Structural Relationship between Cerebral Gray and White Matter Alterations in Degenerative Cervical Myelopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Presenting Symptoms
2.3. Physical Examination
2.4. Radiographical Imaging
2.5. Diffusion Spectral Imaging (DSI) and T1-Weighted Structural MRI Acquisition
2.6. T1-Weighted Structural Image Processing
2.7. DSI Processing
2.8. Whole-Brain Gray Matter Thickness Statistical Analysis
2.9. Region of Interest Subcortical White Matter Analyses
3. Results
3.1. Clinical Summary
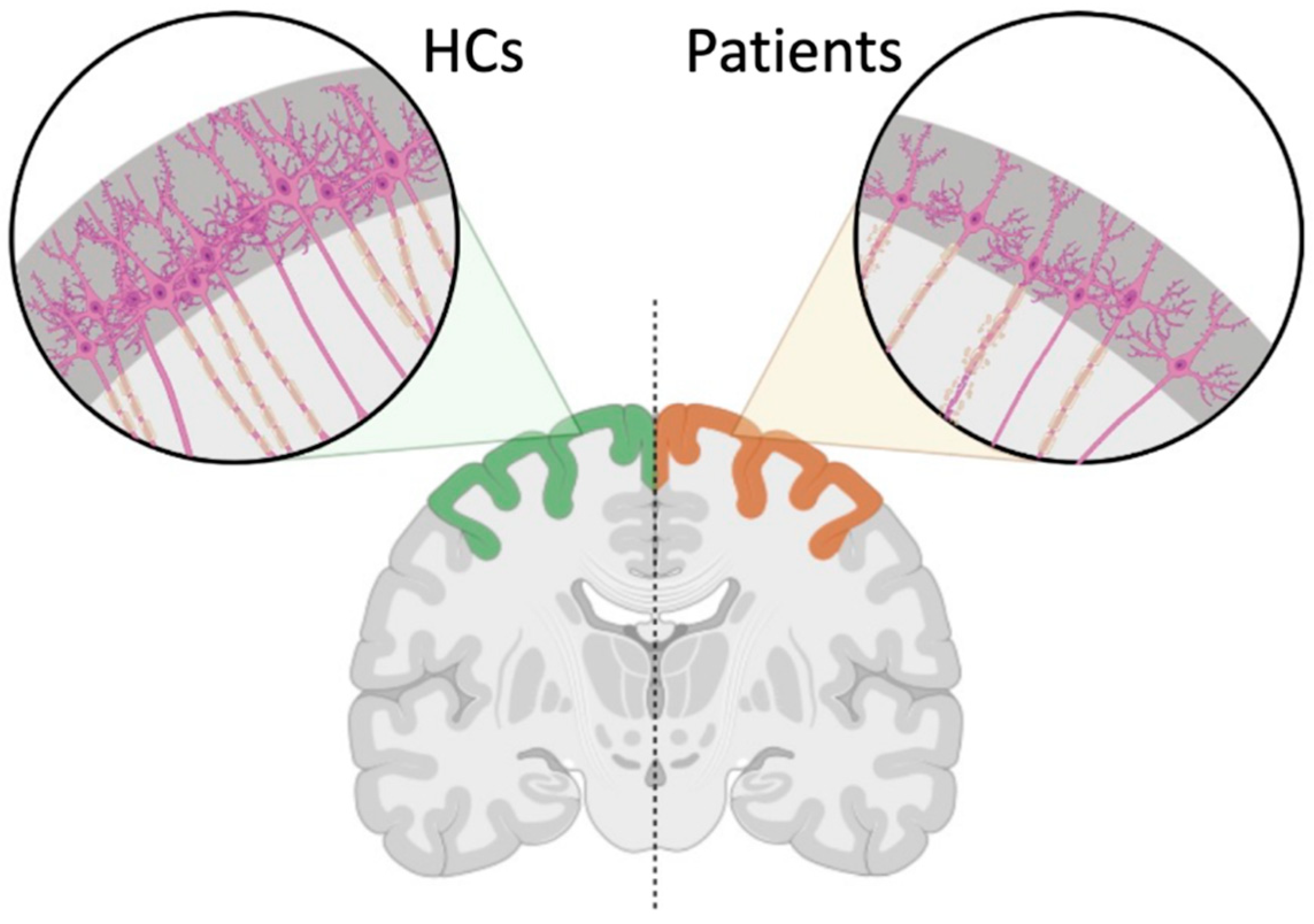
3.2. Structural Alterations
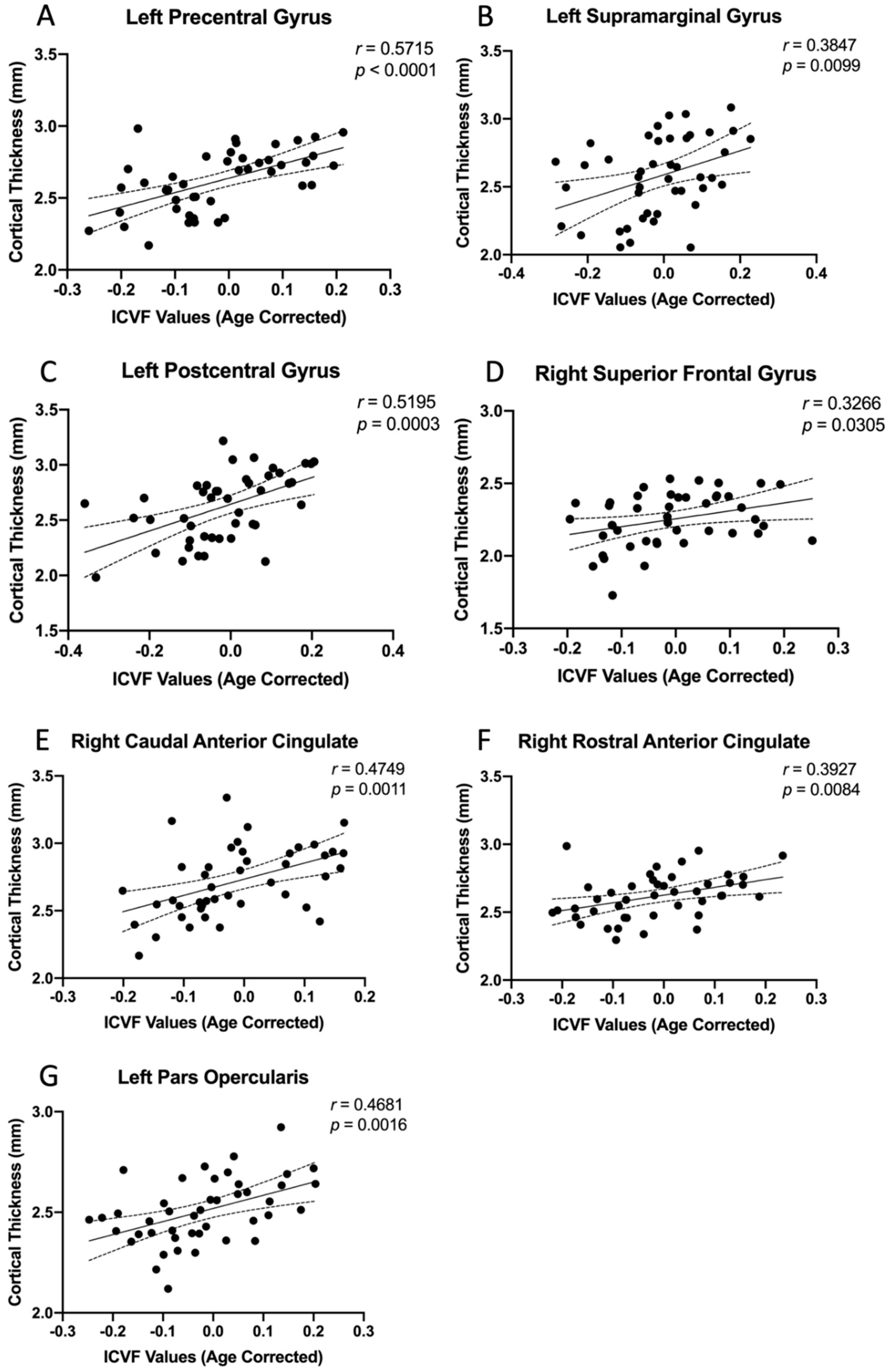
3.3. Region-Specific Structural Relationship
4. Discussion
4.1. Gray and White Matter Alterations Associated with DCM
4.2. Clinical Implications
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Lancet Neurology. A focus on patient outcomes in cervical myelopathy. Lancet Neurol. 2019, 18, 615. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.M.; Yan, R.B.; Xiang, Z.M.; Zhang, H.; Liu, J.; Wu, Y.T.; Zhao, M.; Pan, Q.Y.; Song, L.H.; Zhang, W.; et al. Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience 2014, 266, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Sanz, A.; Molla-Torro, J.V.; Lopez-Celada, S.; Moreno Lopez, P.; Fernandez-Jover, E. MRI evidence of brain atrophy, white matter damage, and functional adaptive changes in patients with cervical spondylosis and prolonged spinal cord compression. Eur. Radiol. 2019, 30, 357–369. [Google Scholar] [CrossRef]
- Wang, L.; Yu, B.; Li, Q.; Qi, F.; Guo, Q. Sensorimotor cortex atrophy in patients with cervical spondylotic myelopathy. Neuroreport 2018, 29, 826–832. [Google Scholar] [CrossRef]
- Zdunczyk, A.; Schwarzer, V.; Mikhailov, M.; Bagley, B.; Rosenstock, T.; Picht, T.; Vajkoczy, P. The Corticospinal Reserve Capacity: Reorganization of Motor Area and Excitability as a Novel Pathophysiological Concept in Cervical Myelopathy. Neurosurgery 2018, 83, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Hrabalek, L.; Hlustik, P.; Hok, P.; Wanek, T.; Otruba, P.; Cechakova, E.; Vaverka, M.; Kanovsky, P. [Effects of spinal cord decompression in patients with cervical spondylotic myelopathy oncortical brain activations]. Rozhl. Chir. 2014, 93, 530–535. [Google Scholar] [PubMed]
