Partial Anomalous Left Pulmonary Artery Anterior Versus Posterior Types: A Systematic Review
Abstract
1. Introduction
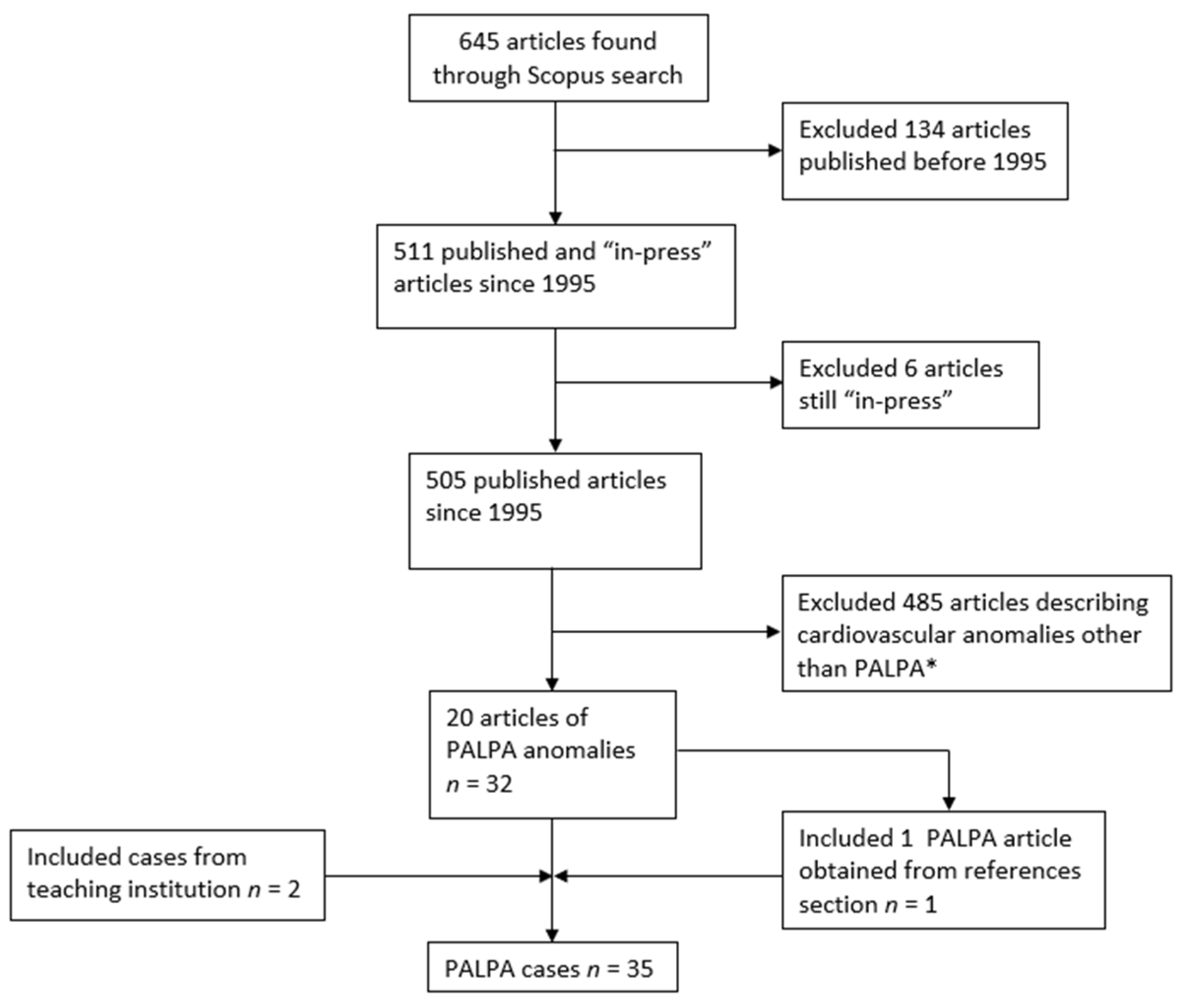
2. Materials and Methods
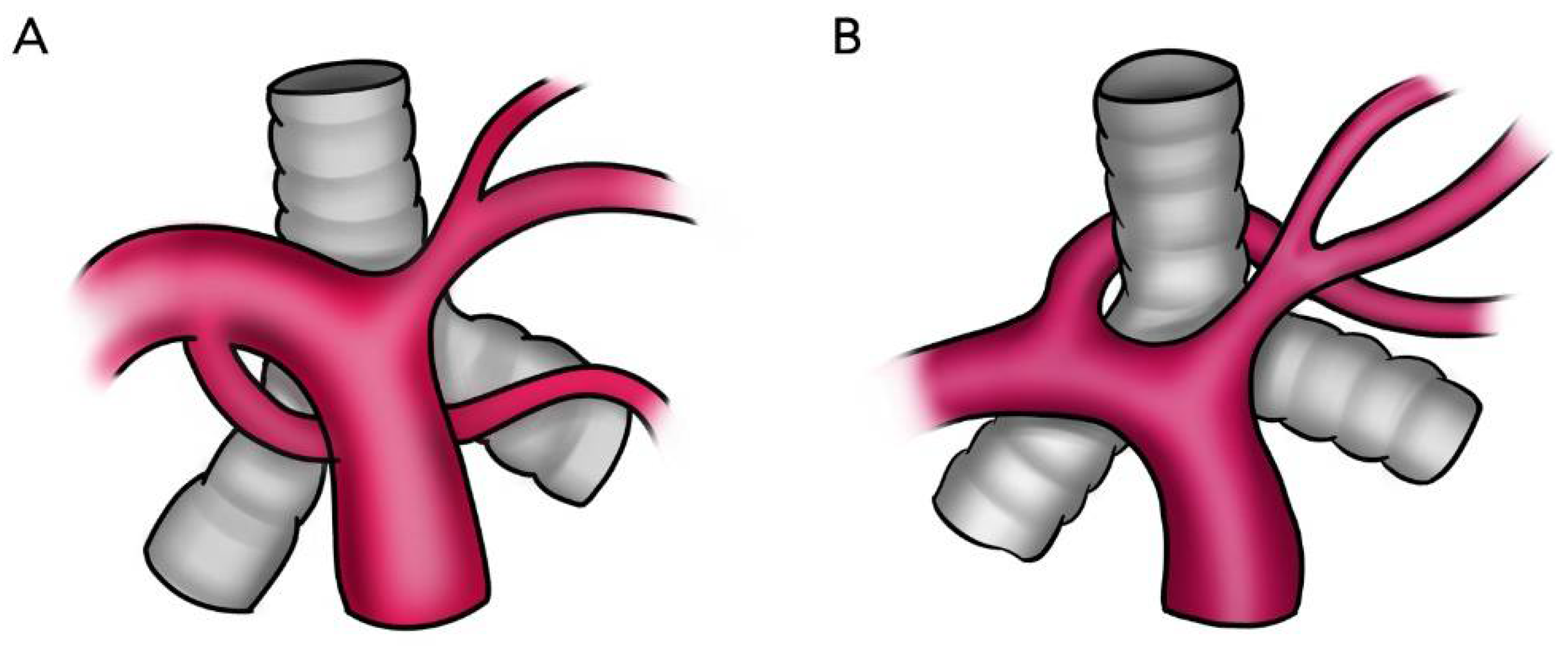
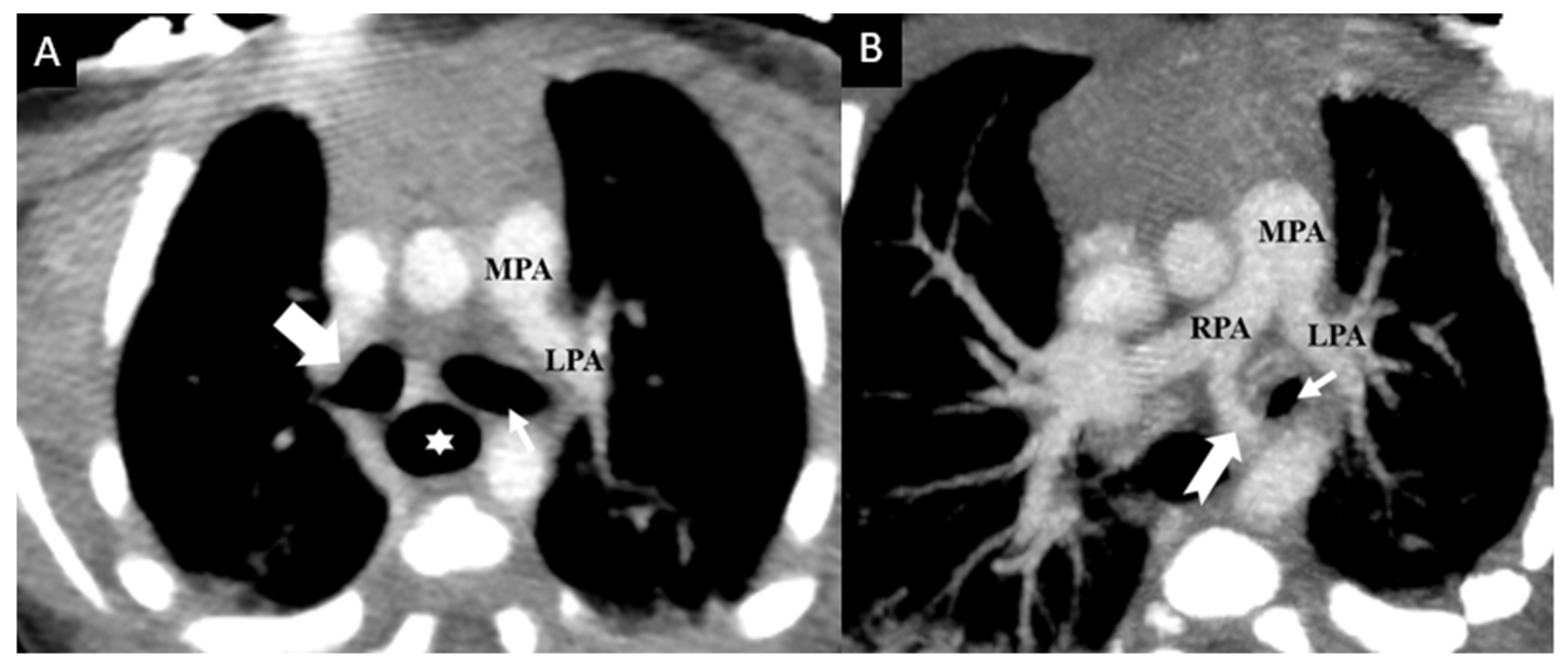
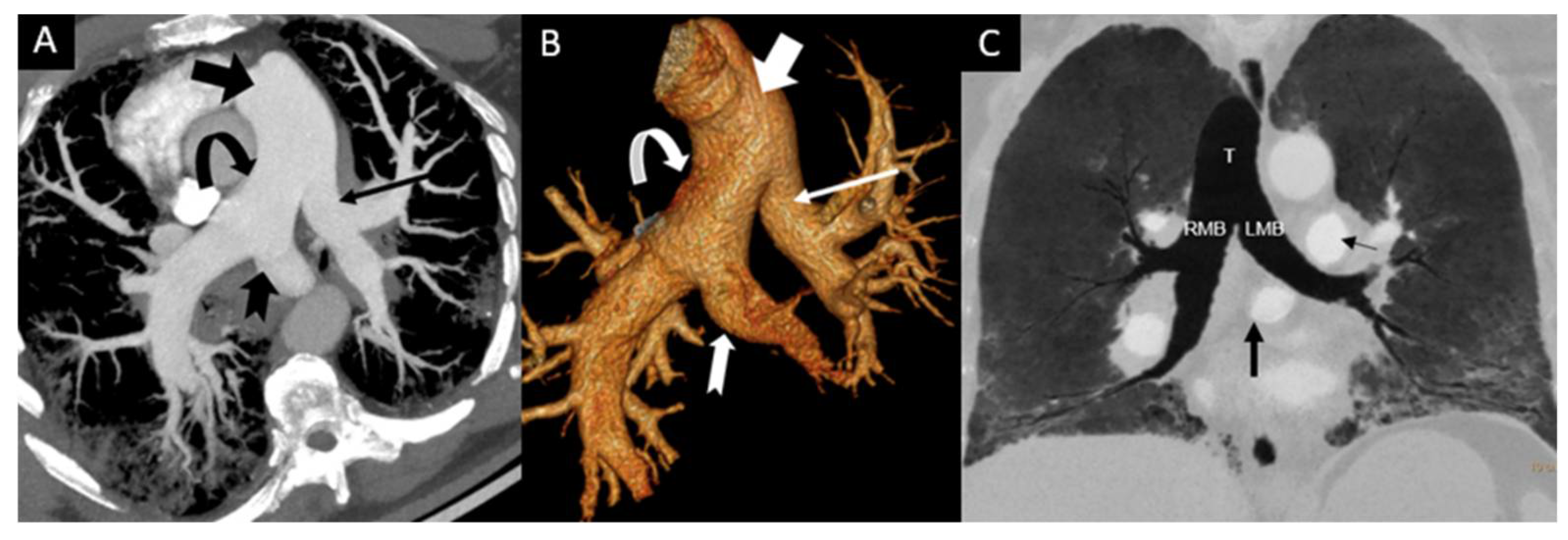
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maldjian, P.D.; Adams, K.R. Partial anomalous left pulmonary artery sling in an adult. J. Clin. Imaging Sci. 2020, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Winlaw, D.S.; Sholler, G.F. Partial anomalous left pulmonary artery: Report of two cases and review of literature. Cardiol. Young 2015, 25, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Gao, W.; Zhong, Y.M.; Sun, A.M.; Wang, Q.; Hu, L.W.; Qiu, H.S.; Li, J.Y.; Jaffe, R.B. Multislice computed tomography assessment of tracheobronchial patterns in partial anomalous left pulmonary artery. J. Comput. Assist. Tomogr. 2017, 41, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Juan, Y.H.; Wang, Q.; Chen, J.; Zhuang, J.; Xie, Z.; Liang, C.; Zhu, Y.; Yu, Z.; Li, J.; et al. Evaluation of left pulmonary artery sling, associated cardiovascular anomalies, and surgical outcomes using cardiovascular computed tomography angiography. Sci. Rep. 2017, 7, 40042. [Google Scholar] [CrossRef]
- Yuan, S.M. Congenital heart defects in Kabuki syndrome. Cardiol. J. 2013, 20, 121–124. [Google Scholar] [CrossRef]
