Repeated Lung Ultrasound versus Chest X-ray—Which One Predicts Better Clinical Outcome in COVID-19?
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Schedule
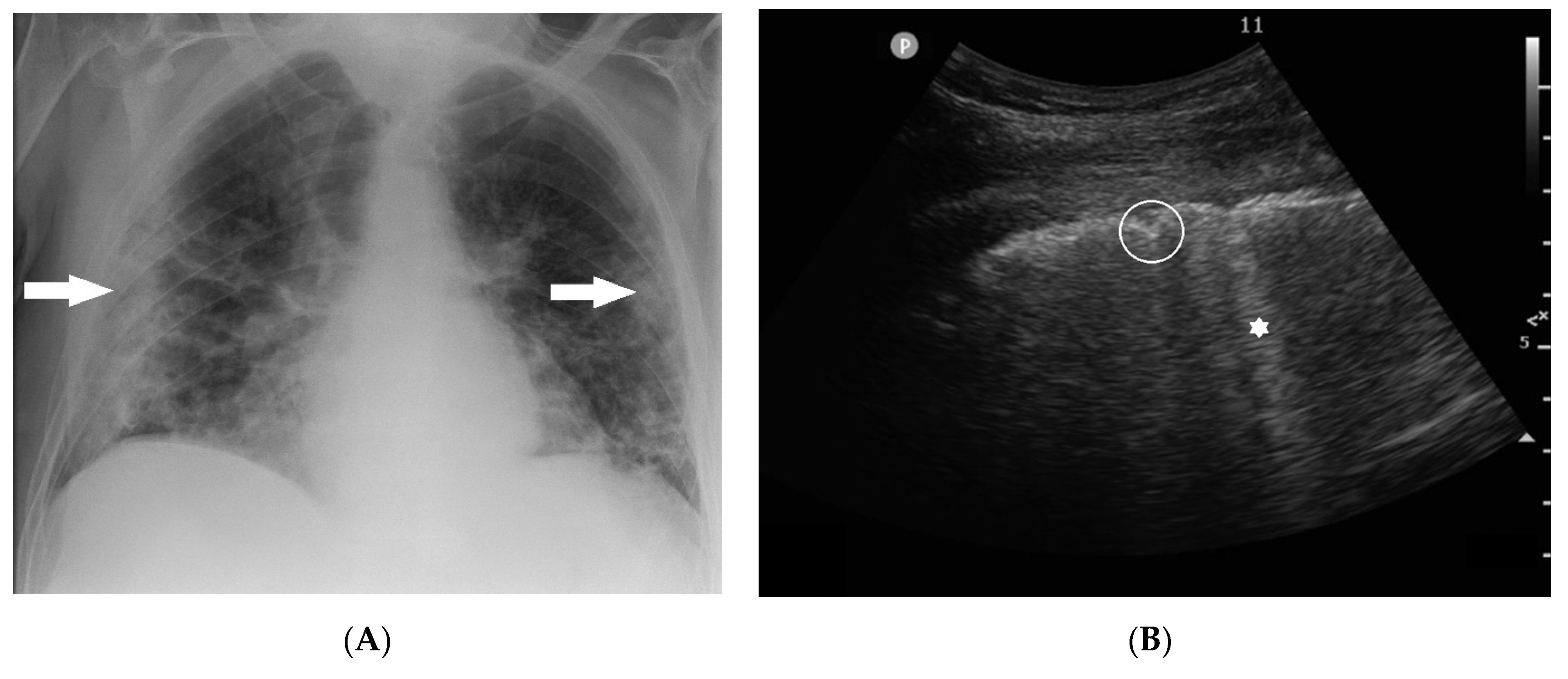
2.2.1. Chest X-ray
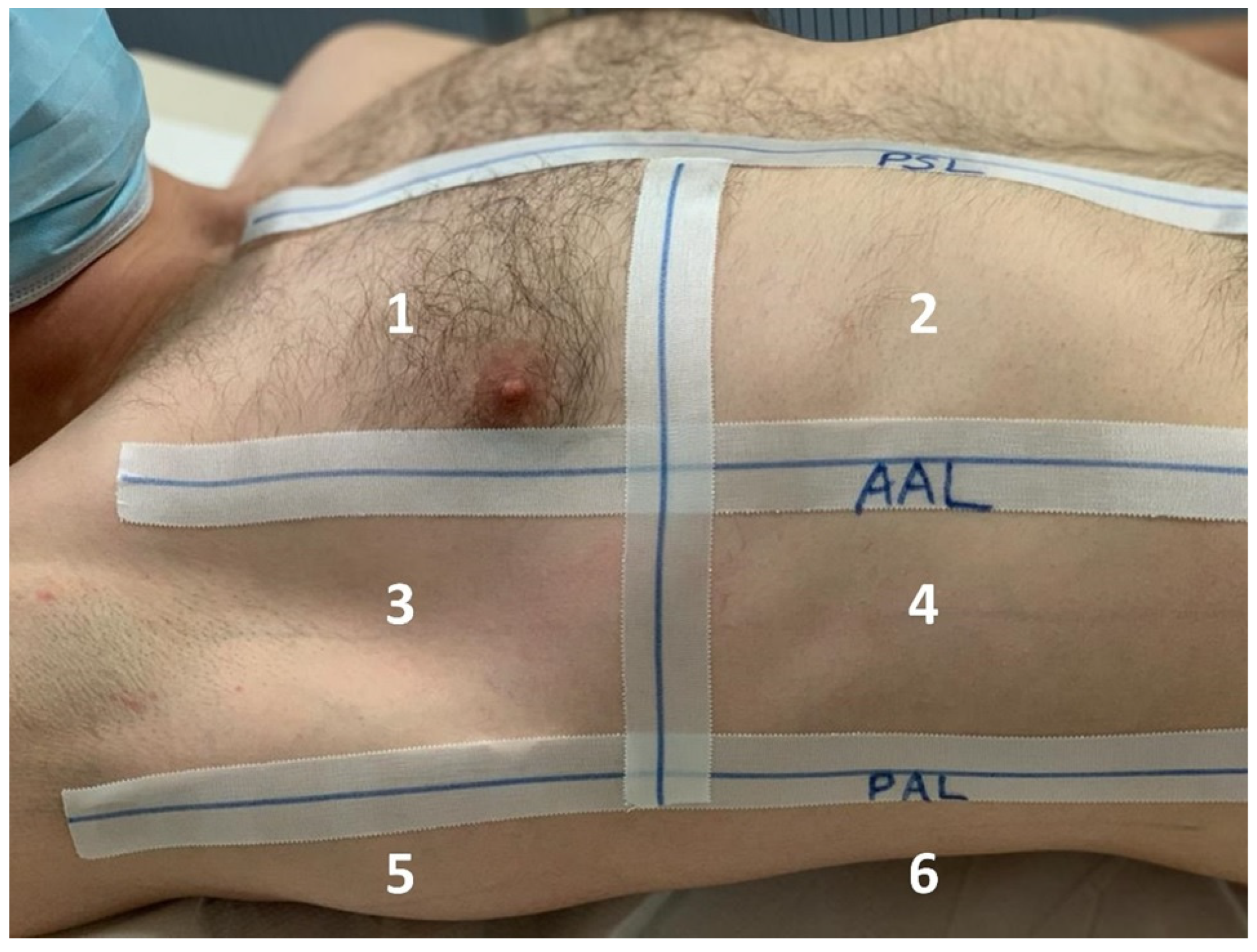
2.2.2. Lung Ultrasound
2.3. Statistical Analysis
3. Results
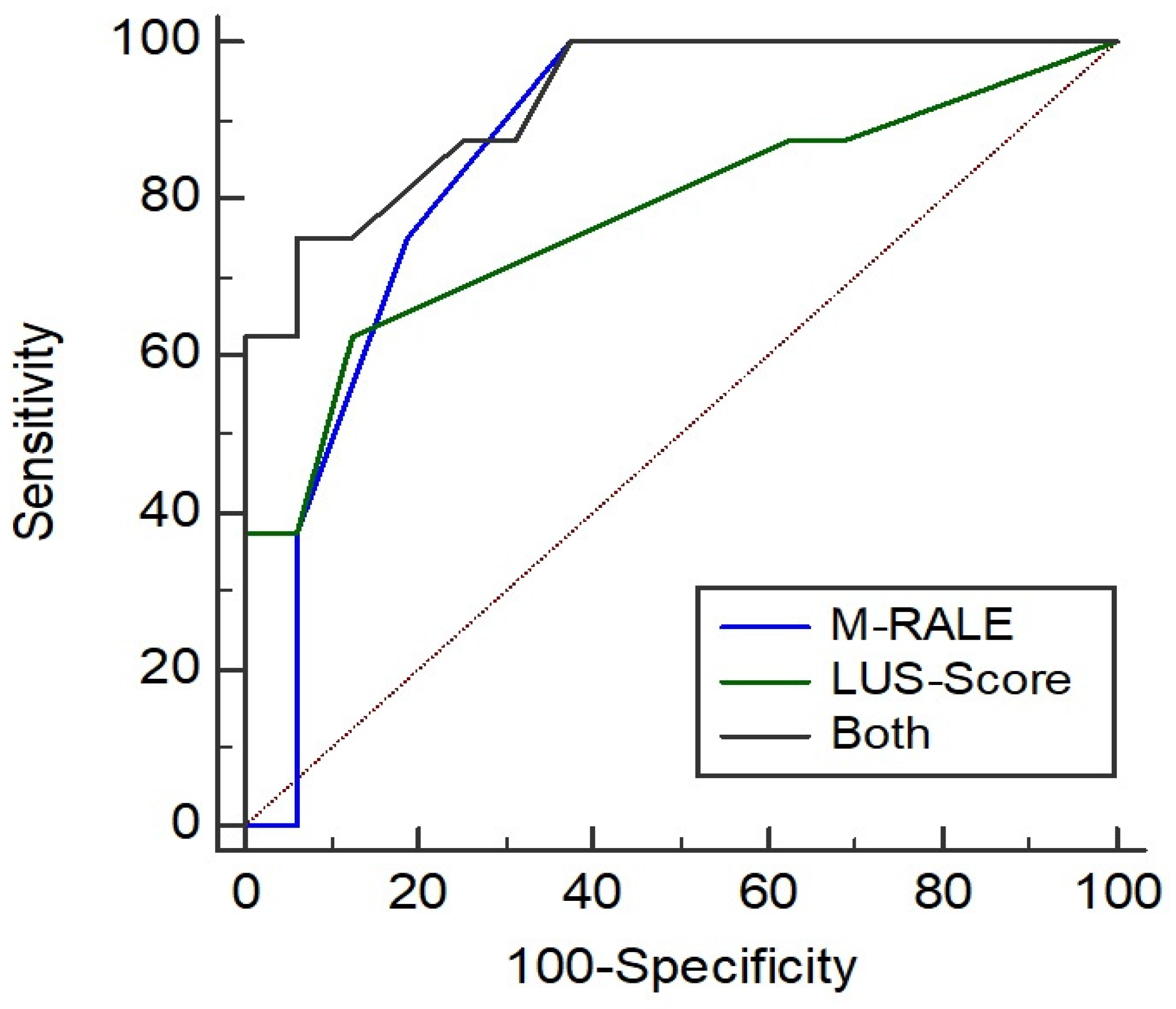
3.1. Association of RALE and Need for ICU Treatment
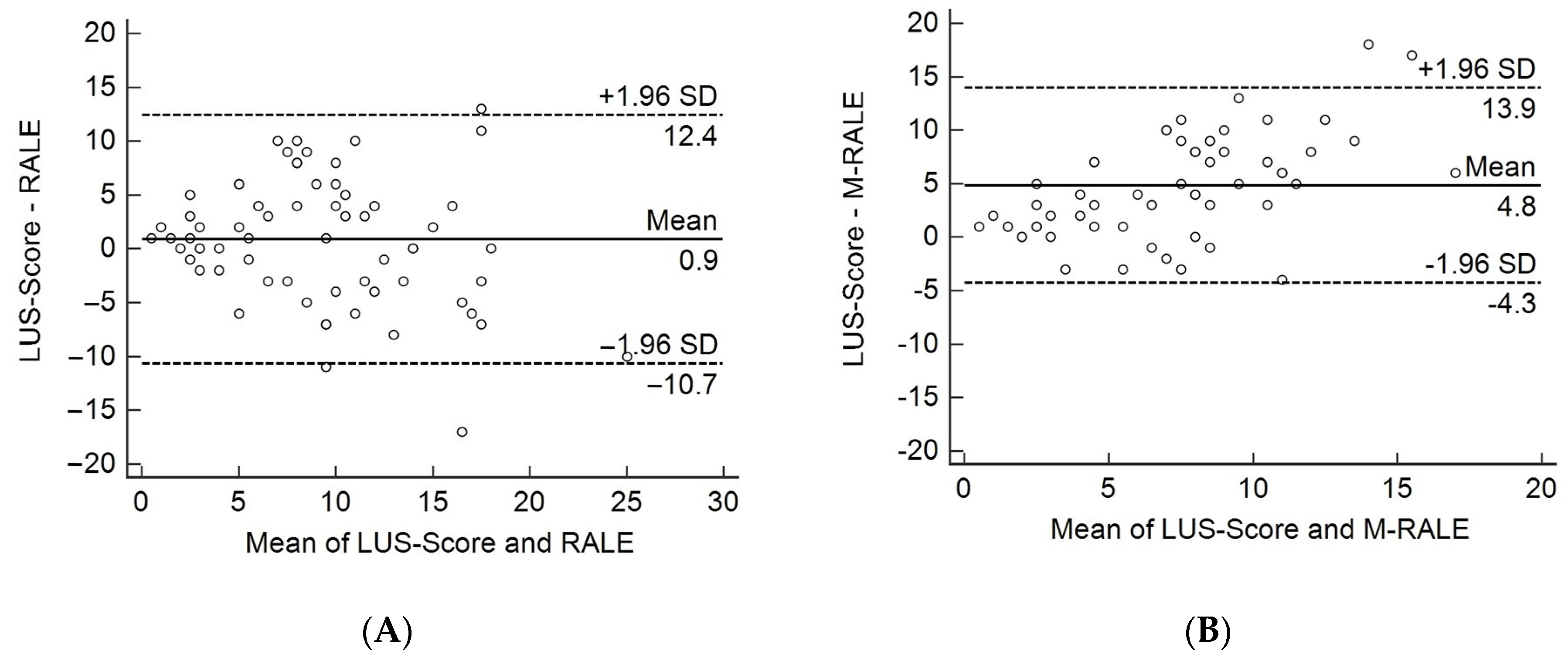
3.2. Comparison of CXR with LUS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell, C.-R.C.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Blazic, I.; Yaacoub, S.; Frija, G.; Chou, R.; Appiah, J.A.; Fatehi, M.; Flor, N.; Hitti, E.; Jafri, H.; et al. Use of Chest Imaging in the Diagnosis and Management of COVID-19: A WHO Rapid Advice Guide. Radiology 2021, 298, E63–E69. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295, 200463. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Prokop, M.; Everdingen, W.; van Rees Vellinga, T.; Quarles van Ufford, H.; Stoger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef]
- Orlandi, M.; Landini, N.; Sambataro, G.; Nardi, C.; Tofani, L.; Bruni, C.; Bellando-Randone, S.; Blagojevic, J.; Melchiorre, D.; Hughes, M.; et al. The role of chest CT in deciphering interstitial lung involvement: Systemic sclerosis versus COVID-19. Rheumatology 2022, 61, 1600–1609. [Google Scholar] [CrossRef]