- Wang, C.; Laiwalla, A.; Salamon, N.; Ellingson, B.M.; Holly, L.T. Compensatory brainstem functional and structural connectivity in patients with degenerative cervical myelopathy by probabilistic tractography and functional MRI. Brain Res. 2020, 1749, 147129. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Holly, L.T.; Oughourlian, T.; Yao, J.; Raymond, C.; Salamon, N.; Ellingson, B.M. Detection of cerebral reorganization associated with degenerative cervical myelopathy using diffusion spectral imaging (DSI). J. Clin. Neurosci. 2021, 86, 164–173. [Google Scholar] [CrossRef]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef]
- Pieri, V.; Sanvito, F.; Riva, M.; Petrini, A.; Rancoita, P.M.V.; Cirillo, S.; Iadanza, A.; Bello, L.; Castellano, A.; Falini, A. Along-tract statistics of neurite orientation dispersion and density imaging diffusion metrics to enhance MR tractography quantitative analysis in healthy controls and in patients with brain tumors. Hum. Brain Mapp. 2021, 42, 1268–1286. [Google Scholar] [CrossRef]
- Caverzasi, E.; Papinutto, N.; Castellano, A.; Zhu, A.H.; Scifo, P.; Riva, M.; Bello, L.; Falini, A.; Bharatha, A.; Henry, R.G. Neurite Orientation Dispersion and Density Imaging Color Maps to Characterize Brain Diffusion in Neurologic Disorders. J. Neuroimaging 2016, 26, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; Mowforth, O.; Gharooni, A.A.; Tetreault, L.; Nouri, A.; Dhillon, R.S.; Bednarik, J.; Martin, A.R.; Young, A.; Takahashi, H.; et al. A New Framework for Investigating the Biological Basis of Degenerative Cervical Myelopathy [AO Spine RECODE-DCM Research Priority Number 5]: Mechanical Stress, Vulnerability and Time. Glob. Spine J. 2022, 12, 78S–96S. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Tessitore, E.; Molliqaj, G.; Meling, T.; Schaller, K.; Nakashima, H.; Yukawa, Y.; Bednarik, J.; Martin, A.R.; Vajkoczy, P.; et al. Degenerative Cervical Myelopathy: Development and Natural History [AO Spine RECODE-DCM Research Priority Number 2]. Glob. Spine J. 2022, 12, 39S–54S. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.W.; Nouri, A.; Wilson, J.R.; Tetreault, L.; Crawley, A.P.; Mikulis, D.J.; Ginsberg, H.; et al. Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ Open 2018, 8, e019809. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Riew, K.D.; Middleton, J.W.; Aarabi, B.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Carette, S.; Chen, R.; et al. A Clinical Practice Guideline for the Management of Patients with Degenerative Cervical Myelopathy: Recommendations for Patients with Mild, Moderate, and Severe Disease and Nonmyelopathic Patients with Evidence of Cord Compression. Glob. Spine J. 2017, 7, 70S–83S. [Google Scholar] [CrossRef]
- Wang, C.; Ellingson, B.M.; Oughourlian, T.C.; Salamon, N.; Holly, L.T. Evolution of brain functional plasticity associated with increasing symptom severity in degenerative cervical myelopathy. EBioMedicine 2022, 84, 104255. [Google Scholar] [CrossRef]
- Yonenobu, K.; Abumi, K.; Nagata, K.; Taketomi, E.; Ueyama, K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine 2001, 26, 1890–1894; discussion 1895. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef]
- Salat, D.H.; Buckner, R.L.; Snyder, A.Z.; Greve, D.N.; Desikan, R.S.; Busa, E.; Morris, J.C.; Dale, A.M.; Fischl, B. Thinning of the cerebral cortex in aging. Cerebral Cortex 2004, 14, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Calamante, F.; Tournier, J.D.; Smith, R.E.; Connelly, A. A generalised framework for super-resolution track-weighted imaging. Neuroimage 2012, 59, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, D.C.; Holly, L.T.; Mayer, E.A.; Salamon, N.; Ellingson, B.M. Alterations in Cortical Thickness and Subcortical Volume are Associated with Neurological Symptoms and Neck Pain in Patients with Cervical Spondylosis. Neurosurgery 2019, 84, 588–598. [Google Scholar] [CrossRef]
- Freund, P.; Weiskopf, N.; Ward, N.S.; Hutton, C.; Gall, A.; Ciccarelli, O.; Craggs, M.; Friston, K.; Thompson, A.J. Disability, atrophy and cortical reorganization following spinal cord injury. Brain 2011, 134, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Bergsland, N.; Lagana, M.M.; Tavazzi, E.; Caffini, M.; Tortorella, P.; Baglio, F.; Baselli, G.; Rovaris, M. Corticospinal tract integrity is related to primary motor cortex thinning in relapsing-remitting multiple sclerosis. Mult. Scler. J. 2015, 21, 1771–1780. [Google Scholar] [CrossRef]