- Erickson, L.C.; Cocalis, M.W.; George, L. Partial anomalous left pulmonary artery: New evidence on the development of the pulmonary artery sling. Pediatr. Cardiol. 1996, 17, 319–321. [Google Scholar] [CrossRef]
- Koch, A.; Hofbeck, M.; Gerling, S.; Buheitel, G.; Singer, H. Partieller fehlabgang der linken pulmonalarterie. Z. Kardiol. 2000, 89, 118–121. [Google Scholar] [CrossRef]
- Fountain-Dommer, R.R.; Shirali, G.S.; Wiles, H.B.; Larsen, R.L. Noninvasive diagnosis of partial anomalous left pulmonary artery. J. Am. Soc. Echocardiogr. 2001, 14, 745–746. [Google Scholar] [CrossRef]
- Divekar, A.; Coe, J.Y.; Saxena, A. Tetralogy of fallot, total anomalous pulmonary venous return, and partial anomalous left pulmonary artery: A rare association. Pediatr. Cardiol. 2004, 25, 430–431. [Google Scholar] [CrossRef]
- Collins, R.T.; Weinberg, P.M.; Goldmuntz, E.; Harris, M. Partial anomalous left pulmonary artery. Circulation 2009, 119, 2405–2407. [Google Scholar] [CrossRef][Green Version]
- Mathias, H.C.; Manghat, N.E. Partial anomalous left pulmonary artery with associated bronchial anomalies in a patient with repaired tetralogy of Fallot. J. Cardiovasc. Comput. Tomogr. 2012, 6, 292–294. [Google Scholar] [CrossRef]
- Bhat, A.H.; Davenport, J.; Cocalis, M. Partial anomalous left pulmonary artery along with aortic coarctation in an infant with Kabuki syndrome. Echocardiography 2012, 29, 145–148. [Google Scholar] [CrossRef]
- Giudici, V.; Kanani, M.; Muthialu, N.; Carr, M.; Calder, A.D.; Owens, C.M.; Cook, A.C.; Marek, J. Duplicated left pulmonary artery: An unknown disease? Three case reports and review of the literature. Cardiol. Young 2016, 26, 340–346. [Google Scholar] [CrossRef]
