Abstract
Atrial size is a predictor of cardiovascular mortality. Non-ECG-gated computed tomography pulmonary angiography (CTPA) is a common test for cardiopulmonary evaluation but normative values for biatrial volumes are lacking. We derived normal CT biatrial volumes using manual and semiautomated segmentation with contemporaneous transthoracic echocardiography (TTE) to confirm normal diastology. Thirty-five consecutive cases in sinus rhythm with no history of cardio-vascular, renal, or pulmonary disease and normal diastolic function were selected. Planimetric CTPA measurements were compared to TTE volumes measured using area length method. TTE and CTPA derived normal LAVi and RAVi were 27 + 5 and 20 + 6 mL/m2, and 30 + 8 and 29 + 9 mL/m2, respectively. Bland–Altman analysis revealed an underestimation of biatrial volumes by TTE. TTE-CT mean biases for LAV and RAV were −5.7 + 12.0 mL and −16.2 + 14.8 mL, respectively. The CT intraclass correlation coefficients (ICC 95% CI) for LA and RA volumes were 0.99 (0.96–1.00) and 0.96 (0.76–0.99), respectively. There was excellent correlation (p < 0.001) between the semiautomated and manual measurements for LA (r 0.99, 95% CI 0.98–0.99) and RA (r 0.99, 95% CI 0.99–1.00). Atrial volumetric assessment on CTPA is easy and reproducible and can provide additional metric in cardiopulmonary assessment.
1. Introduction
There is an increasing trend to use left atrial (LA) size and function as a morphophysiologic expression to predict cardiovascular mortality in a variety of conditions such as atrial fibrillation, cardiomyopathy, ischemic heart disease, and valvular heart disease [1]. Right atrial (RA) size has also been shown to be of prognostic relevance in diverse cardiopulmonary disorders such as pulmonary embolism and pulmonary hypertension [2,3]. Cardiac magnetic resonance imaging (MRI) and echocardiography are the two non-invasive imaging techniques that are traditionally used for cardiac chamber quantification. Echocardiography is widely available and relatively inexpensive but has limited use for thoracic evaluation. MRI, with its unrivalled versatility, is the reference standard [4] and uses steady-state free precession cine imaging rather than angiographic sequences for chamber measurements as it provides high signal contrast between the ventricular blood pool and the myocardium with improved performance for semiautomated edge-detection algorithms. In spite of its numerous advantages such as operator independence, no exposure to ionising radiation, and lack of geometric assumptions to estimate heart size, MRI is not the introductory modality in the investigation of cardiopulmonary disorders and is also resource intensive and time consuming.
Non-ECG-gated computed tomography pulmonary angiography (CTPA) is a commonly performed test for assorted indications pertaining to the cardiopulmonary system including the assessment of dyspnoea and chest pain. Historically, cardiac chamber assessment on CTPA was performed subjectively but with recent technical advances, it is possible to accomplish objective evaluations. However, there is limited published data regarding normal atrial size on CTPA. There are a number of ECG-gated CT angiography [5,6,7] based publications on atrial volumetry but this literature serves to illustrate the methodological differences between the different groups. Such lack of consensus translates to difficulty in establishing normative values. In a recent systematic review by Zuin et al. [8] of the potential role of LA size derived by non-ECG-gated CT angiography in patients with acute pulmonary embolism, LA volumes were evaluated in four studies [9,10,11,12] but none had comparative TTE that could be used for corroboration of the volumes. The results were further limited by the lack of data regarding the presence of atrial fibrillation in the analysed patients.
Given the wide variation in the CT angiography methodology used for atrial measurements, both in terms of the parameters used for estimating atrial size as well as in the techniques employed for the measurements, we performed a small pilot study to examine the feasibility and reproducibility of deriving normative biatrial volumes on CTPAs using both manual contouring and semiautomated volume segmentation with comparative transthoracic echocardiography (TTE) to confirm normal diastology.
