Abstract
[18F]DCFPyL is increasingly used for prostate-specific membrane antigen (PSMA) mediated imaging of men with biochemically recurrent prostate cancer (BRPCa). In this meta-analysis, which is updated with the addition of multiple new studies, including the definitive phase III CONDOR trial, we discuss the detection efficiency of [18F]DCFPyL in BRPCa patients. PubMed was searched on 29 September 2022. Studies evaluating the diagnostic performance of [18F]DCFPyL among patients with BRPCa were included. The overall pooled detection rate with a 95% confidence interval (95% CI) was calculated among all included studies and stratified among patients with PSA ≥ 2 vs. <2 ng/mL and with PSA ≥ 0.5 vs. <0.5 ng/mL. The association of detection efficiency with pooled PSA doubling time from two studies was calculated. Seventeen manuscripts, including 2252 patients, met the inclusion criteria and were used for data extraction. A previous meta-analysis reported that the pooled detection rate was 0.81 (95% CI: 0.77–0.85), while our study showed a pooled overall detection rate of 0.73 (95% CI: 0.66–0.79). An increased proportion of positive scans were found in patients with PSA ≥ 2 vs. <2 ng/mL and PSA ≥ 0.5 vs. <0.5 ng/mL. No significant difference was found in detection efficiency between those with PSA doubling time ≥ 12 vs. <12 months. Detection efficiency is statistically related to serum PSA levels but not to PSA doubling time based on available data. The detection efficiency of [18F]DCFPyL in men with BRPCa has trended down since a previous meta-analysis, which may reflect increasingly stringent inclusion criteria for studies over time.
1. Introduction
Prostate cancer (PCa) remains the most common non-cutaneous cancer among men worldwide [1]. Although the majority of patients with localized disease have a favorable response to initial treatment with either radical prostatectomy or radiation therapy, biochemical recurrence (BCR) remains relatively common and, if left untreated, can progress to incurable metastatic disease. According to the American Urological Association (AUA), BCR after radical prostatectomy is defined as two consecutive serum PSA values of ≥0.2 ng/mL after being undetectable [2], whereas BCR after radiation therapy is defined as a serum prostate-specific antigen (PSA) rise of ≥2.0 ng/mL above the nadir [3]. A key step in selecting the most appropriate form of treatment for BCR is to determine the anatomical distribution and volume of a patient’s disease. Compared to standard clinical parameters alone, the use of molecular imaging with [18F]FACBC PET/CT to guide salvage radiation therapy has been shown to improve outcomes for men with BCR after radical prostatectomy [4]. In the time since completing that trial, molecular imaging with PET radiotracers targeting prostate-specific membrane antigen (PSMA) has emerged as the standard of care for men with BCR. Although a trial is currently underway evaluating the benefits of using PSMA-targeted PET imaging to guide salvage treatment in men with BCR [5,6], most clinicians have already begun using this form of molecular imaging to inform decisions regarding the care of patients with BCR.
Prostate-specific membrane antigen (PSMA) is a transmembrane protein that is localized within the cytoplasm in benign prostatic cells, with malignant transformation resulting in expression on the surface of cells with a resultant large extracellular domain [7]. It is known that PSMA Expression is 100- to 1000-fold higher in PCa versus benign cells, and that small molecules capable of binding to the catalytic domain of the PSMA protein are rapidly internalized within PCa cells [8]. In recent years, it has become possible to not only detect but also treat PCa using radiolabeled small molecules targeting PSMA. For the purpose of diagnostic imaging, the two most commonly used PET imaging agents for targeting PSMA are [18F]DCFPyL and [68Ga]PSMA-11.
In contrast to gallium-68, fluorine-18 has a longer half-life and better spatial resolution, which makes it an ideal agent for cancer detection [9]. Because of these properties, [18F]DCFPyL has emerged as the radiotracer of choice among most clinicians, at least in the United States. A prior meta-analysis of the available medical literature up to December 2020 showed that the agent is associated with a relatively high detection rate among patients with BCR and that the rate of prostate cancer detection is highly dependent on the patient’s serum prostate-specific antigen (PSA) level [10]. In this report, we provided an updated systematic review and meta-analysis of the diagnostic performance of this agent among patients with BCR following definitive local treatment for PCa, with a particular emphasis on correlations to PSA parameters. This updated meta-analysis incorporates the results of several key clinical trials [11,12,13] that have been published since the time of the earlier report and includes data on three times as many patients.
