oral cavity, hence, the study group consisted of 34 subjects.
All the biopsy fragments were fixed in less than 30 minutes after their extraction in 10% buffered paraformaldehyde in order to avoid tissue damage and the inherent enzymatic cascades. Before dissection, we measured the length of the vertex coccyx (LVC) and the head circumference (CC) of each fetus in order to determine the fetal age. The cephalic extremities of the aborted embryos and fetuses were separated from the throat in sagittal plane. A frontal section was made in order to detach the nasal pyramid and make a section corresponding to the sampling box (28.5 × 41 × 6.7 mm). The samples were further processed using a Leica Histocore Pearl automatic histoprocessor using the following reagents: 4% PFA, distilled water, ethanol and xylene. The paraffin embedding was done manually. The paraffin block was shaped and then mounted on the microtome support. A Leica RM2235 manual rotary microtome was used to section the paraffin blocks. The 3 micrometer thick sections were separated and exposed at a temperature of 45-500C. After a few minutes, each section, in the order of cutting, was collected on the microscope slide by immersing it in water at an angle of 45-50 degrees and lifting the section on it. Later, the slides with the sections were dried at room temperature for several hours. The adhesion to slide was made by heating the slides on special racks in preheated oven at 600C.
Histochemical stains, both usual and special, and immunohistochemical stains were used to stain the slides. The histochemical stainings included: hematoxylin eosin (HE), Alcian Blue staining, Periodic Acid Schiff technique, Van Gieson, Trichrome Masson technique and Grimelius staining. Immunohistochemical techniques were used to highlight different phenotypes of the cells and the antibodies that we used were the following: CK5/6, CK8/18, anti-EGFR, anti-S100 protein, anti SOX2, anti-synaptophysin antibody, and anti-p63 antibody. A X40 quantification factor has been used. In order to quantify the intraepithelial immunohistochemical expression, the area of positive reaction relative to the total cross-sectional area of the vomeronasal epithelium was calculated. Using a specific software, we performed the delineation of the positive reaction surface for the given marker. These areas were referenced to the actual thickness of the epithelium in each case. Then, we measured the ratio of positive reaction for each region of interest and calculated the mean to find the percentage of area with positive reaction compared to the entire surface of the epithelium of the VNO. For the values obtained for the fetuses included in the same age group, we also calculated the average to obtain a characteristic result for each group. The entire process of obtaining macroscopic samples and making paraffin blocks is illustrated in Figure 1 and Figure 2.
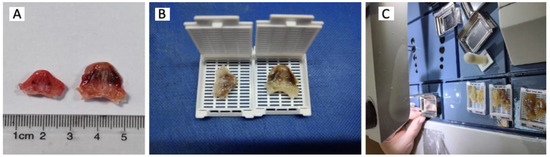
Figure 1.
The macroscopic sections. A – Measuring the sections. A section in the frontal plane was made to detach the nasal pyramid and perform a section corresponding to the sampling box. B -After sectioning and macroscopic orientation, the samples obtained were placed on classic histology cassettes (dimensions 28.5 × 41 × 6.7 mm) to continue the following operations. C -In the last step, the mold containing the sample and the melted paraffin is placed on a cold table where the paraffin will harden. After a few minutes the metal part will be removed and thus the paraffin block is obtained.

Figure 2.
The preparation of paraffin blocks. A -The paraffin block was shaped (its edges that did not contain the sample were excluded) and then it was mounted on the special support of the microtome. B – Obtaining the samples. If the objective section showed the vomeronasal organ, the block was remounted on the microtome and 15 sections as serial as possible were sectioned and collected. C - The 3 micrometer thick sections were separated and then spread out -spread out on a bath of warm water at a temperature of 45-50C. This step ensures that the sections are stretched and practically cancels the folds or overlaps resulting from the sectioning by softening the paraffin and stretching it at the water-air interface. D - After a few minutes, each section was collected on the blade by immersing it in water at an angle of 45-50 degrees and lifting the section on it.