- Duong, P.; Mathur, S.; Miller, O.I. Partial anomalous left pulmonary artery. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 237. [Google Scholar] [CrossRef]
- Chen, C.H.; Tsai, P.S.; Lin, D.C.; Liu, Y.P.; Cheng, K.S. Partial anomalous left pulmonary artery: A case report and literature review. Iran. J. Radiol. 2020, 17, e100510. [Google Scholar] [CrossRef]
- Ge, S.; DeGroff, C.G.; Knudson, O.; Strain, J.; Chan, K.C. Noninvasive assessment of pseudo-pulmonary artery sling by echocardiography and computerized tomography. Circulation 2001, 103, 2–3. [Google Scholar] [CrossRef]
- Sadagopan, S.N.; Roman, K.S.; Salmon, A.P. An accessory left pulmonary artery. Cardiol. Young 2008, 18, 546–547. [Google Scholar] [CrossRef]
- Tissot, C.; Darst, J.R.; Kaza, A.K.; Younoszai, A.K.; da Cruz, E. Partial left pulmonary artery sling associated with multiple ventricular septal defects: A rare congenital anomaly. J. Thorac. Cardiovasc. Surg. 2008, 136, 1085–1087. [Google Scholar] [CrossRef][Green Version]
- Tateishi, A.; Kawada, M. Partial Form of a Pulmonary Artery Sling. Ann. Thorac Surg. 2009, 87, 965. [Google Scholar] [CrossRef]
- Collell, R.; Marimón, C.; Montero, M. Sling parcial de la arteria pulmonar izquierda. Rev. Española Cardiol. 2010, 63, 850. [Google Scholar] [CrossRef]
