Introduction
Chronic wounds have a significant impact on the healthcare system, with associated costs reaching up to
$15 billion in the US. From an epidemiological point of view, there is a tendency to increase the incidence; during their lifetime between 1-2% of the population have an ulcerated lesion in pressure areas or on the lower limbs that remains open for more than 6 weeks [
1,
2]. Associated chronic conditions have a strong impact on wound healing. Thus, the presence of arterial or venous vascular insufficiency, diabetes, neurological deficit, but also local factors such as edema, infection or local pressure can influence the subsequent evolution of a wound, some of which can be influenced by administering a multimodal treatment [
3,
4].
Acute wounds generally have a favorable evolution, with progressive healing following successively the phases of inflammation, cell proliferation and remodeling through which the integrity of the skin is restored. Each of these stages can be negatively influenced, resulting in a chronic condition that cannot be cured without a specific therapeutic approach. Inflammation is the main factor that can lead to the development of chronic wounds. Neutrophils, basophils, and other cells of the innate immune system usually control local infection and provide a favorable environment for cellular and tissue reconstruction. Imbalances that can occur in the case of the primary immune response can lead to the appearance of chronic wounds, which are immunologically characterized by the excessive presence of pro-inflammatory factors (especially macrophages) at the expense of anti-inflammatory factors. Moreover, macrophages cannot perform a good cleaning of neutrophils that have undergone apoptosis processes, which leads to an environment with several pro-inflammatory cytokines (such as TNF-α, IL 1β, but also MMP 2/MMP 9) that degrade the matrix cellular, thus inhibiting the proliferation of fibroblasts [
4,
5,
6].
Many of the chronic wounds are associated with bacterial infections that cause the formation of a conglomerate of cells and local tissue factors, which most of the time cannot be controlled by the administration of antibiotics. The presence of tight networks in which bacteria are enclaved favors the development of resistance to antibacterial treatment. It is a consequence of the fact that many (antibacterial and anti-inflammatory) substances have a low ability to penetrate the microcellular environment, as well as due to the fact that the genetic material that codes resistance to certain antibiotics can be transferred by multiplication from one bacterium to another. From an immunological point of view, the biofilm causes, through its pro-inflammatory effects, the activation of neutrophils and other cells of the immune response. The consequence is the release of TNFα, IL-6 and MMP, which causes the alteration of the cellular environment favoring bacterial growth and the inhibition of the local immune defense, which thus closes the vicious circle with the deposition of new layers in the microbial aggregate that is being formed/ developed [
7,
8,
9].
For an effective and fast treatment, a series of measures must be taken, including the stimulation of neovascularization that leads to an increased intake of oxygen and cells that can heal the septic microenvironment. This corresponds to an efficient local immunomodulation, so that the resulting inflammatory process is adequate. At the same time, the barrier function of the skin must be maintained to prevent superinfection [
10,
11].
Several medical/therapeutic methods are used in the treatment of chronic wounds. Surgical treatment removes the necrotic tissue, while antibiotic therapy sterilizes the wound; immunomodulators and angiogenesis factors favor tissue formation, thus covering the resulting defect [
12]. The surgical methods used in chronic wounds are mainly represented by debridement (which consists in the elimination of necrotic tissue and the bacterial biofilm) which thus allows for adequate epithelization. Most of the time, this intervention is carried out using the scalpel, scissors or curette, which do not cause pain due to non- viable tissues that have no innervation and no blood vessels. A good indicator of proper debridement is the presence of bleeding from the wound bed. The result of this intervention is the transformation of the chronic wound into an acute wound, which can thus resume the normal stages of epithelization, further favored by medical nursing [
13,
14,
15]. Debridement as a therapeutic method is used especially for the complications of diabetes, as well as venous and pressure ulcers. This method must be used with caution in the case of vascular lesions from various pathologies (peripheral arterial disease, vasculitis, etc.) because it can lead to the worsening of the wound by increasing the degree of ischemia and increasing the volume of the ulcer [
16,
17].