- Baratella, E.; Ruaro, B.; Marrocchio, C.; Starvaggi, N.; Salton, F.; Giudici, F.; Quaia, E.; Confalonieri, M.; Cova, M.A. Interstitial Lung Disease at High Resolution CT after SARS-CoV-2-Related Acute Respiratory Distress Syndrome According to Pulmonary Segmental Anatomy. J. Clin. Med. 2021, 10, 3985. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Zhang, H.W.; Yu, J.; Xu, H.J.; Lei, Y.; Pu, Z.H.; Dai, W.C.; Lin, F.; Wang, Y.L.; Wu, X.L.; Liu, L.H.; et al. Corona Virus International Public Health Emergencies: Implications for Radiology Management. Acad. Radiol. 2020, 27, 463–467. [Google Scholar] [CrossRef]
- Heitzman, E.R. Thoracic radiology: The past 50 years. Radiology 2000, 214, 309–313. [Google Scholar] [CrossRef] [PubMed]
- McAdams, H.P.; Samei, E.; Dobbins, J., III; Tourassi, G.D.; Ravin, C.E. Recent advances in chest radiography. Radiology 2006, 241, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.A.; Zhao, Z.; Koyama, T.; Bastarache, J.A.; Shaver, C.M.; Semler, M.W.; Rice, T.W.; Matthay, M.A.; Calfee, C.S.; Ware, L.B. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax 2018, 73, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.Y.F.; Lam, H.Y.S.; Fong, A.H.; Leung, S.T.; Chin, T.W.; Lo, C.S.Y.; Lui, M.M.; Lee, J.C.Y.; Chiu, K.W.; Chung, T.W.; et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology 2020, 296, E72–E78. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Crivelli, P.; Marrocchio, C.; Bozzato, A.M.; Vito, A.; Madeddu, G.; Saderi, L.; Confalonieri, M.; Tenaglia, L.; Cova, M.A. Severity of lung involvement on chest X-rays in SARS-coronavirus-2 infected patients as a possible tool to predict clinical progression: An observational retrospective analysis of the relationship between radiological, clinical, and laboratory data. J. Bras. Pneumol. 2020, 46, e20200226. [Google Scholar] [CrossRef]
- Borghesi, A.; Golemi, S.; Scrimieri, A.; Nicosia, C.M.C.; Zigliani, A.; Farina, D.; Maroldi, R. Chest X-ray versus chest computed tomography for outcome prediction in hospitalized patients with COVID-19. Radiol. Med. 2022, 127, 305–308. [Google Scholar] [CrossRef]
- Bellani, G.; Rouby, J.J.; Constantin, J.M.; Pesenti, A. Looking closer at acute respiratory distress syndrome: The role of advanced imaging techniques. Curr. Opin. Crit. Care 2017, 23, 30–37. [Google Scholar] [CrossRef]
- Bouhemad, B.; Mongodi, S.; Via, G.; Rouquette, I. Ultrasound for "lung monitoring" of ventilated patients. Anesthesiology 2015, 122, 437–447. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Peng, Q.Y.; Wang, X.T.; Zhang, L.N.; Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020, 46, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Piano, A.; Raffaelli, F.; Bonadia, N.; de Gaetano Donati, K.; Franceschi, F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: A case report and potential applications during COVID-19 outbreak. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2776–2780. [Google Scholar] [PubMed]