Results
Histological Aspects of the VNO in Normal and Abnormal Embryos and Fetuses
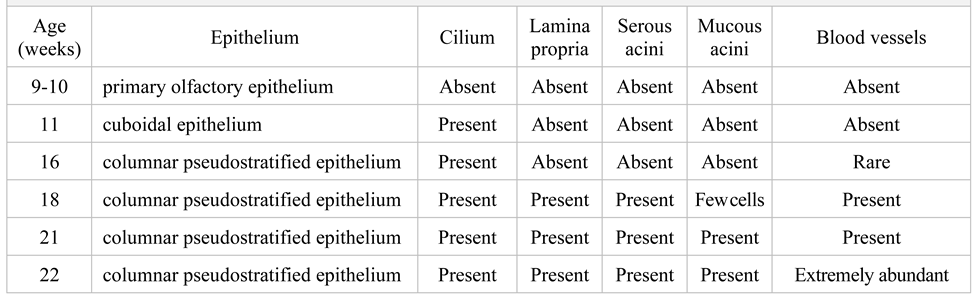
In similar microscopic studies previously published in the literature, which were carried out on normal fetuses, three major stages of development of the VNO were observed. In our study we also identified the same three stages. The presence of the VNO was found at 10 weeks, as the first stage of development. In the second stage, a gradual replacement of the receptor cell population with ciliated cells was observed between 10-14 weeks. In the third stage, the volumetric increase of the vomeronasal epithelium and the luminal expansion was observed from 14 weeks. The VNO subsequently persisted throughout the embryonic and fetal development. In Table 1 we illustrated the histological structure evolution stages. Also, the presence of the VNO was significantly higher in the control group in comparison with to the group with chromosomal abnormalities.

Table 1.
The evolutionary histological stages of the VNO development.
Histological aspects of the VNO, using hematoxylin-eosin staining, in normal and affected embryos and fetuses are illustrated in Figure 3.
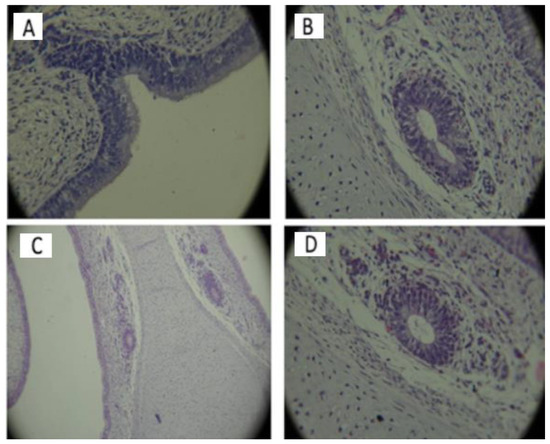
Figure 3.
The VNO detection with hematoxylin-eosin staining. (A) 9-weeks embryo, without chromosomal anomalies, in the initial stage of the formation of the VNO can be observed through the invagination of the mucosa lining the nasal cavity (40x.). (B) 12-week embryo, without chromosomal abnormalities. The basal membrane is located on the outside part, on which are located cubic basal cells in a single layer that include centrally located rounded nuclei. Epithelial cells can be seen delimiting the round-oval lumen on the right side of the image and slightly irregular on the contralateral side. Reduced amount of secretions in the interior of both VNOs (40x.). (C) 16-week fetus with chromosomal anomalies -the presence of bilateral VNO, with different shape and size of the organs. No ciliated cells are observed. The presence of nerve fibers, well delimited, with an upward trajectory is highlighted (10x.). (D) 16-week fetus with chromosomal abnormalities with the presence of the bilateral VNO, with cellular detritus. Nerve threads bilaterally present (40x.).
In the first stage, the VNO is lined by non-ciliated cuboidal epithelium, beginning at 11 weeks of intrauterine life. Later, from 16 weeks, the organ was found to have pseudostratified ciliated columnar epithelium. At 21 weeks of intrauterine life, three types of cells were observed - dark cells, basal cells and clear cells. Blood vessels have also been detected. The microscopic characteristics of the VNO were similar in the both study groups of embryos and fetuses. What differentiated the two groups was, first of all, the presence of nerve fibers adjacent to the VNO, especially in the group of embryos and fetuses with chromosomal anomalies. A more abundant vascularization was also observed starting with lower intrauterine ages, compared to the control group. The blood vessels observed in the previous stages become very numerous starting with the age of 22 weeks in aborted fetuses without malformations, but they are observed in greater numbers in embryos with chromosomal abnormalities from younger ages. The presence of abundant vascularization was evident in the case of malformed embryos where the VNO was absent (Figure 4). Figure 5 represents the microscopic characteristics of the VNO with the following stains: Alcian Blue, Grimellius, PAS, Trichrome Masson.