2. Materials and Methods
2.1. Patient Population
This retrospective study was approved by the Institutional Review Board and need for informed consent was waived. Internal radiology information system/picture archiving and communication system, echocardiography and electronic medical records databases were scrutinised over a 3-year period to select patients who were in sinus rhythm, had no prior history of cardiovascular, renal, or pulmonary disease, and had undergone both non-ECG-gated CTPA and transthoracic echocardiography (TTE) within a 6 month period, irrespective of the indications for the 2 tests.
TTE was first analysed in accordance with current guidelines [13]. Strict exclusion criteria were then applied to identify patients with completely normal diastology (Figure 1: Flow chart of the selection process including exclusion criteria; although age was not an exclusion criterion, all patients with normal diastology in the final analysis were less than 56-years old). If the LV diastolic function was completely normal on TTE, the corresponding CTPA was then retrieved. After excluding cases with thromboembolic disease, the images were analysed for cardiorespiratory motion and contrast opacification that could adversely affect atrial size quantification. As adequate biatrial contrast enhancement is a prerequisite to both manual and semi-automated segmentation, CTPAs with low left atrial enhancement (<150 HU) were excluded. The final analysis included thirty-five cases.
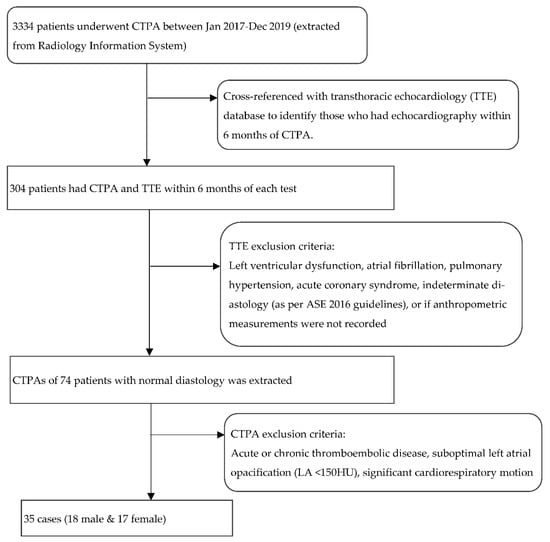
Figure 1.
Flow chart outlining the selection process including the exclusion criteria. TTE: transthoracic echocardiography; LA: left atrium. HU: Hounsfield unit.
2.2. CT Acquisition
CTPA’s were acquired on single-source 128-multislice configuration (Somatom Definition AS+; Siemens AG, Munich, Germany). Non-ECG-gated scanning was performed in craniocaudal direction from lung apices to bases at end-inspiration during a single breath-hold using the following acquisition parameters; table speed 61.4 mm/rotation, pitch 0.8 pitch, tube voltage 80–120 kVp, tube current 100 mAs, rotation time 0.5 s, and 512 × 512 acquisition matrix. A total of 100 mLs Omnipaque 350 (GE Healthcare, Chicago, IL, USA) was injected at 5 mL/s with 20-mL saline chaser. Pulmonary artery visualisation was optimised using an automated bolus-tracking technique with region of interest (ROI) placed within main pulmonary artery and trigger values of 130 Hounsfield units. Images were reconstructed at 1-mm slice thickness at 1 mm interval using SAFIRE (Sinogram Affirmed Iterative Reconstruction, strength 3) iterative reconstruction.
2.3. CT Biatrial Measurement
For manual measurements, CT images were analysed using Vitrea Advanced Visualization multimodal platform (Vital Images, Inc.; Minnetonka, MN, USA). CT parameters were measured in consensus by two radiologists (R1, a cardiovascular radiologist with 15 years’ experience, and R2, a radiology imaging fellow).
CT 2 and 4 chamber planes were created analogous to TTE views. Using multiplanar reformatting (MPR) with true axial stack, 2 and 4 chamber planes were created by positioning long axis reference line through LV apex and mid mitral valve, and short axis reference line parallel and aligned with mitral annular plane. Imaging slice height was adjusted cranio-caudally to remove LV outflow tract/aortic root from view, to avoid creation of TTE equivalent ‘5 chamber view’. The LV short axis plane was used for cross reference to ensure the manually created 2 and 4 chamber planes transected the appropriate and relevant myocardial segments; 2 chamber—anterior and inferior segments, 4 chamber—inferoseptum and anterolateral segment (Figure 2).