Another type of debridement used in chronic wounds is the wet-to-dry method, which relies on the ability of various sterile materials (sterile cotton dressings most often) to stick to necrotic tissue when wet. Due to exposure to body or environmental heat, water evaporates and tissue debris adheres to the sterile material which can be removed by mechanical force. The method described above has as its main shortcoming the lack of differentiation between viable and necrotic tissue (raising at the same time the bacterial biofilm, necrotic tissues, fibroblasts and keratinocytes), thus inducing a high degree of pain [
18].
Autolytic debridement is the removal of devitalized tissue from the adjacent tissue by exposing the wound to a moist environment, so that there is a clear demarcation of the affected tissue (from the healthy one) that can be removed more easily. This process is a long-term form of treatment that can last up to several weeks. However, it appears to be useful in those patients who are at high risk of bleeding and in whom surgical debridement cannot be performed [
19,
20,
21].
After removing the necrotic tissues, it is necessary to cover the wound with a sterile dressing. Along with the evolution of medicine, a wide range of such types of dressings has been developed, having both advantages and disadvantages, as no ideal dressing has been created to date. At the moment, the “ideal dressing” should have as many of the following characteristics as possible: remove exudate but still maintain a moist environment, protect against contamination, not cause trauma while being removed (not leave debris in wound), relieve pain and ensure thermal and water insulation, all this without creating allergic reactions [
22,
23].
In addition to the classic debridement methods, antimicrobial agents such as povidone iodine (one of the most used at the moment, having antibacterial effects but without inhibiting cell proliferation), topical antibiotics (such as neomycin or metronidazole) are used for faster and more efficient wound healing), as well as colloids containing silver salts which have been shown to lead to a significantly better result [
24]. The action mechanism of silver ions is still not very well elucidated in such cases. It is considered that they bind to bacterial membranes inhibiting transmembrane transport, and influence intracellular connections with impact on their genetic material, thus determining the inability to multiply [
25,
26,
27].
Honey has been used since ancient times in the treatment of chronic wounds. Its favorable effects are due to its anti-inflammatory, tissue regeneration and antibacterial action (by inducing low pH, its ability to dehydrate bacterial cells, as well as hydrogen peroxide which appears to have beneficial local effects) [
28]. Antibacterial Manuka honey is made from nectar collected from New Zealand Manuka plants, which are part of the Leptospermum species commonly known as the tea tree family. Honey from this plant source has been found to have a unique antibacterial property that appears not to be found in honey from other floral sources. The main antibacterial property of Manuka honey is derived from the nectar, which contains significant amounts of methylglyoxal (resulting from the dehydration of dihydroxyacetone, phenols and flavonoids). Thus, Manuka honey exhibits selective cytotoxic activity on bacterial cells, inhibiting flagellation and interrupting their division. There are studies that found a direct correlation between the local antiseptic/ bactericidal action and the concentration of methylglyoxal [
28,
29]. Such substances are not affected by catalases or other enzymes and appear to remain active at very low concentrations. This is known as the Non-Peroxide Activity (NPA) or Unique Manuka Factor [
30]. Manuka honey is able to act as a broad-spectrum antibacterial product, being effective against most bacteria that invade wounds (e.g. Staphylococcus Aureus, Pseudomonas Aeruginosa, MRSA and VRE) by disrupting various metabolic processes in the bacterial wall or even in the cytoplasm. The association of Manuka honey with the conventional antibiotic and debridement treatment determines a synergistic effect in terms of the ability to inhibit the local multiplication of bacteria and the production of biofilm, this therapeutic method being especially useful when multiresistant bacteria are present [
30,
31].
The current study evaluates the results obtained by the classical methods of treating chronic wounds (represented by surgical debridement, antibiotic therapy, cotton dressings and povidone-iodine) in comparison with the data obtained by adding dressings containing Manuka Honey, as a complementary treatment for wound coverage. The parameters monitored in this study are represented by the healing time, the appearance or not of wound infections, the average time for the appearance of granulation tissue, as well as the final aesthetic appearance obtained after local healing.