- Gil-Rodriguez, J.; Perez de Rojas, J.; Aranda-Laserna, P.; Benavente-Fernandez, A.; Martos-Ruiz, M.; Peregrina-Rivas, J.A.; Guirao-Arrabal, E. Ultrasound findings of lung ultrasonography in COVID-19: A systematic review. Eur. J. Radiol. 2022, 148, 110156. [Google Scholar] [CrossRef] [PubMed]
- Tung-Chen, Y.; Marti de Gracia, M.; Diez-Tascon, A.; Alonso-Gonzalez, R.; Agudo-Fernandez, S.; Parra-Gordo, M.L.; Ossaba-Velez, S.; Rodriguez-Fuertes, P.; Llamas-Fuentes, R. Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med. Biol. 2020, 46, 2918–2926. [Google Scholar] [CrossRef]
- Vetrugno, L.; Bove, T.; Orso, D.; Barbariol, F.; Bassi, F.; Boero, E.; Ferrari, G.; Kong, R. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography 2020, 37, 625–627. [Google Scholar] [CrossRef]
- Gibbons, R.C.; Magee, M.; Goett, H.; Murrett, J.; Genninger, J.; Mendez, K.; Tripod, M.; Tyner, N.; Costantino, T.G. Lung Ultrasound vs. Chest X-Ray Study for the Radiographic Diagnosis of COVID-19 Pneumonia in a High-Prevalence Population. J. Emerg. Med. 2021, 60, 615–625. [Google Scholar] [CrossRef]
- Martinez Redondo, J.; Comas Rodriguez, C.; Pujol Salud, J.; Crespo Pons, M.; Garcia Serrano, C.; Ortega Bravo, M.; Palacin Peruga, J.M. Higher Accuracy of Lung Ultrasound over Chest X-ray for Early Diagnosis of COVID-19 Pneumonia. Int. J. Environ. Res. Public Health 2021, 18, 3481. [Google Scholar] [CrossRef] [PubMed]