2. Materials and Methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist. A comprehensive search of the literature was performed on 29 September 2022 using the MEDLINE database. Clinical studies in humans evaluating the diagnostic performance of [18F]DCFPyL among patients with BRPCa that were written in the English language were included. The overall pooled detection rate with 95% confidence intervals (95% CIs) was calculated among all included studies. In addition, we compared the pooled detection rates among patients with PSA ≥ 2 ng/mL versus those with PSA < 2 ng/mL, as well as the pooled detection rates in patients with PSA < 0.5 ng/mL versus those with PSA ≥ 0.5 ng/mL. We also stratified the detection rate according to the location of malignant tissue, including local recurrence (prostate bed and seminal vesicles), locoregional lymph node involvement, osseous metastases, and visceral metastases. Meta-analysis for calculation of the pooled proportion of patients with positive findings was performed with R version 4.2.2 (31 October 2022) using a meta package (version 6.0-0) [14].
We also compared the pooled proportion of positive scans in two studies [15,16] according to PSA doubling time < 12 months versus those ≥ 12 months using Review Manager (RevMan) version 5.4. We performed the meta-analysis based on a random-effects model. I2 was calculated to quantify the heterogeneity.
To evaluate the contribution of possible covariates in the heterogeneity, meta-regression analysis was performed with R version 4.2.2 using meta package version 6.0-0 for overall detection. Logit transformation with the inverse variance method was used to perform a meta-analysis of proportions. In addition, Funnel plots were used to assess publication bias. The overall quality of the studies was evaluated based on the revised “Quality Assessment of Diagnostic Accuracy Studies” tool (QUADAS-2) using Review Manager (RevMan) version 5.4 [17]. QUADAS-2 evaluates four domains (patient selection, index test, reference standard, and flow and timing), and each domain was assessed in terms of risk of bias. In addition, the first three domains were considered as a measure to check applicability. The reference standard was considered histopathologic correlation, and if the methods section of an included study clarified that at least some of the lesions were evaluated with pathology, the study was considered a low risk of bias for the reference standard. Otherwise, it was labeled as high risk for reference standards.
3. Results
Fifty-four articles were reviewed individually against our inclusion criteria. Fourteen studies were omitted by reviewing the title or abstract. Full texts of the remaining 40 studies were reviewed, and 22 studies were excluded. As such, a total of 17 manuscripts, including 2252 patients, met the inclusion criteria and were used for data extraction (Figure 1 and Table 1). Two studies by Mena et al. [12,18] showed significant overlap, and only the later study with a greater number of cases was included in the meta-analysis to assess overall detection [12]. However, the smaller and earlier study published in 2020 [18] was used to calculate the pooled proportion of positive cases stratified based on the location of disease, the information of which was not provided in the larger study.
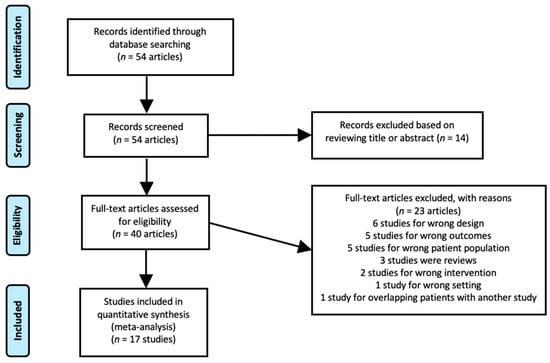
Figure 1.
PRISMA diagram of the study.

Table 1.
Characteristics of the included studies.