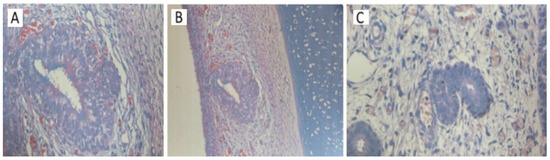
Figure 4.
The absence of the VNO in embryos with or without chromosomal anomalies using hematoxylin-eosin staining. (A) 17-week fetus, without chromosomal anomalies. Specialized olfactory mucosa and the rich vascularization in intimate contact with the cellular structures of the VNO can be observed (40x) (B) 17-week fetus with chromosomal abnormalities. A detail of the VNO, formed by the olfactory mucosa, showing stratifications and in the peripheral area there are nerve-type cells. The luminal cells are ciliated (20x.) (C) The abundant vascularization around the glandular structures formed by a stratified ciliated epithelium is observed (40x.).
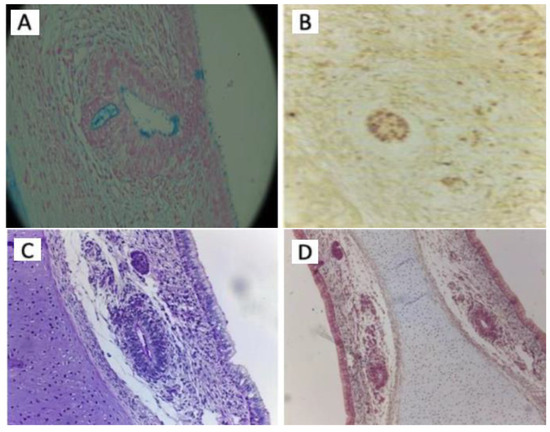
Figure 5.
The microscopic characteristics of the VNO using different stains. (A) 19-week fetus with chromosomal abnormalities. The apical secretion of the epithelial cells and the caliciform cells (Goblet cells) coloured in blue and the intraluminal mucus can be observed. (40x. Alcian Blue stain). (B) 14-week fetus with chromosomal abnormalities. The presence of argyrophilic cells is observed in the loose conjunctival stroma adjacent to the glandular structures of the VNO and in the VNO (40x. Grimellius stain). (C) 14-week fetus with chromosomal abnormalities. No positive PAS inclusions are evident in the VNO (40x. PAS staining). (D) 21-week fetus with chromosomal abnormalities. No capsular densification is observed around the VNO which is bilaterally present (20x. Trichrome Masson staining).
Immunohistochemical Study of the VNO in Normal and Abnormal Embryos and Fetuses
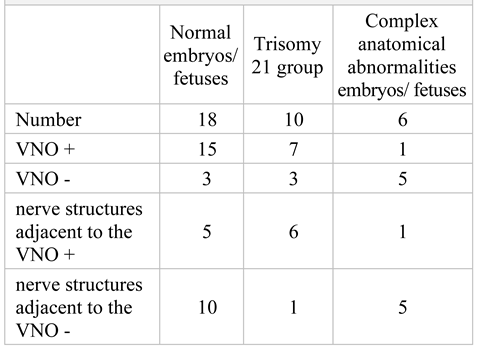
The aim of the immunohistochemical study of the fetal VNO was to characterize this epithelial structure and to obtain a perspective over the time sequence of the immunological development of its different cellular components. Our goal was to clarify the most important and debated characteristic of the VNO epithelium: the presence of intraepithelial nerve structures that could be considered chemoreceptor cells, similar to receptor cells of the organ in other mammals and if there are differences between normal fetuses and those with chromosomal abnormalities in this regard. For the purpose of standardization, the capture of static images of the regions of interest (the areas representing the VNO and adjacent structures and other relevant areas of the section) was performed using a 40x magnification factor. These images were used for immunoexpressing quantification (Table 2).