- Abelardo, E.; Hewitt, R.; Elliott, M.J.; Muthialu, N. Successful surgical repair of complex Christmas-tree pattern tracheo-bronchial anatomy with stenosis. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2161–2163. [Google Scholar] [CrossRef]
- Nagatomo, Y.; Muneuchi, J.; Watanabe, M.; Joo, K.; Ochiai, Y. Repair of Partial Pulmonary Artery Sling in a Symptomatic Adolescent. Ann. Thorac Surg. 2017, 104, e289. [Google Scholar] [CrossRef][Green Version]
- Chao, Y.J.; Chen, M.R.; Lin, S.M. Scimitar syndrome with partial left pulmonary artery sling in a neonate. Acta Cardiol. Sin. 2018, 34, 440–442. [Google Scholar] [CrossRef]
- Moreno, F.; Garcia-Guereta, L.; Benito, F.; Gamallo, C.; Campo, F.; Herranz, F. Aberrant Left Pulmonary Artery with Tracheal Stenosis without Vascular Sling. Pediatr. Cardiol. 1991, 12, 44–45. [Google Scholar] [CrossRef]
- Semiz-Oysu, A.; Basaran, I.; Barutca, H.; Bukte, Y. Anomalous left pulmonary artery without sling formation. Pediatr. Cardiol. 2013, 34, 1928–1931. [Google Scholar] [CrossRef]
- Marins, L.; Anderson, R.H.; Aiello, V.D. Extrapericardial origin of the entirety of the left pulmonary artery in the absence of a pulmonary arterial sling. Cardiovasc. Pathol. 2013, 22, e11–e13. [Google Scholar] [CrossRef]
- Sade, R.M.; Rosenthal, A.; Fellows, K.; Castaneda, A. Pulmonary artery sling. J. Thorac. Cardiovasc. Surg. 1975, 69, 333–346. [Google Scholar] [CrossRef]
- Kussman, B.D.; Geva, T.; Mcgowan, F.X. Cardiovascular causes of airway compression. Paediatr. Anaesth. 2004, 14, 60–74. [Google Scholar] [CrossRef]