- Mateos Gonzalez, M.; Garcia de Casasola Sanchez, G.; Munoz, F.J.T.; Proud, K.; Lourdo, D.; Sander, J.V.; Jaimes, G.E.O.; Mader, M.; Canora Lebrato, J.; Restrepo, M.I.; et al. Comparison of Lung Ultrasound versus Chest X-ray for Detection of Pulmonary Infiltrates in COVID-19. Diagnostics 2021, 11, 373. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bulla, P.; Jodicke, L.; Klein, C.; Bott, S.M.; Keller, R.; Malek, N.; Frohlich, E.; Gopel, S.; Blumenstock, G.; et al. Can follow up lung ultrasound in Coronavirus Disease-19 patients indicate clinical outcome? PLoS ONE 2021, 16, e0256359. [Google Scholar] [CrossRef]
- Ding, X.; Xu, J.; Zhou, J.; Long, Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 2020, 127, 109009. [Google Scholar] [CrossRef]
- Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; Costa, C.; Oliveira, A.G.; Ceia, F. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur. J. Heart Fail. 2004, 6, 807-12–821-2. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit. Care 2020, 24, 198. [Google Scholar] [CrossRef] [PubMed]
- Bouhemad, B.; Brisson, H.; Le-Guen, M.; Arbelot, C.; Lu, Q.; Rouby, J.J. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am. J. Respir. Crit. Care Med. 2011, 183, 341–347. [Google Scholar] [CrossRef]
- Buonsenso, D.; Pata, D.; Chiaretti, A. COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir. Med. 2020, 8, e27. [Google Scholar]
- Kulkarni, S.; Down, B.; Jha, S. Point-of-care lung ultrasound in intensive care during the COVID-19 pandemic. Clin. Radiol. 2020, 75, 710.e1–710.e4. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, S.; Chen, B.; Chen, J.; Xian, J.; Lin, Y.; Shan, H.; Su, Z.Z. A Clinical Study of Noninvasive Assessment of Lung Lesions in Patients with Coronavirus Disease-19 (COVID-19) by Bedside Ultrasound. Ultraschall Med. 2020, 41, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Ruaro, B.; Marrocchio, C.; Poillucci, G.; Pigato, C.; Bozzato, A.M.; Salton, F.; Confalonieri, P.; Crimi, F.; Wade, B.; et al. Diagnostic Accuracy of Chest Digital Tomosynthesis in Patients Recovering after COVID-19 Pneumonia. Tomography 2022, 8, 1221–1227. [Google Scholar] [CrossRef]
- Chou, S.H.; Kicska, G.A.; Pipavath, S.N.; Reddy, G.P. Digital tomosynthesis of the chest: Current and emerging applications. Radiographics 2014, 34, 359–372. [Google Scholar] [CrossRef]
- Meltzer, C.; Fagman, E.; Vikgren, J.; Molnar, D.; Borna, E.; Beni, M.M.; Brandberg, J.; Bergman, B.; Bath, M.; Johnsson, A.A. Surveillance of small, solid pulmonary nodules at digital chest tomosynthesis: Data from a cohort of the pilot Swedish CArdioPulmonary bioImage Study (SCAPIS). Acta Radiol. 2021, 62, 348–359. [Google Scholar] [CrossRef] [PubMed]

| Age, Mean (n, SD) | 70 (13.3) |
| Sex, male (n, %) | 17 (57%) |
| Transfer to ICU (n, %) | 8 (27%) |
| In-hospital mortality (n, %) | 5 (16%) |
| Prior history of heart disease (n, %) | 12 (40%) |
| Prior history of lung disease (n, %) | 11 (37%) |
| Prior history of immunosuppressant therapy (n, %) | 3 (10%) |
| Prior history of cancer (n, %) | 7 (23%) |
| Initial oxygen supplementation (n, %) | 16 (53%) |
| Fever (≥38.3 °C) (n, %) | 8 (27%) |