The proportion of patients showing positive scans, along with the total PSA values, PSA doubling time, and location of PSMA avid lesions, were extracted. A forest plot representing the pooled data from all included studies showing the proportion of patients with positive scans is shown in Figure 2. The overall detection rate between studies was markedly heterogeneous (range: 0.47–0.91, I2: 89%) and was influenced considerably by the absolute PSA level of imaged patients (Figure 3). Higher PSA values were associated with a significantly higher detection rate. In addition, stratifying the data based on PSA values resulted in an improvement of the heterogeneity, for example, a comparison of the proportion of patients showing the proportion of positive [18F]DCFPyL scans among those with PSA < 2 compared to those with PSA ≥ 2 (Figure 3A) showed I2 of 0 and among those with PSA < 0.5 versus PSA ≥ 0.5 showed I2 of 44%. In contrast, we did not observe a difference in the cancer detection efficiency with stratification by PSA doubling time of ≤12 versus >12 months (Figure 4).
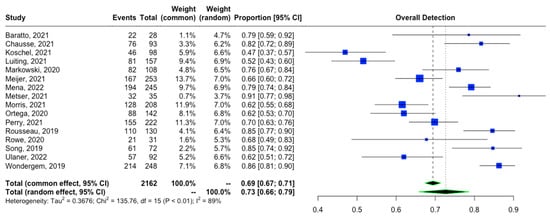
Figure 2.
Overall detection rate of [18F]DCFPyL in patients with BRPCa [11,12,13,15,16,19,20,21,22,23,24,25,26,27,28,29].
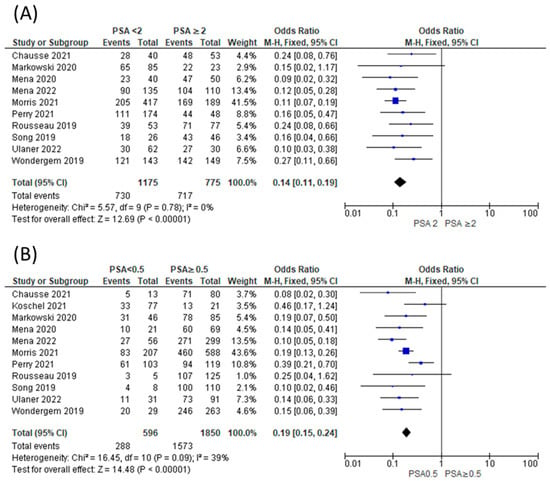
Figure 3.
Comparison of proportion of patients showing positive [18F]DCFPyL scans among those with PSA < 2 compared to those with PSA ≥ 2 (A) [11,12,13,15,16,20,26,27,29] and among those with PSA < 0.5 versus PSA ≥ 0.5 (B) [11,12,13,15,16,20,21,26,27,29].
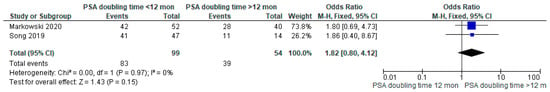
Figure 4.
Comparison of proportion of patients showing positive [18F]DCFPyL scan among those with PSA doubling time <12 months versus >12 months [15,16].
Figure 5 illustrates the pooled proportion of positive scans stratified according to different anatomical regions, including local recurrence, which itself is comprised of prostate bed and seminal vesicles (Figure 5A), locoregional lymph node involvement (Figure 5B), distant lymph node involvement (Figure 5C), bone metastases (Figure 5D), and visceral metastases (Figure 5E). The pooled proportions and 95% CIs of positive scans by anatomical site were 0.26 (95% CI: 0.19–0.32), 0.37 (95% CI: 0.29–0.46), 0.14 (95% CI: 0.06–0.30), 0.17 (95% CI: 0.08–0.33), and 0.08 (95% CI: 0.06–0.10), respectively. These results were associated with moderate to high heterogeneity; the lowest heterogeneity in detection rate was among those with visceral metastasis (I2 = 55%), and the highest heterogeneity was among those with locoregional lymphadenopathy (I2 = 99%).