- Gikonyo, B.M.; Jue, K.L.; Edwards, J.E. Pulmonary vascular sling: Report of seven cases and review of the literature. Pediatr. Cardiol. 1989, 10, 81–88. [Google Scholar] [CrossRef]
- Henry, B.M.; Cheruiyot, I.; Wong, L.M.; Keet, K.; Mutua, V.; Chhapola, V.; Tubbs, R.S. The bridging bronchus: A comprehensive review of a rare, potentially life-threatening congenital airway anomaly associated with cardiovascular defects. Pediatr. Pulmonol. 2019, 54, 1895–1904. [Google Scholar] [CrossRef]
- Wells, T.R.; Gwinn, J.L.; Landing, B.H.; Stanley, P. Reconsideration of the anatomy of sling left pulmonary artery: The association of one form with bridging bronchus and imperforate anus. Anatomic and diagnostic aspects. J. Pediatr. Surg. 1988, 23, 892–898. [Google Scholar] [CrossRef]
- Digilio, M.C.; Marino, B.; Toscano, A.; Giannotti, A.; Dallapiccola, B. Congenital heart defects in Kabuki syndrome. Am. J. Med. Genet. 2001, 100, 269–274. [Google Scholar] [CrossRef] [PubMed]
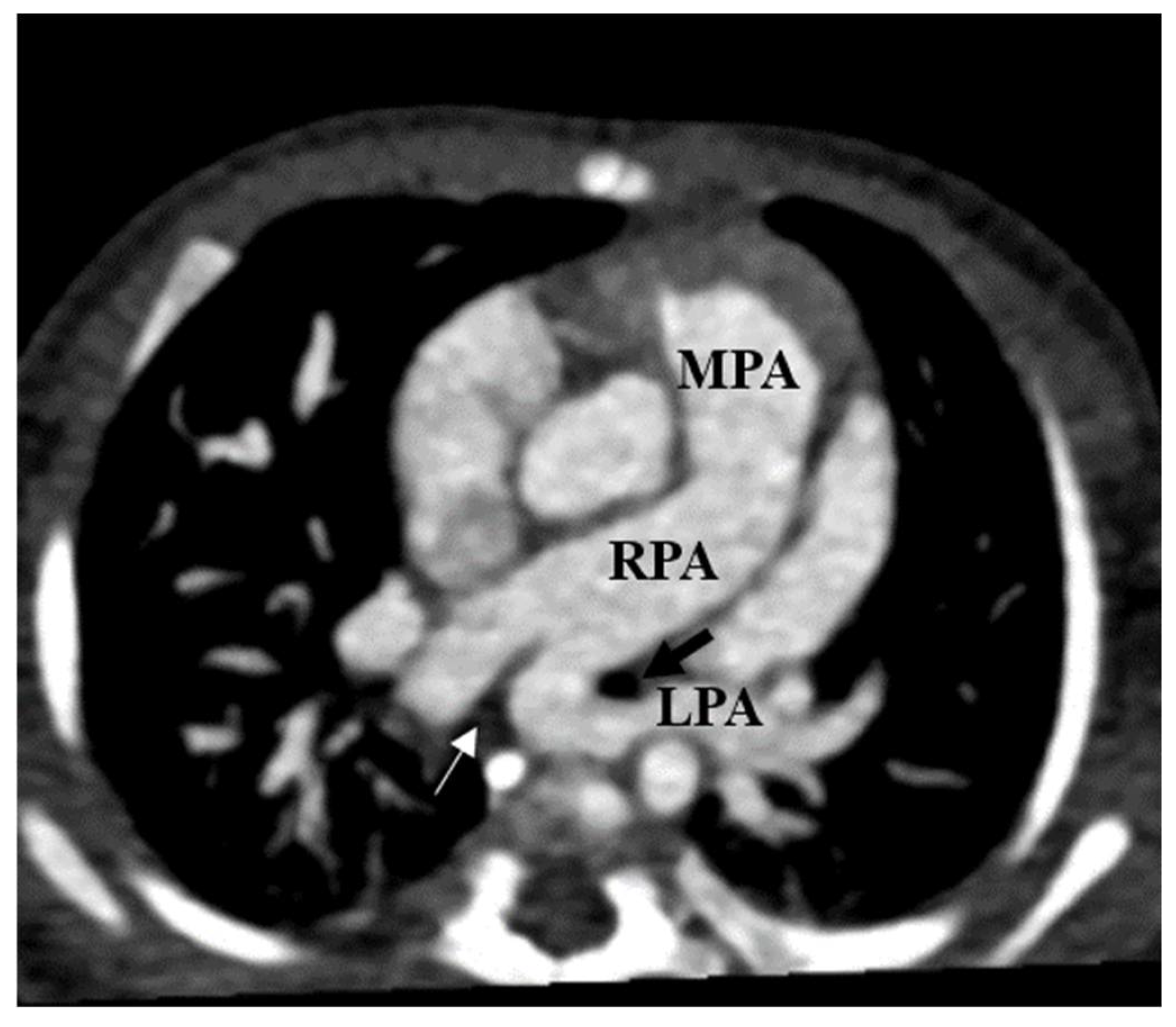
| Case | Reference | Age | PALPA Relation to TB Tree | Feeding Pulmonary Segment | TB Tree Anomalies | Respiratory Issues (YN) | CVD OT PALPA M/S/N | Other Anomaly | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | New case | 10 d | Anterior | ? | N | N | HLHS, DORV, CoA | |
| 2 | M | New case | 51 y | Anterior | ? | N | N | N | |
| 3 | M | Erickson, 1996 [6] | 30 m | Anterior | LLL | N | N | VSD | Hypospadias |
| 4 | M | Koch, 2000 [7] | 5 m | Anterior | LLL | N | N | CoA, MS | Imperforate anus |