Table 2.
The presence of VNO in embryos and foetuses with genetic abnormalities versus normal embryos. (VNO – vomeronasal organ, + = positive, - = negative).
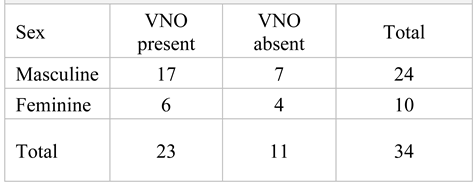
Of the total of 34 samples included in the study, the VNO was detected in 23 cases (67%). In the control group, the presence of the VNO was recorded in 83% of the samples. In the group with chromosomal anomalies, the incidence of the VNO was 70% and in the group with morphological anomalies, VNO was detected in 16% of cases. The total number of male fetuses was higher than that of females (sex ratio 2,4:1). The sex ratio for the presence of the VNO was 2.8:1 (Table 3). We did not find any statistically significant difference between presence of the VNO and the sex of the fetus (p>0,05).

Table 3.
The presence of the vomeronasal organ according to the sex of the embryos included in the study.
We observed that positive surface for cytokines in the control group decreased with age, and in group with anatomical anomalies, the number of positive cells was low. On the other hand, in the group with chromosomal anomalies, the positivity, although a slight downward trend was recorded, it remained high.
In the case of cytokeratin 5/6 (CK5/6), in the first age group, a positive reaction was observed in our study, mainly located in the upper layers of the epithelium. In this area lacking nuclei, the positive reaction was continuous and concentrated on the apical part of the cells, as presented in Figure 6.
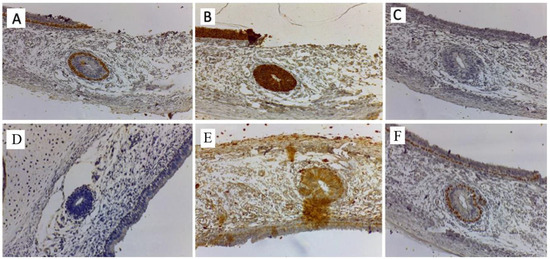
Figure 6.
Immunohistochemical study of the VNO. (A) Basal cells of the olfactory epithelium positive for CK5/6. The basal layer is positive, attesting its epithelial origin and its relationship with the normal nasal mucosa (40x). (B) The olfactory epithelium positive for cytokeratin CK 18/8 underlying and in intimate contact with the epithelium. Bowman’s glands are observed. The structures of the VNO have only the cells in the basal portion positive for this immunomarker (40x.). (C) Rare positive cells for chromogranin in the olfactory epithelium and in the structure of the VNO (40x.). (D) EGFR negative in the VNO. (40x.). (E) S100 positive around the VNO area and the nerve threads approaching the basal layer of the organ. It attests its communication with nerve fibers. The nasal mucosa is negative for S100. (40x.). (F) Intense nuclear immunoreactivity for p63 of the basal cells of the olfactory epithelium as well as of the basal cells of the glandular structures of the VNO. (40x.).
A series of immunopositively structures emerged from these cells heading towards the basement membrane, but none of them reach their target. Thus, here we see a polarized aspect, where the basal layer is completely negative, the intermediate structures show partial immunopositivity and the luminal area of the epithelium is intensely positive. This aspect was not observed in the respiratory epithelium, where there was an almost homogeneous immunoexpressing over the entire thickness of the epithelium, contrasting with the epithelium of the VNO. In the second age group, the positive reaction extended almost uniformly throughout the epithelium, including its lower components but with the absence of the basal cells. Between 22 and 23 weeks, the intensely positive reaction extended to the entire surface of the vomeronasal epithelium, with increased immunopositivity of the superficial layer.