- Jacobs, H.I.; van Boxtel, M.P.; Gronenschild, E.H.; Uylings, H.B.; Jolles, J.; Verhey, F.R. Decreased gray matter diffusivity: A potential early Alzheimer’s disease biomarker? Alzheimer’s Dement. 2013, 9, 93–97. [Google Scholar] [CrossRef]
- Parker, T.D.; Slattery, C.F.; Zhang, J.; Nicholas, J.M.; Paterson, R.W.; Foulkes, A.J.M.; Malone, I.B.; Thomas, D.L.; Modat, M.; Cash, D.M.; et al. Cortical microstructure in young onset Alzheimer’s disease using neurite orientation dispersion and density imaging. Hum. Brain Mapp. 2018, 39, 3005–3017. [Google Scholar] [CrossRef]
- Hulshoff Pol, H.E.; Schnack, H.G.; Posthuma, D.; Mandl, R.C.; Baare, W.F.; van Oel, C.; van Haren, N.E.; Collins, D.L.; Evans, A.C.; Amunts, K.; et al. Genetic contributions to human brain morphology and intelligence. J. Neurosci. 2006, 26, 10235–10242. [Google Scholar] [CrossRef]
- Boomsma, D.; Busjahn, A.; Peltonen, L. Classical twin studies and beyond. Nat. Rev. Genet. 2002, 3, 872–882. [Google Scholar] [CrossRef]
- Glahn, D.C.; Thompson, P.M.; Blangero, J. Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Hum. Brain Mapp. 2007, 28, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Lenroot, R.K.; Schmitt, J.E.; Ordaz, S.J.; Wallace, G.L.; Neale, M.C.; Lerch, J.P.; Kendler, K.S.; Evans, A.C.; Giedd, J.N. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum. Brain Mapp. 2009, 30, 163–174. [Google Scholar] [CrossRef] [PubMed]


| Subject | Age (Mean SD) [Min, Max] | Subgroup | mJOA (Mean SD) [Min, Max] |
|---|---|---|---|
| Patient Cohort
(n = 44) | 59.9 ± 11.6 [37, 81] |
DCM (n = 37) | 14.7 ± 2.3 [9, 17] |
|
Asymptomatic with spinal cord compression (n = 7) | 18 | ||
| HCs (n = 17) | 41.0 ± 14.0 [25, 62] | N/A | 18 |
| Cortical Regions | Average Age Corrected ICVF | ||
|---|---|---|---|
| Study Patients | HCs | p-Value | |
| Precentral Gyrus l | −0.0193 | 0.1735 | <0.0001 ** |
| Precentral Gyrus r | −0.0073 | 0.1259 | <0.0001 ** |
| Postcentral Gyrus l | −0.0196 | 0.1854 | <0.0001 ** |
| Caudal Anterior Cingulate r | −0.0134 | 0.1531 | <0.0001 ** |
| Rostral Anterior Cingulate r | −0.0165 | 0.1698 | <0.0001 ** |
| Supramarginal Gyrus l | −0.0142 | 0.1617 | <0.0001 ** |
| Superior Frontal Gyrus r | −0.0078 | 0.1203 | <0.0001 ** |
| Insular r | −0.0159 | 0.1774 | <0.0001 ** |
| Pars Opercularis l | −0.0209 | 0.1760 | <0.0001 ** |
| Pars Triangularis l | −0.0133 | 0.1519 | <0.0001 ** |