| 5 | N/A | Fountain-Dommer, 2001 [8] | Newborn | Anterior | LLL | N | N | HLHS, DORV, VSD, CoA, L sided A arch, R subclavian from descending aorta | |
| 6 | N/A | Fountain-Dommer, 2001 [8] | 19 d | Anterior | LLL | N | N | CoA | |
| 7 | N/A | Divekar, 2004 [9] | Newborn | Anterior | LLL | N | N | TOF, TAPVR | |
| 8 | F | Collins, 2009 [10] | 48 m ** | Anterior | LLL | LMB stenosis | N | ASD | Skeletal malformations |
| 9 | M | Mathias, 2012 [11] | 28 y | Anterior | LLL | RTB, R main bronchial diverticulum | N | TOF | |
| 10 | M | Bhat, 2012 [12] | Newborn ** | Anterior | LLL | N | Y (“issues with airway”) | CoA | Dysmorphic features, maxillary teeth, undescended testes |
| 11 | N/A | Giudici, 2016 [13] | 84 m ** | Anterior | LLL | N | N | MS | SLE, small left kidney, hypothyroidism, GERD |
| 12 | F | Wang, 2017 [3] | 9 m | Anterior | LUL | RTB | N | PDA | |
| 13 | M | Wang, 2017 [3] | 4 m ** | Anterior | LLL | LMB stenosis | Y (cough) | CoA, MS, MR | |
| 14 | M | Wang, 2017 [3] | 38 m | Anterior | LLL | N | N | DORV, VSD, PS, SA, TAPVC | |
| 15 | F | Wang, 2017 [3] | 2 m | Anterior | LLL | N | N | VSD, ASD | |
| 16 | F | Wang, 2017 [3] | 40 m | Anterior | LUL | Mild trachea stenosis | Y (cough) | PA, VSD, RAoA | |
| 17 | M | Wang, 2017 [3] | 18 m | Anterior | LLL | N | N | CoA, PDA, VSD | |
| 18 | F | Wang, 2017 [3] | 27 m | Anterior | LLL | RTB, mild LMB stenosis | Y (SOB) | TOF, LSVC, PDA | |
| 19 | M | Duong, 2018 [14] | 11 m | Anterior | LLL | N | N | VSD | |
| 20 | F | Chen, 2020 [15] | 8 m ** | Anterior | LUL | LMB stenosis | N | N | Facial anomaly, low set ears, funnel chest |