In the case of cytokeratin 18/8 (CK18/8), the appearance was different from CK5/6 because it outlines all the cellular layers of the epithelium, offering a positive reaction at the periphery of the cytoplasm (Figure 6). In the first age group, a positive reaction was observed, that extended over the entire epithelial surface, from the apical part and including the basal cells. In the second age group there was a strong immunopositivity in the basal cells and in the non-nucleated apical part of the epithelium. In general, CK 18/8 immunopositivity did not show significant variations during the fetal periods.
Regarding chromogranin, in the case of embryos with chromosomal abnormalities, positive nerve structures could be observed at the level of the VNO. Chromogranin induced negative reactions in case of non-affected embryos and fetuses.
The positive immunoexpression for the S100 protein was located near the different structures of the submucosa and around the nerve bundles. A positive reaction was observed around certain exocrine glands of the nasal submucosa. In the first age group, immunopositively structures partially surround the medial part of the VNO, in close connection with its outer contour. The S100 reaction in the submucosa of the olfactory epithelium expressed immunopositivity characteristic of the olfactory nerve, establishing connections that pass through the basal membrane in the epithelium. After 16 weeks, there was a decrease in the immunodepression around the VNO, and by the age of 23 weeks, only a few isolated spots of positivity could be observed in its surrounding area. In the case of embryos and fetuses with chromosomal abnormalities, the positivity was maintained in all age groups.
Synaptophysin was detected in the sensory nerve endings of the peripheral nervous system. In our study, it showed negative immunoreactivity in the VNO and in all the tissues of the fetal nasal cavity. In the first age group, there may be small areas of positive reaction in the area of the organ, but these are located at some distance from the basal membrane.
Comparing the immunohistochemistry aspects in both study groups, we observed that the images were similar: basal cells were positive for CK5/6, while epithelial cells were negative; CK 8/18 showed normal positive marking in the respiratory epithelium (control group) and at the level of the VNO epithelium; the basal layer showed positive reaction for p63 in both groups.
In the control group, the VNO was negative for S100 mark. No nerve fibers could be observed at the level of the lamina propria surrounding the organ. On the other hand, in the group of embryos with chromosomal abnormalities, in addition to the fact that the positive internal control was present, positively labelled nerve bundles/filaments with cytoplasmic and nuclear expression located in the lamina propria surrounding the VNO were observed. Chromogranin and synaptophysin markers were negative in both groups. Epithelial cells have shown positive immunomarking for SOX2, with nuclear expression, in both groups.
Discussions
The fact that the VNO was found in 67% of the analyzed samples is highly suggestive for its inconstant presence under normal conditions, which raises many questions about the phylogenetic significance of the organ for the human species. The VNO was observed as bilateral epithelial invaginations during the initial period of development. The greatest development has been observed between 12-14 weeks. Then, there is a gradual replacement of the receptor population with non-uniform ciliated cells. At 11 weeks will appear the cuboidal epithelium and at 16 weeks will appear the cylindrical ciliated epithelium [12]. All these aspects have also been observed in our study.
In our study, we observed the persistence of the VNO during the fetal period in both groups. The VNO increases its volume until the second trimester, after which it begins to involute in normal fetuses. In the group with chromosomal abnormalities, although the size of VNO tends to shrink, it remains visible. Also, the presence of neuroepithelial elements and nerve filaments persists even after these elements have disappeared in non-affected fetuses. Neurofilaments begin to appear in the central and peripheral nervous system of the mouse after days 9-10 of embryonic development, which corresponds to the time of initial axonal expansion [13]. These filaments are characteristic of differentiated neurons, and they replace vimentin in the cytoskeleton of dividing neuronal precursors. Both types of intermediate filaments can be co-expressed for a short period of time [14]. In our study, the presence of neurofilaments could not be confirmed in any of the examined specimens. The lack of a major neuronal component raises major questions about the ability of the vomeronasal epithelium to develop and differentiate into a neuroepithelium [13,14,15].