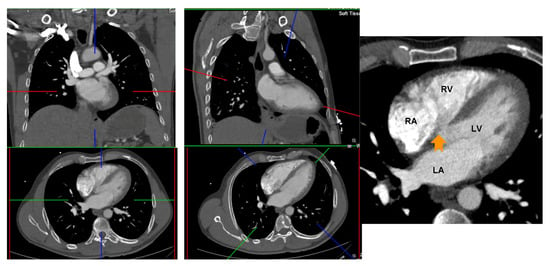
Figure 2.
Creation of 4 and 2 chamber planes on non-ECG-gated CTPA. Left panel: Source data prior to image manipulation. Middle panel: Alignment of crosshairs with long axis reference line through LV apex and mid mitral valve and short axis reference line parallel and aligned with mitral annular plane. Right panel: Avoid creation of a 5 chamber view by adjusting the image height (arrow points to left ventricular outflow tract). RA: right atrium; LA: left atrium; RV: right ventricle; LV: left ventricle.
LA area planimetry was performed using freehand ROI tool. Pulmonary veins and LA appendage were excluded. Using TTE area length method, LA volume was estimated by: (0.85 × area 1 (2 chamber) × area 2 (4 chamber)) ÷ shortest LA long axis length [14]. Whilst limitations of area length method (relating to LA shape geometric assumptions) are acknowledged, it was selected over the Simpson modified biplane method to allow expeditious CT LA volume estimation without the need for additional software computation. Direct LA area was not measured from the straight axial stack due to divergence of LA long axis plane from the standard axial plane which would result in a degree of systematic bias and limit direct comparability with TTE. This would be further compounded by single plane 4 chamber LA measurements being smaller than 2 chamber [15]. RA volume was also measured from same CT 4 chamber plane without alteration of the horizontal imaging plane to emphasise the RA. Area planimetry was performed using freehand ROI tool with exclusion of RA appendage. Single plane RA volume was estimated by: (0.85 × (RA 4 chamber area)2) ÷ RA long axis length [14,16]. (Figure 3 and Figure 4).
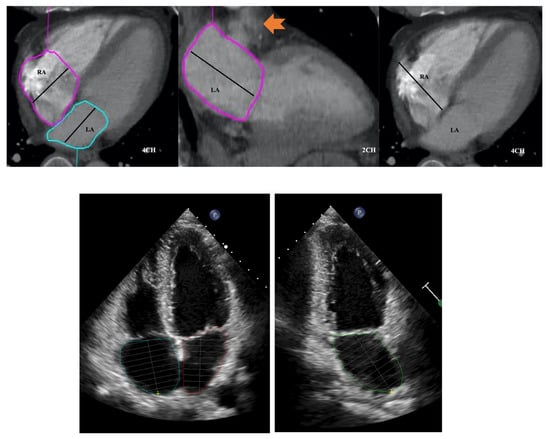
Figure 3.
Four and two chamber planes from a non-ECG-gated CTPA (top) and corresponding echocardiograpy images (bottom). Atrial areas planimetered with exclusion of right and left atrial appendages and pulmonary veins. Arrow in middle panel indicates left atrial appendage. RA: right atrium; LA: left atrium.
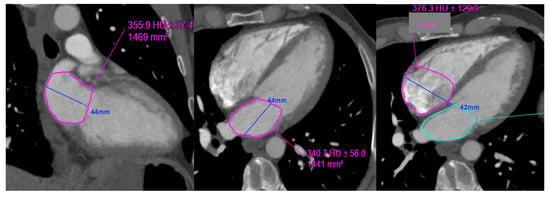
Figure 4.