| 21 | N/A | Ge, 2001 [16] | 2 m | Posterior | LLL | N | N | DORV, VSD, PS, MS, ASD, Hypoplastic LV | |
| 22 | N/A | Sadagopan, 2008 [17] | 36 w | Posterior | LLL | N | N | VSD, CoA | Pelvicalyceal dilatation of kidneys, dysmorphic features (micrognathia, clinodactyly) |
| 23 | M | Tissot, 2008 [18] | Newborn | Posterior | LLL | N | Y (inc. work of breathing) | Swiss cheese VSD, PDA | Imperforate anus, cleft palate, two neonatal teeth, dysmorphic facies, small right pelvic kidney, left hydronephrosis, hypothyroidism |
| 24 | N/A | Tateishi, 2009 [19] | 4 m | Posterior | LLL | TS | Y (dyspnea) | “AV septal defect with regurg” | |
| 25 | F | Collell, 2010 [20] | 5 y | Posterior | LLL | Yes; unspecified | Y (stridor) | N | |
| 26 | F | Abelardo, 2013 [21] | 3 m | Posterior | LUL | BB, LSTBS (Christmas tree) | Y (stridor) | N | Imperforate anus, rectovaginal fistula |
| 27 | M | Sen, 2015 [2] | “Baby” | Posterior | LLL | N | N | ASD, MA, VSD, BAV, CoA | Anal atresia, T3 butterfly vertebra, left T4 hemivertebra |
| 28 | M | Sen, 2015 [2] | 1 d | Posterior | LLL | N | N | TOF | CHARGE association |
| 29 | N/A | Giudici, 2016 [13] | 36 m | Posterior | “Left lung” | RTB, BB (Christmas tree) | Y (pulmonary infections, cough) | ASD | |
| 30 | N/A | Giudici, 2016 [13] | 48 m ** | Posterior | “Left lung” | LMB stenosis | Y (pulmonary infections, pulmonary hypertension) | VSD | Microcephaly, cleft palate, developmental delay |