In the present work, immunohistochemistry was performed on several specimens of different ages, in order to describe the changes over the time and the neuronal properties of the vomeronasal epithelium. The results obtained with immunohistochemistry with chromogranin and synaptophysin clearly demonstrated that in small fetuses there are populations of cells that can be considered neurons or neuroendocrine cells. Regardless of the type of cells expressing the specific immunomarkers in the vomeronasal epithelium, the relative amount of immunoexpression shows a decreasing trend with age, and from 23 weeks it is already insignificant. This information correlates with the results of other authors who demonstrated a reduced immuno-expression in the epithelium of the VNO in human adults [16]. Chromogranin is present in neuroendocrine cells in the body. It is the standard immunohistochemical marker for neuroendocrine tumors, and elevated levels of chromogranin in the blood are characteristic for these tumors [17,18,19]. However, in our study, in fetuses with chromosomal abnormalities, we found chromogranin positive cells suggesting the possibility that these cells have neuroendocrine characteristics.
Synaptophysin is associated with the membranes of synaptic vesicles and is detected in the sensory nerve endings of the peripheral nervous system. Negative reactions in both the VNO and the respiratory epithelium further emphasize the lack of production and storage of biomolecules necessary for information transmission [20].
The use of CK 18/8 was considered to expand the possibilities of identifying all cell populations with epithelial properties. The CK18/8 results of this study show that during the fetal age ranges studied almost all epithelial cells of the VNO showed structural components associated with cytokeratin, suggesting the gradual transformation or replacement of neural precursors into epithelial cells [21].
S100 protein is normally expressed by cells that develop from the neural crest, such as Schwann cells, melanocytes, glial cells and other cells, such as chondrocytes, myoepithelial cells, Langerhans cells [22]. S100 protein (as a glial cell marker) could not be highlighted close to the adult VNO. However, the immunoexpression of S100 protein has been demonstrated in the vicinity of the olfactory epithelium of the olfactory nerve fibers from the epithelium [15]. There were found nerve bundles surrounded by S100 positive glial cells in early fetal stages. Furthermore, in later stages, these cells became less obvious. The above statements are confirmed by the immunoexpression of S100 in the vicinity of the olfactory epithelium, where the connections with nerve fibers could be clearly demonstrated.
The antibodies used in our study have been developed in order to be used in adult human tissues, which allowed us to detect the first appearance of fully differentiated cell types, at least in the youngest fetuses. We observed several changes in the distribution of immunopositivity over time, which demonstrated a change in the immunological properties and epitope display of the affected cells [11,23,24]. The novelty of our study consists in the fact that in the group of embryos and fetuses with chromosomal anomalies, although the morphological and immunohistochemical characteristics were similar to the control group, the specific structures of the VNO persisted beyond 22 weeks, when in the other group, this structure regressed. Moreover, the presence of nerve threads and their connections with VNO were obvious, as well as of the nerve fibers with higher centers can be suspected due to their trajectory to the superior, towards the olfactory bulb.
Explanations for the persistence of the VNO in fetuses with Down syndrome are controversial, as is the entire theory regarding its role and function. The persistence of the organ can explain the specific pattern of behavior of children and young people with Down syndrome [25,26,27].
Conclusions
The microscopic characteristics of the VNO were similar in both study groups. But what differentiated the two groups was, first of all, the presence of nerve fibers adjacent to the VNO, observed in the group with chromosomal anomalies. In this group, we also observed a more abundant vascularization of the VNO, beginning from younger intrauterine ages, compared to the control group. A possible explanation for the absence of nervous or neuroendocrine structures in normal fetuses could be that the migration occurred in the sixth week, and it was ended by the age at which the samples were collected. On the other hand, in fetuses with chromosomal anomalies, the migration defects allowed the persistence of this structures at the level of the VNO and the nasal cavity.
The immunohistochemical study revealed that the vomeronasal epithelium initially contains a population of cells with neuronal properties and shows a potential to differentiate into a chemosensory epithelium, but the neuronal population decreases dramatically with advancing fetal age. Despite the initially well-represented neuronal population, there are no obvious connections with neighboring nerve fibers. Negative immunoexpression of chromogranin and synaptophysin excluded the possible explanation of neuronal marker immunopositivity with the existence of neuroendocrine cells. Epithelial markers demonstrated the presence of cell types with different cytoskeletal components, but they become predominant at later stages, indirectly confirming the disappearance of neural structures. The results of the present work support the theory of neuroepithelium regression, that is present in the initial stages of fetal development.