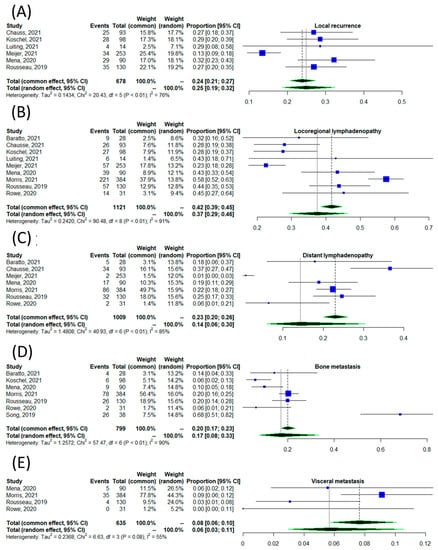
Figure 5.
The detection rate of [18F]DCFPyL scan according to the site of disease, including local recurrence (A) [18,20,21,22,23,27], locoregional lymphadenopathy (B) [11,18,19,20,21,22,23,27,28], distant lymphadenopathy (C) [11,18,19,20,23,27,28], osseous metastases (D) [11,16,18,19,21,27,28], and visceral metastases (E) [18,20,21,22,23,27].
To further assess the heterogeneity, a meta-regression was performed using histopathologic validation as the standard of truth. We found that ten studies did not provide histopathologic validation, while seven studies verified at least a portion of lesions with radiotracer uptake via tissue sampling (Table 1). Only one study compared all imaging findings with the ground truth of histopathology [13]. Meta-regression did not show a statistically significant difference.
A funnel plot was used for the evaluation of possible publication bias. By visual inspection, there is no subjective asymmetry among the studies in overall detection rate evaluation (Figure 6).
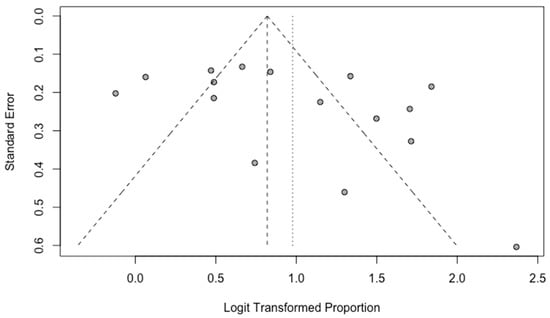
Figure 6.
Funnel plot for the studies included in the evaluation of the overall detection rate.
Assessment of the risk of bias using QUADAS-2 showed that, in addition to the reference standard, overall, the studies showed a low risk of bias (Figure 7). Six studies did not include any pathologic correlation as a measure of the reference standard, and four studies did not clarify their approach.
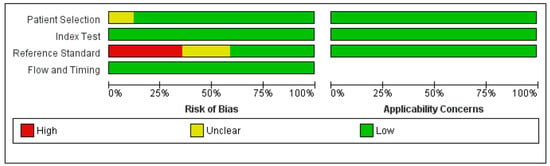
Figure 7.
Risk of bias and applicability concerns summary based on QUADAS-2.
4. Discussion
In this systematic review and meta-analysis, we provide updated estimates of the pooled sensitivity of [18F]DCFPyL PET/CT for detecting sites of disease in men with BRPCa following local definitive therapy. This is also the first meta-analysis to include the definitive phase III trial (CONDOR) that helped establish [18F]DCFPyL as a standard of care in men with BRPCa. Additionally, we stratified the data according to the PSA level and PSA doubling time and showed that the proportion of positive scans is significantly higher among those with PSA values ≥ 2 ng/mL versus <2 ng/mL and among those with PSA values ≥ 0.5 versus <0.5. These findings confirm that higher PSA levels improve detection efficiency. This may impact decision-making regarding the timing of when to image a patient, but also demonstrates that there is no cut-off below which there is not at least a moderate detection efficiency. In contrast, the rates of cancer detection by PSA doubling time did not show a significant difference at the conventional p-value threshold of 0.05. Although this finding may represent reality, it is more likely that our study was simply underpowered to detect an association between this parameter and cancer detection.