Example of left and right atrial planimetry in a 50-year-old male with body surface area of 1.9 (height 173 cm, weight 76 kg).
| Left atrial volume: (0.85 × area 1 (2 chamber) × area 2 (4 chamber)) ÷ shortest LA long axis length: |
| 0.85 × 14.7 × 14.4 ÷ 4.4 = 40.8 mL. LAVi = 21.4 mL/m2 |
| Right atrial volume: (0.85 × (RA 4 chamber area)2) ÷ RA long axis length: |
| 0.85 × (16.1) 2) ÷ 4.2 = 52.45 mL. RAVi: 27.6 mL/m2 |
LAVi: Left atrial volume indexed; RAVi: Right atrial volume indexed.
Semiautomated biatrial measurements were performed offline using commercially available software (CVI42 version 5.12.1, Circle Cardiovascular Imaging, Calgary, AB, Canada). The biatrial endocardial borders were manually delineated in the apical four- and two-chamber views using a point-and-click approach before the automated tracking algorithm was applied. The pulmonary veins and atrial appendages were again excluded from the analysis. Maximum and minimum volumes were calculated based on the biplane area-length method [14] and indexed to body surface area.
The time required to load the CT data into the PACS and software interface and to complete the manual and semiautomated image analyses was also recorded.
To maintain methodological comparability with CT, atrial volumes on TTE were measured using the atrial length method by a single experienced echocardiologist (KL).
2.4. Statistical Analysis
Continuous normal data are presented as mean (±1 standard deviation). Non-normal data are presented as median (inter-quartile range). Categorical variables are displayed as n (%). Continuous data was compared with the (paired) two tailed Student’s t test. Intraclass correlation coefficient (ICC) estimates and their 95% confidence intervals for inter-observer variability in manual CT atrial measurement were calculated. TTE and manual CT measurement of biatrial volumes was compared with Bland–Altman analysis. Manual CT and semiautomated CT measurement of biatrial volumes was also compared with Bland–Altman analysis. Correlation analysis was performed using Pearson correlation coefficient. A p-value of <0.05 was considered statistically significant.
3. Results
The baseline characteristics and TTE features demonstrating normal diastology are delineated in Table 1 and Table 2, respectively. Manual CT and TTE biatrial measurements are outlined in Table 3.

Table 1.
Baseline characteristics of the thirty-five cases.

Table 2.
Transthoracic echocardiography (TTE) measures of normal diastology.

Table 3.
Manual CT and transthoracic echocardiography (TTE) atrial measurements.
3.1. TTE versus CT Biatrial Volumes
TTE and CTPA derived normal LAVi and RAVi were 27 + 5 and 20 + 6 mL/m2, and 30 + 8 and 29 + 9 mL/m2, respectively. Bland–Altman analysis (Figure 5) revealed an underestimation of biatrial volumes by TTE compared to CT. TTE-CT mean biases for (non-indexed) LAV and RAV were −5.7 + 12.0 mL and −16.2 + 14.8 mL, respectively. The intraclass correlation coefficients (ICC 95% CI) for the two readers for CT manual atrial measurements were 0.99 (0.96–1.00) and 0.96 (0.76–0.99) for LA and RA volumes, respectively.
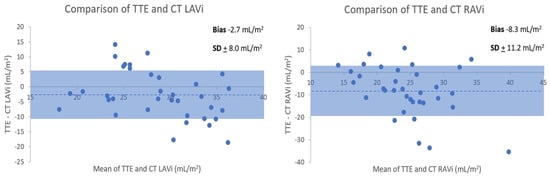
Figure 5.
Bland–Altman comparison of normal transthoracic echocardiography (TTE) and CT (indexed) LA and RA volume measurements.
Shading indicates ±1 standard deviation. Dotted line denotes mean bias.
Whist majority of cases had smaller LAV with TTE, some patients had larger volumes with TTE than CT. This would relate to TTE LAV being measured in end-systole at maximal atrial volume and CT being ungated. LA: left atrium. RA: LAV: left atrial volume. Right atrium. RAV: right atrial volume.
3.2. Manual versus Semiautomated CT Biatrial Volumes
The indexed manual and semiautomated biatrial volumes are outlined in Table 4.

Table 4.
Manual automated CT atrial measurements.
Excellent correlation (p < 0.001) was seen for LA (r 0.99, 95% CI 0.98–0.99) and RA (r 0.99, 95% CI 0.99–1.00), as displayed in Figure 6.