| 31 | F | Wang, 2017 [3] | 5 m | Posterior | LLL | BB, LSTS | Y (cough, SOB) | N | |
| 32 | M | Wang, 2017 [3] | 5 m | Posterior | LLL | RTB, LSTS | N (not reported) | N | |
| 33 | M | Nagatomo, 2017 [22] | 16 y | Posterior | LLL | Dynamic TS, LTB | Y (dyspnea on exertion) | N | |
| 34 | F | Chao, 2018 [23] | 1 d | Posterior | LLL and part of LUL | LMB stenosis, hypoplastic R lung | Y (severe respirator distress) | Scimitar, dextrocardia | T12 hemivertebra |
| 35 | M | Maldjian, 2020 [1] | 72 y | Posterior | LUL | N | N | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restrepo, C.S.; Gonzalez, T.V.; Baxi, A.J.; Saboo, S.S. Partial Anomalous Left Pulmonary Artery Anterior Versus Posterior Types: A Systematic Review. Tomography 2022, 8, 1947-1958. https://doi.org/10.3390/tomography8040163
Restrepo CS, Gonzalez TV, Baxi AJ, Saboo SS. Partial Anomalous Left Pulmonary Artery Anterior Versus Posterior Types: A Systematic Review. Tomography. 2022; 8(4):1947-1958. https://doi.org/10.3390/tomography8040163
Chicago/Turabian StyleRestrepo, Carlos S., Tomas V. Gonzalez, Ameya J. Baxi, and Sachin S. Saboo. 2022. "Partial Anomalous Left Pulmonary Artery Anterior Versus Posterior Types: A Systematic Review" Tomography 8, no. 4: 1947-1958. https://doi.org/10.3390/tomography8040163
APA StyleRestrepo, C. S., Gonzalez, T. V., Baxi, A. J., & Saboo, S. S. (2022). Partial Anomalous Left Pulmonary Artery Anterior Versus Posterior Types: A Systematic Review. Tomography, 8(4), 1947-1958. https://doi.org/10.3390/tomography8040163