The explanations for the persistence of the VNO in fetuses with Down syndrome are controversial, as is the entire theory regarding its role and functionality. The persistence of VNO could dictate the specific behavior pattern of children and young people with Down syndrome. Therefore, through this work we can once again launch the hypothesis that in the absence of the normal functioning of the olfactory system and cognitive abilities, the presence of the VNO could explain the social-emotional behavior and superior nonverbal communication abilities in children or adults with Down syndrome, but more detailed studies, on larger cohorts, are needed in future in order to confirm this hypothesis.
This study is still ongoing in order to identify the increased frequency of VNO in fetuses. In the future, the detection of cell types and cell counting in fetuses with or without chromosomal abnormalities can be done with the simultaneous observation of the same cells in the hypothalamus, in order to detect their migration. Adult specimens obtained during nasal surgery, could also be used in the future in order to confirm the persistence of the VNO in adults. Further immunohistochemical studies using specific markers may also be useful in order to identify sensory neurons and thus confirm their role as a sensory organ.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. CCM and MAM designed the study. Data were collected by CCM and CIC. Analysis was carried out by CCM, MAM and LD. LP and AN take final responsibility for writing the original manuscript.
Informed Consent Statement
This study obtained the approval of the hospital’s ethics committee, thus complying with the ethical standards of the 1975 Declaration of Helsinki, revised in 2000, as well as the national legislation. Written informed consent for participation was signed by the parents in accordance with the national legislation and institutional requirements.
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Bruintjes, T.D.; Bleys, R.L.A.W. The clinical significance of the human vomeronasal organ. Surg. Radiol. Anat. 2023, 45, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Katreddi, R.R.; Forni, P.E. Mechanisms underlying pre-and postnatal development of the vomeronasal organ. Cell. Mol. Life Sci. 2021, 78, 5069–5082. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, G.S.; Sapundzhiev, N.R.; Tonchev, A.B. The vomeronasal organ: history, development, morphology, and functional neuroanatomy. Handbook Clin. Neurol. 2021, 182, 283–291. [Google Scholar]
- Vasuki, A.K.; Fenn, T.K.; Devi, M.N.; Hebzibah, T.D.; Jamuna, M.; Sundaram, K.K. Fate and Development of Human Vomeronasal Organ – A Microscopic Fetal Study. J. Clin. Diagn. Res. 2016, 10, AC08–11. [Google Scholar] [CrossRef] [PubMed]
- Trotier, D.; Eloit, C.; Wassef, M.; Talmain, G.; Bensimon, J.; Døving, K.; Ferrand, J. The Vomeronasal Cavity in Adult Humans. Chem. Senses 2000, 25, 369–380. [Google Scholar] [CrossRef]
- Trotier, D. Vomeronasal organ and human pheromones. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 184–190. [Google Scholar] [CrossRef]
- Bordoni, B.; Zanier, E. Cranial nerves XIII and XIV: nerves in the shadows. J. Multidiscip. Heal. 2013, 6, 87–91. [Google Scholar] [CrossRef]
- Tucker, E.S.; Lehtinen, M.K.; Maynard, T.; Zirlinger, M.; Dulac, C.; Rawson, N.; Pevny, L.; LaMantia, A.-S. Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development 2010, 137, 2471–2481. [Google Scholar] [CrossRef]
- Taroc, E.Z.M.; Prasad, A.; Lin, J.M.; Forni, P.E. The terminal nerve plays a prominent role in GnRH-1 neuronal migration independent from proper olfactory and vomeronasal connections to the olfactory bulbs. Biol. Open 2017, 6, 1552–1568. [Google Scholar] [CrossRef]