Materials and Methods
The study was carried out in Department of Clinical Surgery, St. Pantelimon Emergency Clinical Hospital, Bucharest, Romania, from March 2021 to October 2022. Inclusion criteria were represented by the presence of chronic wounds on a patient with type 2 diabetes recently diagnosed (during the last 2 years), but without other major comorbidities (such as: myocardial infarction, congestive heart disease, stroke, peritonitis or other malignancies of the digestive system), presence of pressure ulcers of any grade and ulcers secondary to vascular diseases. Following the application of the inclusion and exclusion criteria, a study group of 20 patients was formed, who were randomly divided into two subgroups. These were represented by the patients who will follow the standard therapy (in which there will be no adjuvant treatment methods such as special dressings) and the patients who will use special dressings containing Manuka honey.
After the admission of the patients to the general surgery department, routine investigations were carried out (inflammatory markers, blood sugar and glycosylated hemoglobin, assessment of renal, hepatic and pulmonary functions, etc.). Bacteriological samples were collected from the wound, after which a broad-spectrum antibiotic treatment was initiated (Ceftriaxone 1g every 12 hours) while modified biological parameters were corrected. Surgical treatment was represented by the removal of necrotic or modified tissues, with the achievement of a clean wound bed represented by viable tissue with hemorrhagic scars. The resulting integumentary defect was measured, after which a dressing made of sterile compresses with betadine was made.
Control group
For the first 10 days, the wound was treated twice a day under the supervision of the surgeon. Then, for 11 weeks, daily local care was performed with povidone-iodine solution and sterile absorbent soft silicone dressing.
The treatment plan followed by the patients enrolled in this group also included several prophylactic methods. These were represented by ensuring a good mechanical protection of the affected region (by avoiding possible trauma), a good hygiene behavior with daily washing (in the case of wounds located on the perineal region with washing after every stool), as well as regular mobilization in the case of pressure ulcers.
The general treatment was represented in this group by the administration of appropriate antibiotics (after the evaluation of the antibiogram) and analgesia according to the degree of pain perception of the patients (from non-steroidal anti-inflammatory drugs to morphine derivatives, for patients who had large ulcers and/or positioned in well innervated areas such as the inguinal or perineal regions. In association with the local treatment, specific treatment for comorbidities was administered, if necessary. Thus, various deficits were corrected such as hyperglycemia (by maintaining blood glucose values in the range of 100-120mg/dl, using fast and slow-acting insulin), hypertensive drugs were administered (for systolic blood pressure values higher than 140 mmHg.), as well as oxygen (in patients who had sleep apnea syndrome or chronic obstructive bronchitis and who were dependent on oxygen therapy). Patients generally had a slow but favorable evolution due to rigorous control of local infection, which allowed local development of granulation tissue.
After approximately 10 days, the patients were discharged, with a good local and general evolution. The wound had granulation tissue without signs of local infection (such as erythema or pus), and a new antibiogram was performed before discharge. If the infection was still present, the patient was discharged from hospital where there was the possibility of ambulatory antibiotic treatment. In patients who had an infection with bacteria resistant to antibiotics that can be administered orally, the treatment was continued in the hospital until a sterile local fluid was collected.
The recommendations made by the surgeon at discharge were recorded in the file for the family doctor. These recommendations included daily dressing changes, administration of antibiotics (if the wound secretions were not sterile) or pain control medication, adapted diet and good personal hygiene. All these parameters were controlled by a family physician.
Study group
In the study group, the same local and general treatment procedures were used as in the case of the control group. In addition, after surgical debridement of the altered tissues on the first day, a conventional dressing was performed daily (starting on the second day), followed by irrigation of the wound with normal saline and application of Manuka honey directly to the wound surface, which was protected on the surface with absorbent dressings. These were changed daily for a week or until the secretions were completely stopped. Another antibiogram was collected from the secretions if they still persisted, and after discharge local and systemic treatment was continued as described for the control group.