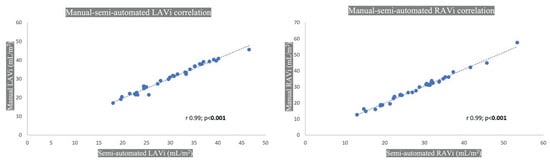
Figure 6.
CT manual and semiautomated atrial volume correlation. LAVi: left atrial volume indexed; RAVi: right atrial volume indexed.
The mean time for biatrial analysis with the manual approach was 12 min (+/−3 min). Automated image analysis was faster with an average of 6–8 min.
4. Discussion
Due to the paucity of published CTPA literature on normative atrial volumes, we performed this pilot study and derived biatrial volumes on 35 patients with comparative volumes on contemporaneous TTE. Our results showed excellent correlation between CT manual and semiautomated volumetric analysis for both atria. However, compared with CT, there was an underestimation of biatrial volumes by TTE. This is not surprising as previous studies using ECG-gated CT datasets have shown similar results [17,18,19].
Quantifying LA size is difficult, in part because of the complex geometry and the variable contributions of its appendage and pulmonary veins. Furthermore, atria have an amorphous morphology and their dimensions are affected by parameters such as age and body mass index [20,21,22]. LA volume increases markedly in patients with severe renal dysfunction [23]. Taking these factors in to consideration, we carefully selected our TTE-CTPA cohort of normal diastology, ensuring normal renal function and sinus rhythm. Although we did not set an age cut-off, patients with normal diastolic function on the TTE were younger, with an upper limit of 55 years.
Axial diameter has previously been shown to be a simple and quick CT measurement of LA size with a cut-off of 4.0 cm for predicting diastolic dysfunction with a sensitivity of 68% and a specificity of 74% [24]. However, this and other studies [24,25,26] acknowledge that volumetric analysis provides a more accurate representation of LA size. Furthermore, in patients with sinus rhythm, indexed LA volume is a more robust marker of cardiovascular events than LA area or diameter [27]. This is because LA is an asymmetrical cavity and so volumetric analysis gives a more precise size estimation. Finally, LA dilatation might not be evenly distributed in all planes, and so a simple antero-posterior dimension is likely to be insensitive to LA size change [25].
Whilst there are established reference ranges for normative RV volumes on TEE, there is very limited data on measurement of RA size and in particular, RA volumes by CT. A cut-off of >35 mm, in the transverse diameter, perpendicular to the interatrial septum, measured from septal wall to lateral wall has been proposed for RA enlargement, but this is based on historic data [28]. Few studies have used ECG-gated CT datasets for RA volumetry [6,29,30] and have shown that similar to LA, the CT-derived RA volumes also tend to be overestimated when compared with MRI [29] and echocardiography [30]. The differences in the temporal resolution may account for the inter-technique variability in the measurements. A recent prospective study of 609 patients with acute PE [2] demonstrated the feasibility of performing fully automated RA volumetric analysis on non-ECG-gated CTPA. Whilst RA dilation was a frequent finding in this population, its prognostic performance was inferior compared to other risk stratification markers. Moreover, in the above study [2], there were patients with co-morbidities that affected the atrial volumes (elderly male patients with cardiovascular comorbidities had higher volumes whilst cancer patients had lower volumes) and only 25% of the cases had corresponding echocardiography which adds to the difficulty of comparing CT measurement accuracy to other techniques.
The complex RA anatomy and the suboptimal delineation of the RA wall on the CT due to streak artifacts from the intravenous contrast medium contribute to the difficulties in RA volumetric analysis. Furthermore, the RA size may be affected by the high flow rate of the administered contrast medium, as well as alterations in venous return with inspiratory breath-holds. Notwithstanding these challenges, we were able to perform both a manual and semiautomated assessment of the RA volumes with excellent correlation between the two methodologies. Similar to LA volumes, TTE underestimated the RA volumes compared to CT but with a higher magnitude of difference. This greater underestimation of RA volumes with TTE is likely due to the lack of right ventricle (RV)/ RA focused imaging as in routine clinical practice; the standard TTE four chamber imaging emphasises the left heart.