- Beites, C.L.; Kawauchi, S.; Calof, A.L. Olfactory Neuron Patterning and Specification. Dev Neurobiol. 2009, 7, 145–156. [Google Scholar] [CrossRef]
- Bhatnagar, K.P.; Smith, T.D. The human vomeronasal organ. III. Postnatal development from infancy to the ninth decade. Am. J. Anat. 2001, 199(Pt. 3), 289–302. [Google Scholar] [CrossRef]
- Døving, K.B.; Trotier, D. Structure and function of the vomeronasal organ. J. Exp. Biol. 1998, 201(Pt. 21), 2913–2925. [Google Scholar] [CrossRef]
- Cochard, P.; Paulin, D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J. Neurosci. 1984, 4, 2080–2094. [Google Scholar] [CrossRef] [PubMed]
- Tapscott, S.J.; Bennett, G.S.; Holtzer, H. Neuronal precursor cells in the chick neural tube express neurofilament proteins. Nature 1981, 292, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, G.P.; Balalau, O.D.; Comandasu, D.E.; Stanescu, A.D.; Tomescu, D.R. Best therapeutic practices in the management of obstetric sepsis. J. Mind Med Sci. 2023, 10, 304–311. [Google Scholar] [CrossRef]
- Takami, S.; Getchell, M.L.; Chen, Y.; Monti-Bloch, L.; Berliner, D.L.; Stensaas, L.J.; Getchell, T.V. Vomeronasal epithelial cells of the adult human express neuron-specific molecules. NeuroReport 1993, 4, 375–378. [Google Scholar] [CrossRef]
- De Camilli, P.; Vitadello, M.; Canevini, M.; Zanoni, R.; Jahn, R.; Gorio, A. The synaptic vesicle proteins synapsin I and synaptophysin (protein P38) are concentrated both in efferent and afferent nerve endings of the skeletal muscle. J. Neurosci. 1988, 8, 1625–1631. [Google Scholar] [CrossRef]
- Motofei, I.G. Biology of cancer; from cellular and molecular mechanisms to developmental processes and adaptation. Semin. Cancer Biol. 2021, 86(Pt. 3), 600–615. [Google Scholar] [CrossRef]
- Nobels, F.R.; Kwekkeboom, D.J.; Bouillon, R.; Lamberts, S.W. Chromogranin A: its clinical value as marker of neuroendocrine tumours. Eur. J. Clin. Invest. 1998, 28, 431–440. [Google Scholar] [CrossRef]
- Balalau, D.O.; Augustin, F.-E.; Bogheanu, D.-M.; Negulescu, A.-G.; Sima, R.-M.; Dumitriu, A.S.; Paunica, S.; Ples, L. Recurrent pregnancy loss -a life changing condition for women. J. Mind Med Sci. 2023, 10, 8–14. [Google Scholar] [CrossRef]
- Dénes, L.; Pap, Z.; Szántó, A.; Gergely, I.; Pop, T.S. Human vomeronasal epithelium development: An immunohistochemical overview. Acta Microbiol. Et Immunol. Hung. 2015, 62, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Manetti, M.; Sgambati, E. Immunolocalization of VEGF/VEGFR system in human fetal vomeronasal organ during early development. Acta Histochem. 2019, 121, 94–100. [Google Scholar] [CrossRef]
- Balalau, O.D.; Loghin, M.G.; Bogheanu, D.M.; Bacalbasa, N.; Stanescu, A.D.; Bălan, D.G.; Păunică, I.; Olaru, O.G. Management of systemic lupus erythematosus in pregnancy. J. Mind Med Sci. 2022, 9, 236–241. [Google Scholar] [CrossRef]
- Kasari, C.; Freeman, S.F.N. Task-Related Social Behavior in Children With Down Syndrome. Am. J. Ment. Retard. 2001, 106, 253–64. [Google Scholar] [CrossRef]
- Lioubinski, O.; Alonso, M.T.; Alvarez, Y.; Vendrell, V.; Garrosa, M.; Murphy, P.; Schimmang, T. FGF signalling controls expression of vomeronasal receptors during embryogenesis. Mech. Dev. 2006, 123, 17–23. [Google Scholar] [CrossRef]
- Witt, M.; Hummel, T. Vomeronasal versus olfactory epithelium: is there a cellular basis for human vomeronasal perception? Int Rev Cytol. 2006, 248, 209–259. [Google Scholar] [CrossRef]
© 2024 by the authors. 2024 Carmen Constantina Martinescu, Marius Alexandru Moga, Codrut Ioan Ciurea, Lorena Dima, Liana Ples, Andreea Neculau.