This meta-analysis is an update to a previously published study by Sun et al. [10], which included nine studies in their meta-analysis of [18F]DCFPyL overall detection rate in BCR. Our study compiled 17 studies for this purpose, including the most up-to-date studies during the last year. Sun et al. reported that the pooled detection rate was 0.81 (95% CI: 0.77–0.85), while our study showed a pooled overall detection rate of 0.73 (95% CI: 0.67–0.79), which may reflect an overall trend toward a more stringent definition of BCR in study inclusion criteria. In addition, we noticed an increase in heterogeneity, with I2 being 53.2% in the prior study and 90.0% in ours. Subjective evaluation of the outliers on the forest plot (Figure 2) showed that the possible contributors are the studies by Koschel et al. [18] and Luiting et al. [19]. In addition, heterogeneity was further assessed with meta-regression based on the presence of histopathologic validation as the standard of truth. The bubble plot in Figure 6 demonstrated that the difference in the standard of truth at least partially could explain the heterogeneity.
Stratification of the results according to the site of disease was comparable to the findings of the study by Sun et al. [10]. Similar to our study, Sun et al. also found regional lymphadenopathy to be the most common site of disease and osseous metastasis as the least common site of metastasis. The slightly higher proportions in their study could be at least partly related to regression to the mean.
Limitations of the current analysis include the high heterogeneity among trials and the fundamental differences in truth standards that were utilized. The majority of the studies did not stratify the results based on prior radiation and/or radical prostatectomy, which likely contributed to the high heterogeneity observed in our results. Nonetheless, this study serves as an important update on the importance of PSMA-targeted imaging with [18F]DCFPyL in men with BRPCa.
5. Conclusions
Our meta-analysis shows that the detection efficiency of [18F]DCFPyL in men with BRPCa remains overall high but has trended down from earlier estimates in the literature, leading to increased heterogeneity in the reporting of this outcome. This observation may be related to the use of increasingly stringent inclusion criteria in studies over time, such as the requirement for negative conventional imaging as in the CONDOR trial. Another factor may be the trend toward imaging of men with lower PSA values in more recent studies. Indeed, our analysis has shown that PSA level at the time of imaging is unmistakably linked to cancer detection rates, whereas PSA doubling time was not. Finally, differences in the required truth standard for test positivity have likely also contributed to the high degree of heterogeneity in the literature, and this must be taken into account when comparing cancer detection rates across studies.
Author Contributions
Conceptualization, M.S.S., M.G.P., M.A.G. and S.P.R.; methodology, M.S.S., S.S. and A.A.-Z.; formal analysis, M.S.S., S.S. and A.A.-Z.; investigation, M.S.S., S.S., A.A.-Z., L.B.S., J.D.O. and G.A.U.; resources, S.P.R.; data curation, M.S.S., S.S., A.A.-Z. and L.B.S.; writing—original draft preparation, M.S.S.; writing—review and editing, S.S., A.A.-Z., L.B.S., M.G.P., J.D.O., G.A.U., M.A.G. and S.P.R.; supervision, M.G.P., J.D.O., G.A.U., M.A.G. and S.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge funding from the National Institutues of Health, CA134675.
Informed Consent Statement
Informed consent was not obtained as this study was a meta-analysis of existing data and no new patients were accrued or included in the data analysis.
Data Availability Statement
No new data were generated in this study.
Conflicts of Interest
Under a license agreement between Progenics (a wholly owned subsidiary of Lantheus) and Johns Hopkins University, M.G.P., and the University are entitled to royalties on an invention described in this article. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. M.A.G. has served as a consultant to Progenics. S.P.R. is a consultant to Progenics.