Statistical data analysis was performed with IBM SPSS Statistics for Windows, version 19.0. The measurement data was expressed by x ±s, and conducted with t test. The data material was expressed by cases (percentage), and conducted with χ2 test. The results were considered significant if p value was <0.05.
Results
Patients in the control group had a mean age of 64.3 years. Six of them had diabetes and 3 patients had pressure ulcers, while one patient had a chronic wound secondary to chronic venous disease. Following the collection of the bacteriological examination at admission, Staphylococcus aureus was identified in 6 out of 10 cases. Two patients had chronic wounds infected with Streptococcus pyogenes, 1 patient with Proteus spp. and one patient with Pseudomonas Aeruginosa. Eight of the 10 patients had an inflammatory syndrome on admission, with leukocytosis, neutrophilia and a C-reactive protein/ CRP above 110 mg/l, while 2 of the patients had elevated CRP values but no leukocytosis or neutrophilia.
Hyperglycemia over 200 mg/l was identified in 7 of the patients included in the control group, 6 being known to be diabetic; creatinine values were increased in 4 of them, due to consecutive renal damage. Glycosylated hemoglobin was used as a marker of glycemic control for the last 30 days, so that values above 7 were obtained in 5 of the 7 patients diagnosed with diabetes.
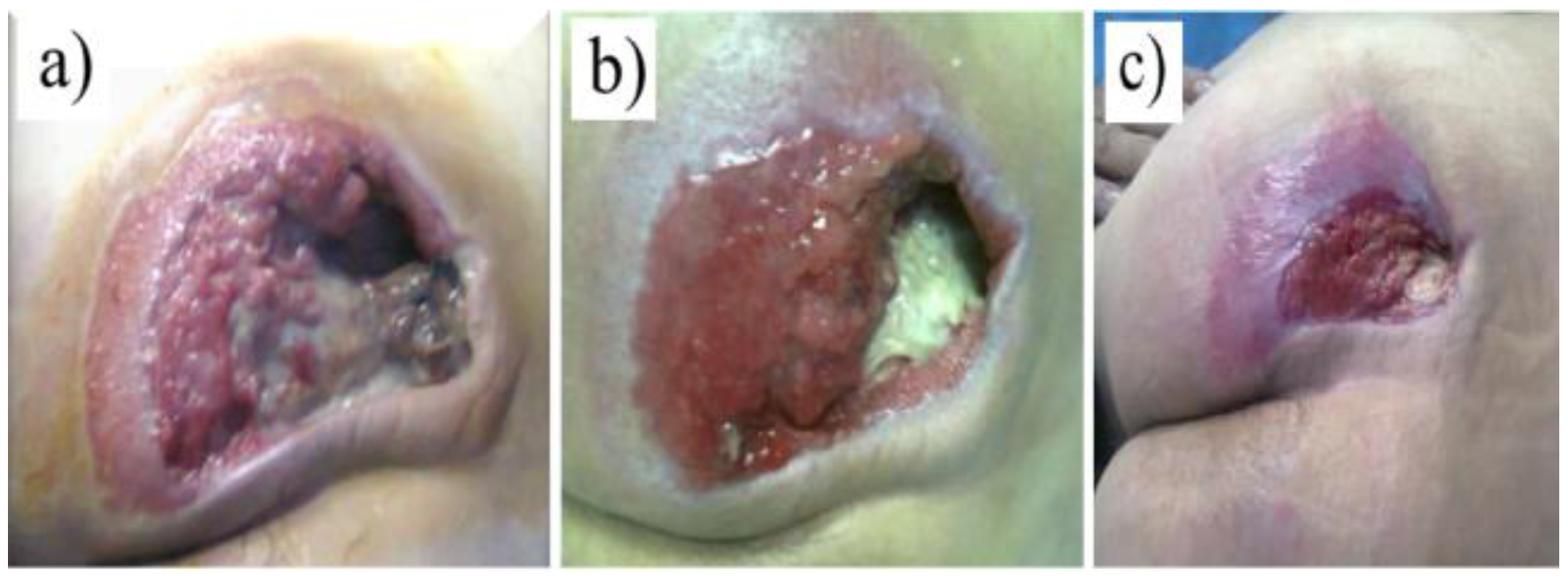
Excision of necrotic tissue resulted in a skin defect with a mean area between 4.22 cm2 and 24.23 cm2 in patients with pressure ulcers. The patients had a slow but favorable evolution, the average time until the generation of granulation tissue being about 5.2 days.