Our study has demonstrated the feasibility and reproducibility of deriving normative values on routine CTPA. A main limitation is the small sample size. We identified 304 patients with contemporaneous CTPA and TTE but a large proportion in this retrospective study group was not suitable due to diastolic dysfunction. However, it must be emphasised that the current sample size of 35 patients satisfies the central limit theorem to demonstrate Gaussian/normal distribution for assessment of CT atrial normative values. Moreover, to our knowledge, it is the only study to have corroborative TTE in all cases to ensure completely normal diastology. We also acknowledge that atrial chamber quantification is gender specific, but our work is intended as a pilot study and Gaussian distribution cannot be demonstrated if our small cohort is divided into male and female subgroups. Whilst ECG-gating may potentially improve the accuracy of the atrial volumes, we believe our approach reflects real-world clinical practice as most institutions perform CTPA without ECG-gating. Manual atrial volumetric assessment is operator dependent but our quantitative data demonstrated excellent intraclass correlation. However, whilst manual measurements without recourse to expensive and dedicated analysis software were shown to be as good as the more sophisticated semiautomated measurements, the latter are quicker and simpler to perform and hence more easily adaptable in clinical practice.
In conclusion, atrial measurements on CTPA are undergoing an evolutionary process from subjective evaluation to objective quantification. Knowledge of normal values of atrial volumes is required to differentiate between pathological conditions and normal state as well as grade the disease severity and monitor treatment response. The present work has shown that CT atrial volumetric assessment is easy and reproducible and can provide an additional metric in the CTPA assessment of cardiopulmonary diseases. Current trends using artificial intelligence algorithms are apposite for automated atrial volumetric analysis to be incorporated into routine practice. However, prospective large volume studies will be needed to validate the normative atrial volumes on CTPA.
Author Contributions
Conceptualisation, D.G., W.A. and P.L.; methodology, D.G., J.R. and K.L.; software, D.G., J.R., K.L. and S.A.; validation, D.G., J.R., K.L., S.A. and B.A.; formal analysis, D.G., J.R., S.A. and K.L.; data curation, D.G., K.L. and B.A., writing—D.G.; Editing—J.R., K.L., S.A. and B.A.; supervision, W.A. and P.L.; project administration, D.G., B.A., W.A. and P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Imperial College Hospital NHS Trust (IRAS 280472, 18 March 2020).
Informed Consent Statement
Patient consent was waived as there are no patient identifiable data in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available as the study was not registered on a public domain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoit, B.D. Left Atrial Size and Function. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaumer, M.H.; Ebner, M.; Ritter, C.O.; Steimke, L.; Rogge, N.I.; Sentler, C.; Thielmann, A.; Hobohm, L.; Keller, K.; Lotz, J.; et al. Prognostic value of right atrial dilation in patients with pulmonary embolism. ERJ Open Res. 2021, 7, 00414–02020. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, F.; Mandawat, A.; Il’giovine, Z.J.; Shaw, L.K.; Siddiqui, I.; Tapson, V.F.; Arges, K.; Rivera, D.; Romano, M.M.; Velazquez, E.J.; et al. Clinical Utility and Prognostic Value of Right Atrial Function in Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2018, 11, e006984. [Google Scholar] [CrossRef]
- Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; Manning, W.J.; et al. ACCF/ACR/AHA/NASCI/SCMR 2010 Expert Consensus Document on Cardiovascular Magnetic Resonance: A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J. Am. Coll. Cardiol. 2010, 55, 2614–2662. [Google Scholar] [CrossRef]
- Stojanovska, J.; Cronin, P.; Patel, S.; Gross, B.H.; Oral, H.; Chughtai, K.; Kazerooni, E.A. Reference Normal Absolute and Indexed Values From ECG-Gated MDCT: Left Atrial Volume, Function, and Diameter. Am. J. Roentgenol. 2011, 197, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Pagali, S.R.; Hamirani, Y.S.; Chen, A.; Cheu, G.; Gao, Y.; Li, D.; Mao, S. Sex-Specific Biatrial Volumetric Measurements Obtained with Use of Multidetector Computed Tomography in Subjects with and without Coronary Artery Disease. Tex. Heart Inst. J. 2014, 41, 286–292. [Google Scholar] [CrossRef]
- Lin, F.Y.; Devereux, R.B.; Roman, M.J.; Meng, J.; Jow, V.M.; Jacobs, A.; Weinsaft, J.W.; Shaw, L.J.; Berman, D.S.; Callister, T.Q.; et al. Cardiac Chamber Volumes, Function, and Mass as Determined by 64-Multidetector Row Computed Tomography: Mean Values Among Healthy Adults Free of Hypertension and Obesity. JACC Cardiovasc. Imaging 2008, 1, 782–786. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Turchetta, S.; Zonzin, P.; Zuliani, G.; Roncon, L. Left atrial size measured on CT pulmonary angiography: Another parameter of pulmonary embolism severity? A systematic review. J. Thromb. Thrombolysis 2020, 50, 181–189. [Google Scholar] [CrossRef]
- Guo, Z.-J.; Liu, H.-T.; Bai, Z.-M.; Lin, Q.; Zhao, B.-H.; Xu, Q.; Zeng, Y.-H.; Feng, W.-Q.; Zhou, H.-T.; Liang, F.; et al. A new method of CT for the cardiac measurement: Correlation of computed tomography measured cardiac parameters and pulmonary obstruction index to assess cardiac morphological changes in acute pulmonary embolism patients. J. Thromb. Thrombolysis 2018, 45, 410–416. [Google Scholar] [CrossRef]
- Aviram, G.; Soikher, E.; Bendet, A.; Ziv-Baran, T.; Berliner, S.; Shmueli, H.; Friedensohn, L.; Milwidsky, A.; Sadovnik, O.; Topilsky, Y. Automatic assessment of cardiac load due to acute pulmonary embolism: Saddle vs. central and peripheral emboli distribution. Heart Lung 2016, 45, 261–269. [Google Scholar] [CrossRef]
- Aviram, G.; Soikher, E.; Bendet, A.; Shmueli, H.; Ziv-Baran, T.; Amitai, Y.; Friedensohn, L.; Berliner, S.; Meilik, A.; Topilsky, Y. Prediction of Mortality in Pulmonary Embolism Based on Left Atrial Volume Measured on CT Pulmonary Angiography. Chest 2016, 149, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Aviram, G.; Sirota-Cohen, C.; Steinvil, A.; Keren, G.; Banai, S.; Sosna, J.; Berliner, S.; Rogowski, O. Automated volumetric analysis of four cardiac chambers in pulmonary embolism. Thromb. Haemost. 2012, 108, 384–393. [Google Scholar] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Hahn, R.T.; Jin, Z.; Homma, S.; Sacco, R.L.; Di Tullio, M.R. Comparison of Echocardiographic Single-Plane versus Biplane Method in the Assessment of Left Atrial Volume and Validation by Real Time Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 954–960. [Google Scholar] [CrossRef]
- DePace, N.L.; Ren, J.-F.; Kotler, M.N.; Mintz, G.S.; Kimbiris, D.; Kalman, P. Two-dimensional echocardiographic determination of right atrial emptying volume: A noninvasive index in quantifying the degree of tricuspid regurgitation. Am. J. Cardiol. 1983, 52, 525–529. [Google Scholar] [CrossRef]