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [PubMed]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Jani, A.B.; Schreibmann, E.; Goyal, S.; Halkar, R.; Hershatter, B.; Rossi, P.J.; Shelton, J.W.; Patel, P.R.; Xu, K.M.; Goodman, M.; et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): A single centre, open-label, phase 2/3 randomised controlled trial. Lancet 2021, 397, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Armstrong, W.R.; Kishan, A.U.; Booker, K.M.; Hope, T.A.; Fendler, W.P.; Elashoff, D.; Nickols, N.G.; Czernin, J. Update from PSMA-SRT Trial NCT03582774: A Randomized Phase 3 Imaging Trial of Prostate-specific Membrane Antigen Positron Emission Tomography for Salvage Radiation Therapy for Prostate Cancer Recurrence Powered for Clinical Outcome. Eur. Urol. Focus 2021, 7, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Zhu, S.; Hirmas, N.; Eiber, M.; Hadaschik, B.; Stuschke, M.; Herrmann, K.; Czernin, J.; Kishan, A.U.; Nickols, N.G.; et al. Phase 3 multicenter randomized trial of PSMA PET/CT prior to definitive radiation therapy for unfavorable intermediate-risk or high-risk prostate cancer [PSMA dRT]: Study protocol. BMC Cancer 2021, 21, 512. [Google Scholar] [CrossRef]
- Wright, G.L., Jr.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA Theranostics: Review of the Current Status of PSMA-Targeted Imaging and Radioligand Therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef]
- Gorin, M.A.; Pomper, M.G.; Rowe, S.P. PSMA-targeted imaging of prostate cancer: The best is yet to come. BJU Int. 2016, 117, 715–716. [Google Scholar] [CrossRef]
- Sun, J.; Lin, Y.; Wei, X.; Ouyang, J.; Huang, Y.; Ling, Z. Performance of 18F-DCFPyL PET/CT Imaging in Early Detection of Biochemically Recurrent Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 649171. [Google Scholar] [CrossRef]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.C.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef]
- Mena, E.; Rowe, S.P.; Shih, J.H.; Lindenberg, L.; Turkbey, B.; Fourquet, A.; Lin, F.I.; Adler, S.; Eclarinal, P.; McKinney, Y.L.; et al. Predictors of 18F-DCFPyL PET/CT Positivity in Patients with Biochemical Recurrence of Prostate Cancer After Local Therapy. J. Nucl. Med. 2022, 63, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Thomsen, B.; Bassett, J.; Torrey, R.; Cox, C.; Lin, K.; Patel, T.; Techasith, T.; Mauguen, A.; Rowe, S.P.; et al. 18F-DCFPyL PET/CT for Initially Diagnosed and Biochemically Recurrent Prostate Cancer: Prospective Trial with Pathologic Confirmation. Radiology 2022, 305, 419–428. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Sedhom, R.; Fu, W.; Gray, J.C.R.; Eisenberger, M.A.; Pomper, M.G.; Pienta, K.J.; Gorin, M.A.; Rowe, S.P. Prostate Specific Antigen and Prostate Specific Antigen Doubling Time Predict Findings on 18F-DCFPyL Positron Emission Tomography/Computerized Tomography in Patients with Biochemically Recurrent Prostate Cancer. J. Urol. 2020, 204, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Harrison, C.; Duan, H.; Guja, K.; Hatami, N.; Franc, B.L.; Moradi, F.; Aparici, C.M.; Davidzon, G.A.; Iagaru, A. Prospective Evaluation of 18F-DCFPyL PET/CT in Biochemically Recurrent Prostate Cancer in an Academic Center: A Focus on Disease Localization and Changes in Management. J. Nucl. Med. 2020, 61, 546–551. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Mena, E.; Lindenberg, M.L.; Turkbey, I.B.; Shih, J.H.; Harmon, S.A.; Lim, I.; Lin, F.; Adler, S.; Eclarinal, P.; McKinney, Y.L.; et al. 18F-DCFPyL PET/CT Imaging in Patients with Biochemically Recurrent Prostate Cancer After Primary Local Therapy. J. Nucl. Med. 2020, 61, 881–889. [Google Scholar] [CrossRef]