After 10 days, the persistence of the bacterial infection was detected in 2 of the 10 patients. They showed Proteus or Enterococcus resistant to the initial antibiotic but sensitive to Moxifloxacin on the antibiogram, the average depth of the wounds being 5.21 mm. After 11 weeks the lesion had completely healed with an unaesthetic keloid scar due to the lack of soft tissue. The scar was retractile and deforming, being scheduled for a later corrective surgical intervention.
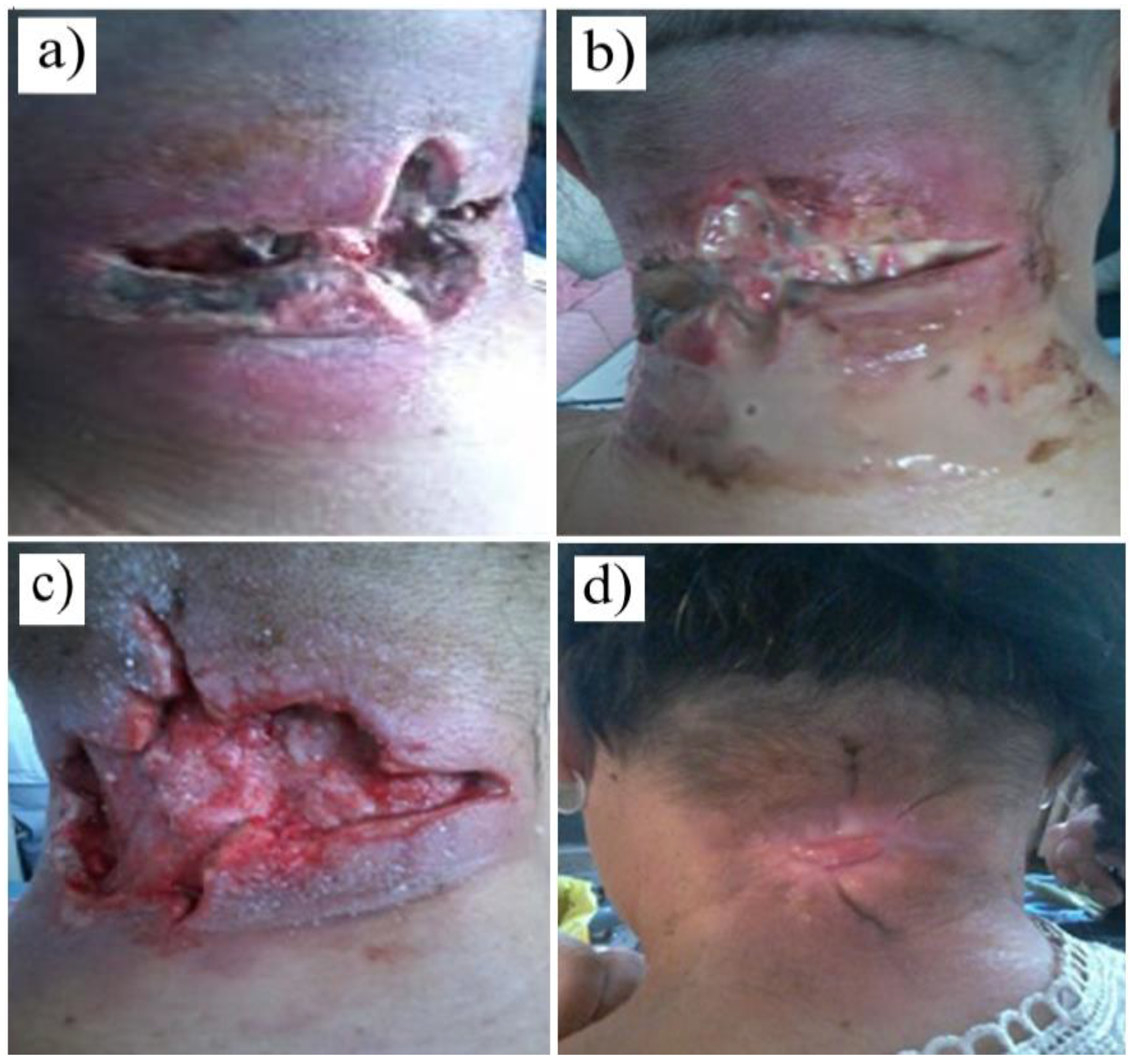
Figure 1.
Figure 1. Postoperative appearance (after 11 weeks of treatment) of a chronic wound caused by an anthracoid furuncle (the control group).
Figure 1.
Figure 1. Postoperative appearance (after 11 weeks of treatment) of a chronic wound caused by an anthracoid furuncle (the control group).
The study group included 5 men and 5 women, with an average age of about 65.8 years. The etiology of chronic wounds was secondary to poorly managed diabetes in 5 cases, 4 patients presented with pressure ulcers and 1 patient had vascular disease. The initial bacteriological examination revealed the presence of Staphylococcus aureus in 5 out of 10 cases, 3 patients had different Proteus species, while 2 out of 10 patients had Pseudomonas aeruginosa infections.
Figure 2.
Figure 2. Evolution of the chronic wound (pressure ulcer) of a patient in the control group (for 4 weeks)
Figure 2.
Figure 2. Evolution of the chronic wound (pressure ulcer) of a patient in the control group (for 4 weeks)