- Koka, A.R.; Yau, J.; Van Why, C.; Cohen, I.S.; Halpern, E.J. Underestimation of Left Atrial Size Measured with Transthoracic Echocardiography Compared With 3D MDCT. Am. J. Roentgenol. 2010, 194, W375–W381. [Google Scholar] [CrossRef] [PubMed]
- Koka, A.R.; Gould, S.D.; Owen, A.N.; Halpern, E.J. Left Atrial Volume: Comparison of 2D and 3D Transthoracic Echocardiography with ECG-gated CT Angiography. Acad. Radiol. 2012, 19, 62–68. [Google Scholar] [CrossRef]
- Kataoka, A.; Funabashi, N.; Takahashi, A.; Yajima, R.; Takahashi, M.; Uehara, M.; Takaoka, H.; Saito, M.; Yamaguchi, C.; Lee, K.; et al. Quantitative evaluation of left atrial volumes and ejection fraction by 320-slice computed-tomography in comparison with three- and two-dimensional echocardiography: A single-center retrospective-study in 22 subjects. Int. J. Cardiol. 2011, 153, 47–54. [Google Scholar] [CrossRef]
- Liu, X.-K.; Jahangir, A.; Terzic, A.; Gersh, B.J.; Hammill, S.C.; Shen, W.-K. Age- and sex-related atrial electrophysiologic and structural changes. Am. J. Cardiol. 2004, 94, 373–375. [Google Scholar] [CrossRef]
- Pan, N.-H.; Tsao, H.-M.; Chang, N.-C.; Chen, Y.-J.; Chen, S.-A. Aging Dilates Atrium and Pulmonary Veins. Chest 2008, 133, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, A.M.; Jacobsen, S.J.; Mahoney, D.W.; Rodeheffer, R.J.; Bailey, K.R.; Redfield, M.M. Left atrial volume as an index ofleft atrial size: A population-based study. J. Am. Coll. Cardiol. 2003, 41, 1036–1043. [Google Scholar] [CrossRef]
- Damman, K.; Testani, J.M. The kidney in heart failure: An update. Eur. Heart J. 2015, 36, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Lick, A.N.; Danrad, R.; Smith, D.L.; Lammi, M.R. Left Atrium Measurements via Computed Tomography Pulmonary Angiogram as a Predictor of Diastolic Dysfunction. J. Comput. Assist. Tomogr. 2017, 41, 792–797. [Google Scholar] [CrossRef]
- Lester, S.J.; Ryan, E.W.; Schiller, N.B.; Foster, E. Best method in clinical practice and in research studies to determine left atrial size. Am. J. Cardiol. 1999, 84, 829–832. [Google Scholar] [CrossRef]
- Takemoto, Y.; Barnes, M.E.; Seward, J.B.; Lester, S.J.; Appleton, C.A.; Gersh, B.J.; Bailey, K.R.; Tsang, T.S. Usefulness of Left Atrial Volume in Predicting First Congestive Heart Failure in Patients ≥65 Years of Age with Well-Preserved Left Ventricular Systolic Function. Am. J. Cardiol. 2005, 96, 832–836. [Google Scholar] [CrossRef]
- Tsang, T.S.; Abhayaratna, W.P.; Barnes, M.E.; Miyasaka, Y.; Gersh, B.J.; Bailey, K.R.; Cha, S.S.; Seward, J.B. Prediction of Cardiovascular Outcomes with Left Atrial Size: Is Volume Superior to Area or Diameter? J. Am. Coll. Cardiol. 2006, 47, 1018–1023. [Google Scholar] [CrossRef]
- Tardivon, A.A.; Musset, D.; Maitre, S.; Brenot, F.; Dartevelle, P.; Simonneau, G.; Labrune, M. Role of CT in Chronic Pulmonary Embolism: Comparison with Pulmonary Angiography. J. Comput. Assist. Tomogr. 1993, 17, 345–351. [Google Scholar] [CrossRef]
- Rheinheimer, S.; Reh, C.; Figiel, J.; Mahnken, A.H. Assessment of right atrium volume by conventional CT or MR techniques: Which modality resembles in vivo reality? Eur. J. Radiol. 2016, 85, 1040–1044. [Google Scholar] [CrossRef]
- Takahashi, A.; Funabashi, N.; Kataoka, A.; Yajima, R.; Takahashi, M.; Uehara, M.; Takaoka, H.; Saito, M.; Yamaguchi, C.; Lee, K.; et al. Quantitative evaluation of right atrial volume and right atrial emptying fraction by 320-slice computed tomography compared with three-dimensional echocardiography. Int. J. Cardiol. 2011, 146, 96–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).