| Dyspnea (n, %) | 14 (47%) |
| Tachypnea (≥20/min) (n, %) | 24 (80%) |
| Oxygen saturation (<95%) (n, %) | 17 (57%) |
| Early warning score ≥8 (n, %) | 14 (47%) |
| Baseline Median (IQR) | Follow-Up 1 Median (IQR) | Follow-Up 2 Median (IQR) | |
|---|---|---|---|
| RALE | 5.5 (2–13) | 10 (4–14) | 10 (6–14) |
| M-RALE | 3.5 (2–7) | 5 (2–8) | 5 (3–7) |
| LUS score | 8 (4–12) | 10 (6–13) | 10 (5–12.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spogis, J.; Fusco, S.; Hagen, F.; Kaufmann, S.; Malek, N.; Hoffmann, T. Repeated Lung Ultrasound versus Chest X-ray—Which One Predicts Better Clinical Outcome in COVID-19? Tomography 2023, 9, 706-716. https://doi.org/10.3390/tomography9020056
Spogis J, Fusco S, Hagen F, Kaufmann S, Malek N, Hoffmann T. Repeated Lung Ultrasound versus Chest X-ray—Which One Predicts Better Clinical Outcome in COVID-19? Tomography. 2023; 9(2):706-716. https://doi.org/10.3390/tomography9020056
Chicago/Turabian StyleSpogis, Jakob, Stefano Fusco, Florian Hagen, Sascha Kaufmann, Nisar Malek, and Tatjana Hoffmann. 2023. "Repeated Lung Ultrasound versus Chest X-ray—Which One Predicts Better Clinical Outcome in COVID-19?" Tomography 9, no. 2: 706-716. https://doi.org/10.3390/tomography9020056
APA StyleSpogis, J., Fusco, S., Hagen, F., Kaufmann, S., Malek, N., & Hoffmann, T. (2023). Repeated Lung Ultrasound versus Chest X-ray—Which One Predicts Better Clinical Outcome in COVID-19? Tomography, 9(2), 706-716. https://doi.org/10.3390/tomography9020056