Unlike the control group, all patients presented with positive inflammatory markers in the blood count and C-reactive protein, the latter having an average value of 103.3mg/L. Hyperglycemia was identified in 8 of the 10 patients, with a glycosylated hemoglobin level above 6.5 in 7 cases. In 3 of them, diabetes was associated with chronic kidney disease at admission, thus negatively influencing the evolution of chronic wounds.
Removal of devitalized tissue resulted in wounds that ranged in area from 6.22 cm2 to 26.81 cm2, with a mean time to granulation tissue appearance of approximately 4.8 days.
The mean wound depth observed after 10 days was 4.82 mm. At the end of the fourth week the average decrease in wound depth was about 0.92 mm, with subsequent complete epithelialization of the wound. In the antibiograms of the patients performed after 10 days, the presence of detectable microbes was not identified.
Discussions
In the present study, patients with pressure ulcers, with wounds resulting from trauma or after surgery (in association with increased blood sugar, vascular diseases, varicose ulcers or other vasculopathies that presented chronic ischemic lesions) were included. Each of these pathological entities requires an individualized treatment primarily based on the removal of devitalized and necrotic tissues, but in association with specific treatment to reduce inflammation and infection in the affected area. Thus, for a good healing of chronic wounds, it is necessary to interrupt the pro-inflammatory vicious circle described in the Introduction. For this purpose, different techniques can be used, from very simple methods (such as the surgical removal of necrotic/devitalized tissues) to the use of artificial enzyme preparations that determine the biological removal of cellular debris [
32,
33,
34].