- Baratto, L.; Song, H.; Duan, H.; Hatami, N.; Bagshaw, H.P.; Buyyounouski, M.; Hancock, S.; Shah, S.; Srinivas, S.; Swift, P.; et al. PSMA- and GRPR-Targeted PET: Results from 50 Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2021, 62, 1545–1549. [Google Scholar] [CrossRef]
- Chausse, G.; Ben-Ezra, N.; Stoopler, M.; Levett, J.Y.; Niazi, T.; Anidjar, M.; Abikhzer, G.; Probst, S. Diagnostic performance of 18F-DCFPyL positron emission tomography/computed tomography for biochemically recurrent prostate cancer and change-of-management analysis. Can. Urol. Assoc. J. 2021, 15, 173–178. [Google Scholar] [CrossRef]
- Koschel, S.; Taubman, K.; Sutherland, T.; Yap, K.; Chao, M.; Guerrieri, M.; Benson, A.; Starmans, M.; Byrne, G.; Ong, G.; et al. Patterns of disease detection using [18F]DCFPyL PET/CT imaging in patients with detectable PSA post prostatectomy being considered for salvage radiotherapy: A prospective trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Luiting, H.B.; Remmers, S.; Meijer, D.; Vis, A.N.; Donswijk, M.; Oprea-Lager, D.E.; Emmett, L.; Rauscher, I.; Van der Poel, H.G.; Roobol, M.J.; et al. External Validation of Two Nomograms Developed for 68Ga-PSMA-11 Applied to the Prostate-specific Membrane Antigen Tracer 18F-DCFPyl: Is Prediction of the Optimal Timing of Salvage Therapy Feasible? Eur. Urol. Open Sci. 2021, 28, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Meijer, D.; van Leeuwen, P.J.; Oosterholt, P.M.J.; Bodar, Y.J.L.; van der Poel, H.G.; Hendrikse, N.H.; Donswijk, M.L.; Wondergem, M.; Vellekoop, A.E.; van Moorselaar, R.J.A.; et al. Management impact of 18F-DCFPyL PET/CT in hormone-sensitive prostate cancer patients with biochemical recurrence after definitive treatment: A multicenter retrospective study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Ortega, C.; Hussey, D.; Chan, R.; Berlin, A.; Finelli, A.; Veit-Haibach, P. 18F-DCFPyL (PSMA) PET in the Management of Men with Biochemical Failure after Primary Therapy: Initial Clinical Experience of an Academic Cancer Center. Curr. Oncol. 2021, 28, 3251–3258. [Google Scholar] [CrossRef]
- Ortega, C.; Schaefferkoetter, J.; Veit-Haibach, P.; Anconina, R.; Berlin, A.; Perlis, N.; Metser, U. 18F-DCFPyL PET/CT in Patients with Subclinical Recurrence of Prostate Cancer: Effect of Lesion Size, Smoothing Filter, and Partial-Volume Correction on PROMISE Criteria. J. Nucl. Med. 2020, 61, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Talwar, A.; Taubman, K.; Ng, M.; Wong, L.M.; Booth, R.; Sutherland, T.R. [18F]DCFPyL PET/CT in detection and localization of recurrent prostate cancer following prostatectomy including low PSA < 0.5 ng/mL. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2038–2046. [Google Scholar]
- Rousseau, E.; Wilson, D.; Lacroix-Poisson, F.; Krauze, A.; Chi, K.; Gleave, M.; McKenzie, M.; Tyldesley, S.; Goldenberg, S.L.; Benard, F. A Prospective Study on 18F-DCFPyL PSMA PET/CT Imaging in Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. 2019, 60, 1587–1593. [Google Scholar] [CrossRef]
- Rowe, S.P.; Campbell, S.P.; Mana-Ay, M.; Szabo, Z.; Allaf, M.E.; Pienta, K.J.; Pomper, M.G.; Ross, A.E.; Gorin, M.A. Prospective Evaluation of PSMA-Targeted 18F-DCFPyL PET/CT in Men with Biochemical Failure After Radical Prostatectomy for Prostate Cancer. J. Nucl. Med. 2020, 61, 58–61. [Google Scholar] [CrossRef]
- Wondergem, M.; Jansen, B.H.E.; van der Zant, F.M.; van der Sluis, T.M.; Knol, R.J.J.; van Kalmthout, L.W.M.; Hoekstra, O.S.; van Moorselaar, R.J.A.; Oprea-Lager, D.E.; Vis, A.N. Early lesion detection with 18F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1911–1918. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).