| Caudal Middle Frontal Gyrus r | −0.0139 | 0.1504 | <0.0001 ** |
| Superior Temporal Gyrus r | −0.0210 | 0.1773 | <0.0001 ** |
| Cortical Regions | Correlation with Age Corrected ICVF | |||
|---|---|---|---|---|
| Study Patients | HCs | |||
| r-Value | p-Value | r-Value | p-Value | |
| Precentral Gyrus l | 0.5715 | <0.0001 ** | 0.1632 | 0.5314 |
| Precentral Gyrus r | 0.2111 | 0.1691 | 0.0080 | 0.9757 |
| Postcentral Gyrus l | 0.5195 | 0.0003 * | 0.3932 | 0.1184 |
| Caudal Anterior Cingulate r | 0.4748 | 0.0011 * | 0.3351 | 0.1886 |
| Rostral Anterior Cingulate r | 0.3927 | 0.0084 * | 0.2652 | 0.3038 |
| Supramarginal Gyrus l | 0.3847 | 0.0099 * | 0.4317 | 0.0836 |
| Superior Frontal Gyrus r | 0.3266 | 0.0305 * | 0.4773 | 0.0527 |
| Insular r | −0.0186 | 0.9059 | 0.0574 | 0.8267 |
| Pars Opercularis l | 0.4681 | 0.0014 * | 0.2617 | 0.3102 |
| Pars Triangularis l | 0.2006 | 0.1922 * | 0.1852 | 0.4767 |
| Caudal Middle Frontal Gyrus r | 0.1222 | 0.4296 | 0.0670 | 0.7897 |
| Superior Temporal Gyrus r | 0.1052 | 0.4969 | 0.2366 | 0.3605 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Sanvito, F.; Oughourlian, T.C.; Islam, S.; Salamon, N.; Holly, L.T.; Ellingson, B.M. Structural Relationship between Cerebral Gray and White Matter Alterations in Degenerative Cervical Myelopathy. Tomography 2023, 9, 315-327. https://doi.org/10.3390/tomography9010025
Wang C, Sanvito F, Oughourlian TC, Islam S, Salamon N, Holly LT, Ellingson BM. Structural Relationship between Cerebral Gray and White Matter Alterations in Degenerative Cervical Myelopathy. Tomography. 2023; 9(1):315-327. https://doi.org/10.3390/tomography9010025
Chicago/Turabian StyleWang, Chencai, Francesco Sanvito, Talia C. Oughourlian, Sabah Islam, Noriko Salamon, Langston T. Holly, and Benjamin M. Ellingson. 2023. "Structural Relationship between Cerebral Gray and White Matter Alterations in Degenerative Cervical Myelopathy" Tomography 9, no. 1: 315-327. https://doi.org/10.3390/tomography9010025
APA StyleWang, C., Sanvito, F., Oughourlian, T. C., Islam, S., Salamon, N., Holly, L. T., & Ellingson, B. M. (2023). Structural Relationship between Cerebral Gray and White Matter Alterations in Degenerative Cervical Myelopathy. Tomography, 9(1), 315-327. https://doi.org/10.3390/tomography9010025