Type II diabetes causes a thickening of the basement membranes of the capillaries, which leads to a degree of tissue ischemia. In such a tissue microenvironment, the healing of chronic wounds is much longer than in a person with normal blood glucose levels. The cause of the thickening of the basal membranes at the level of the cellular components is the inadequate synthesis of collagen, secondary to the presence of advanced glycation end-products (which have a pro-inflammatory effect by stimulating the synthesis of IL-1 and TNF-α). In addition, due to increased glucose levels, a decrease in keratinocyte proliferation and maturation was observed [
35,
36].
Patients in the present study who had associated diabetes accounted for more than half of the subjects. The location of the lesions was represented most of the times at the level of the lower limbs (especially the distal part of the forefoot), secondary to the traumas resulting during daily activities (5 of the 8 with limb lesions). Other affected areas were the calcaneus and the proximal portion of the sole. All these local lesions could have had a good evolution, if the patients had inspected their lower limbs every day and applied simple but early therapeutic methods (thus preventing infection and chronicity of the lesions). From the group of diabetics, 3 had undergone previous surgery, 2 of them required minor amputations (fingers 4 and 5), with difficult healing, while 1 patient presented to the emergency room with a furuncle in the nuchal region.
In terms of the therapeutic approach, in diabetic patients where Manuka honey was used as an adjunctive method of therapy, the evolution towards healing seems to have been faster. Thus, 5 patients had an average granulation time of 4.1 days compared to those who used classical treatment methods, in which the presence of newly formed tissue was observed after 5.7 days. The use of Manuka honey also led to a faster reduction in wound depth 10 days after admission compared to the control group, the difference between them being about 0.82 mm.
Pressure ulcers are caused by the pressure exerted by the weight of the body on the integument and subcutaneous tissue, which is placed between a bony prominence and a hard body in the environment (in the hospital being represented by the mattress or transport trolley). Due to pressures up to 32 mmHg, at the cellular level there is a decrease in both the oxygen supply to the epidermal cells (as well as the elimination of carbon dioxide and other metabolic products), which leads to ischemia and subsequent cell necrosis [
37,
38]. If very rapid reperfusion occurs at this level, reactive oxygen species appear that cause a stronger inflammatory response than the initial one, leading to increased cellular distress at this level. In addition to the constant pressure applied to the soft tissues, the angle at which this pressure is applied is also important. Thus, through the oblique positioning of different segments of the body, frictional and tearing injuries can occur that stretch and break the capillaries, which leads to even greater ischemia and tissue suffering (the time of appearance of pressure injuries being shorter while these lesions show an increased degree of severity).
In our study, patients who were treated with Manuka Honey had an average granulation time of 5.1 days compared to those who were treated with the classical treatment method, where it was 5.5 days. The localization of pressure ulcers found in our group of patients was predominantly in the presacral region in 4 patients, 2 patients presented in both the sacrum and the left ischial tuberosity, and 1 patient had multiple locations (sacrum, ischial tuberosity and calcaneus). These patients were brought to the emergency room from a family environment where there were no people qualified to provide specific medical care. Such patients were immobilized in beds secondary to stroke in 3 cases, 2 patients had hip fractures that required immobilization and 2 patients could not be mobilized due to joint injuries that occurred during the evolution of rheumatoid arthritis.
Regarding the size of the wounds resulting from the surgical treatment, it had smaller values in the control group (with a mean area of 14.26 cm2) compared to the Manuka group (where the mean area was 18.81 cm2). The maximum wound depth 10 days after admission was 4.12 mm for the study group versus 4.53 mm for the control group.
Along with the changes in the microcirculation level, fibrosis occurs and the release of pro-inflammatory mediators that determine the differentiation of fibroblasts into myofibroblasts, which causes a traction of the tissues that predisposes to the appearance of chronic injuries. Another effect of venous stasis is the loading of macrophages with iron secondary to the degradation of the hemoglobin
Venous ulcers are a complication of chronic venous insufficiency, which most commonly occurs due to the inability of the valves in the superficial venous system to maintain a favorable return circulation of blood. This causes the distal reflux of blood with the appearance of local tension, which will lead to the appearance of a chronic inflammatory response with the deterioration of microcirculation. These microcirculatory changes lead to fibrosis and the release of local proinflammatory mediators, which cause the differentiation of fibroblasts into myofibroblasts, which further causes tissue traction that predisposes to chronic injury. Another effect of venous stasis is loading of macrophages with iron secondary to hemoglobin degradation [
39,
40,
41].
In our study, 2 patients presented with varicose ulcers, each of them receiving a separate therapeutic regimen. Better therapeutic results were observed in the patient in whom Manuka honey was used. Thus, 10 days after admission, no germs were detected in the antibiogram, while the granulation time and the maximum depth of the wound were relatively similar.
In the case of the two groups (study and control), the results of the Student's test show that chronic wounds that were treated with Manuka honey showed a significantly greater reduction in depth and surface area compared to those treated classically (the p indicator was less than 0.05 with 95% confidence, similar results being also found in the case of the average granulation and healing time).
The presented study highlights the multiple factors that contribute to the difficulty of treating chronic and infected wounds. This usually requires advanced technologies, invasive monitoring, anesthetic procedures, metabolic sustainability on the part of the patient, as well as therapies (to facilitate a good outcome of surgical procedures in severe conditions, in elderly, immunosuppressed and often debilitated patients). Appropriate care of the chronically infected wound patient includes fluid and electrolyte rebalancing, hemodynamic monitoring, nutritional support, and specific antibiotic therapy. Another important factor to consider is the lack of knowledge about type 2 diabetes and its complications, or about nursing methods of an immobilized patient. Consequently, appropriate nursing interventions regarding self-management of disease symptoms, treatment regimen and lifestyle changes inherent to living with chronic conditions would influence not only the quality of life of a patient, but also the healing period in case of complications.
Other complementary methods to obtain a better therapeutic result in such patients are represented by hyperbaric oxygen therapy, the main effect being represented by the stimulation of local angiogenesis and the induction of vasodilatation (by increasing the cellular intake of nitrogen monoxide) [
42,
43,
44,
45]. There are also products that can be applied topically that have an increased content of tissue growth factors. Becaplermin 0.01% gel contains a high concentration of platelet-derived growth factors, which leads to good results on chronic wounds of the diabetic patient, but with increased risk of cancer occurrence [
46,
47].
Such complementary treatments for chronic wounds must meet several requirements. These must be useful regardless of the etiopathology of the chronic wound, the risk of adverse reactions must be minimal (in the short and long term), most biological products must be used as adjunctive treatment with minimal costs, in order to be accessible [
48,
49,
50].
The current study has some limitations. First of all, products containing Manuka honey are not yet standardized (the amount of active substance can be different from one dose to another), which means that its effectiveness in distinct patients can be different. Another shortcoming of this study is the rather small group on which it was conducted. By introducing a larger number of patients, more obvious conclusions can be drawn. Consequently, we consider this study only a preliminary step that may indicate new therapeutic directions to investigate.
Conclusions
The treatment of chronic wounds must be personalized according to the etiology, the evolutionary stage, the factors and comorbidities that can contribute to their aggravation, as well as according to the patient's behavior. When all these factors are not taken into account, a quick healing is not obtained, the evolution of the wound being slow, often involving expensive and painful treatments, which can thus lead to a decrease in the quality of the patient’s life.
Manuka honey could have multiple therapeutic actions (antibacterial, antimicrobial, antioxidant, antiseptic, anti-inflammatory and antifungal), which must be verified on a large scale. In addition, this product can play an important role in autolytic debridement, maintaining a moist environment at the wound bed, reducing tissue trauma during dressing changes and helping to reduce unpleasant odors. Our study suggests that the use of Manuka honey as a raw dressing can not only significantly improve the quality of life, but also contribute to reducing the cost of hospitalization.