Tuberculosis of the Heart: A Diagnostic Challenge
Abstract
1. Introduction
2. Pathogenesis of Tuberculosis of the Heart
3. Clinical Presentation of Tuberculosis of Heart
4. Laboratory Diagnostic Test
5. Role of Cardiac MRI
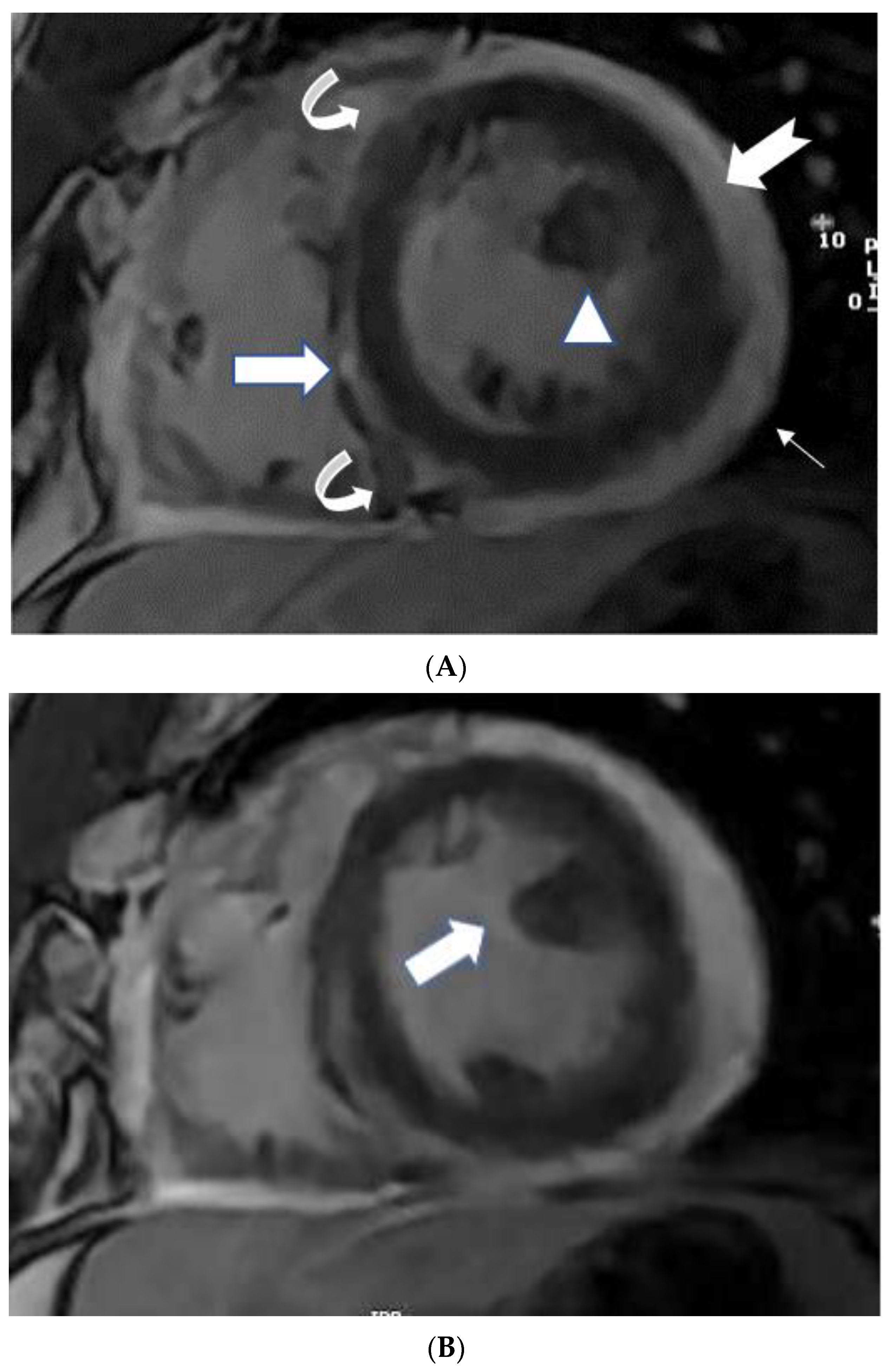
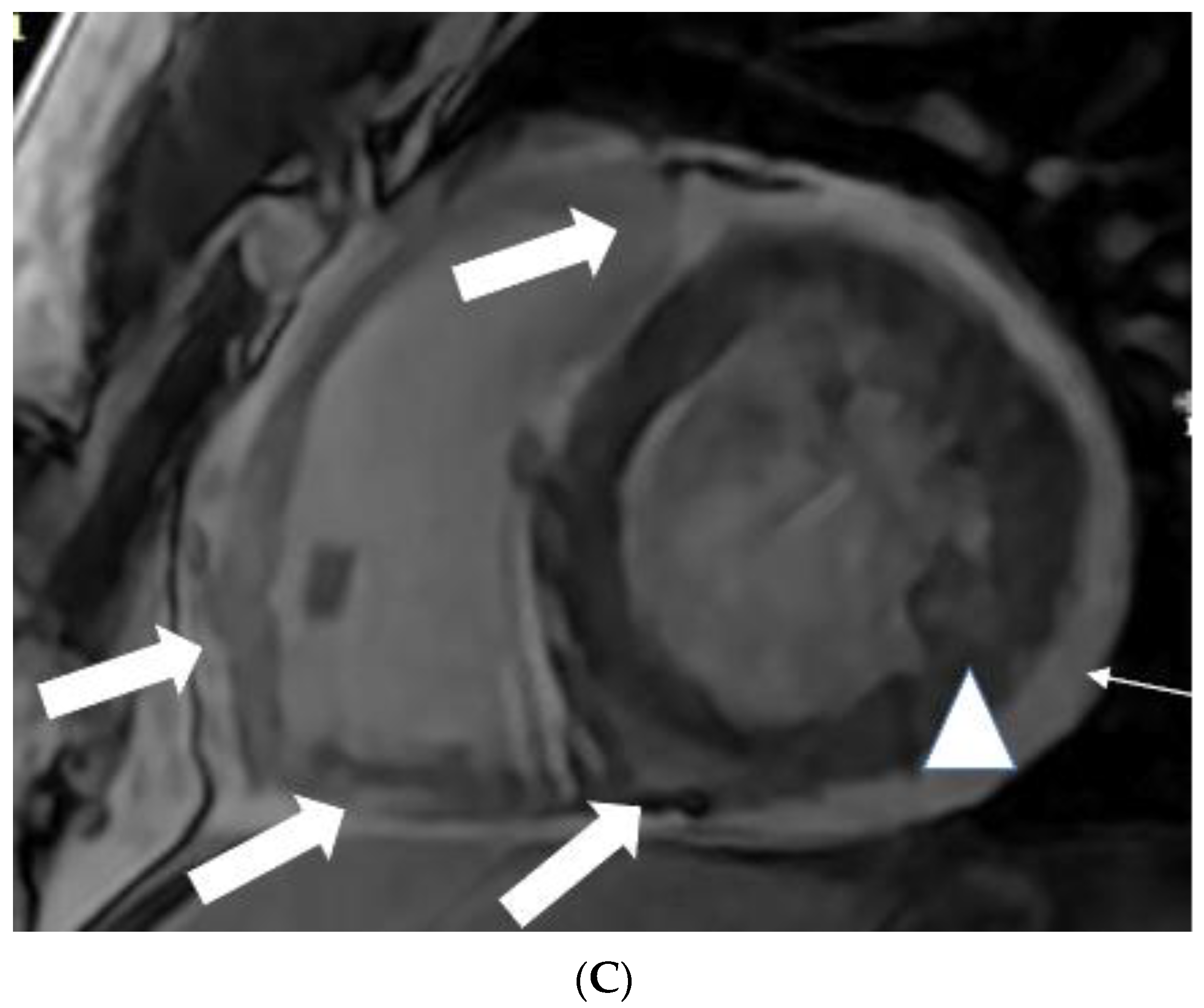
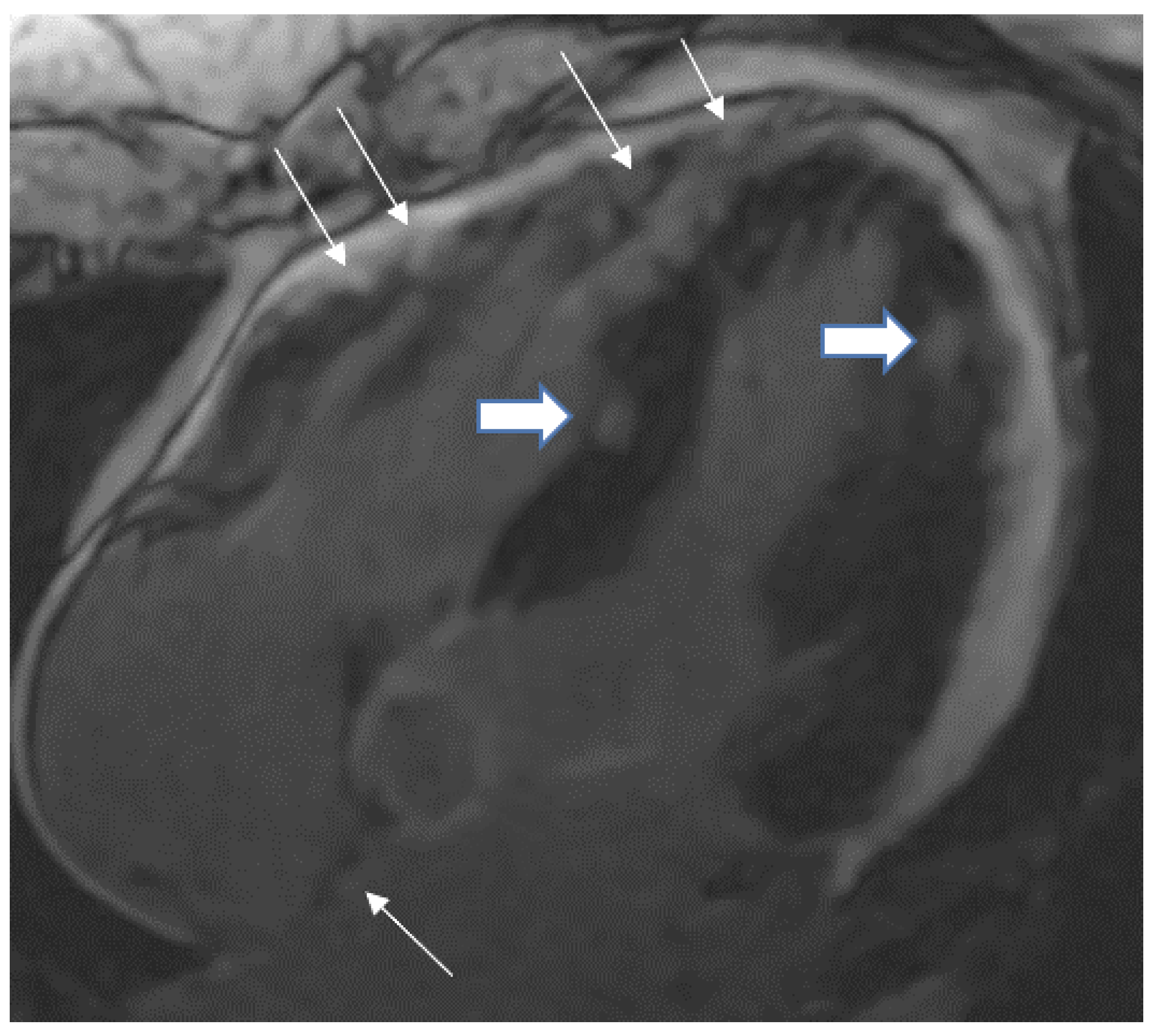
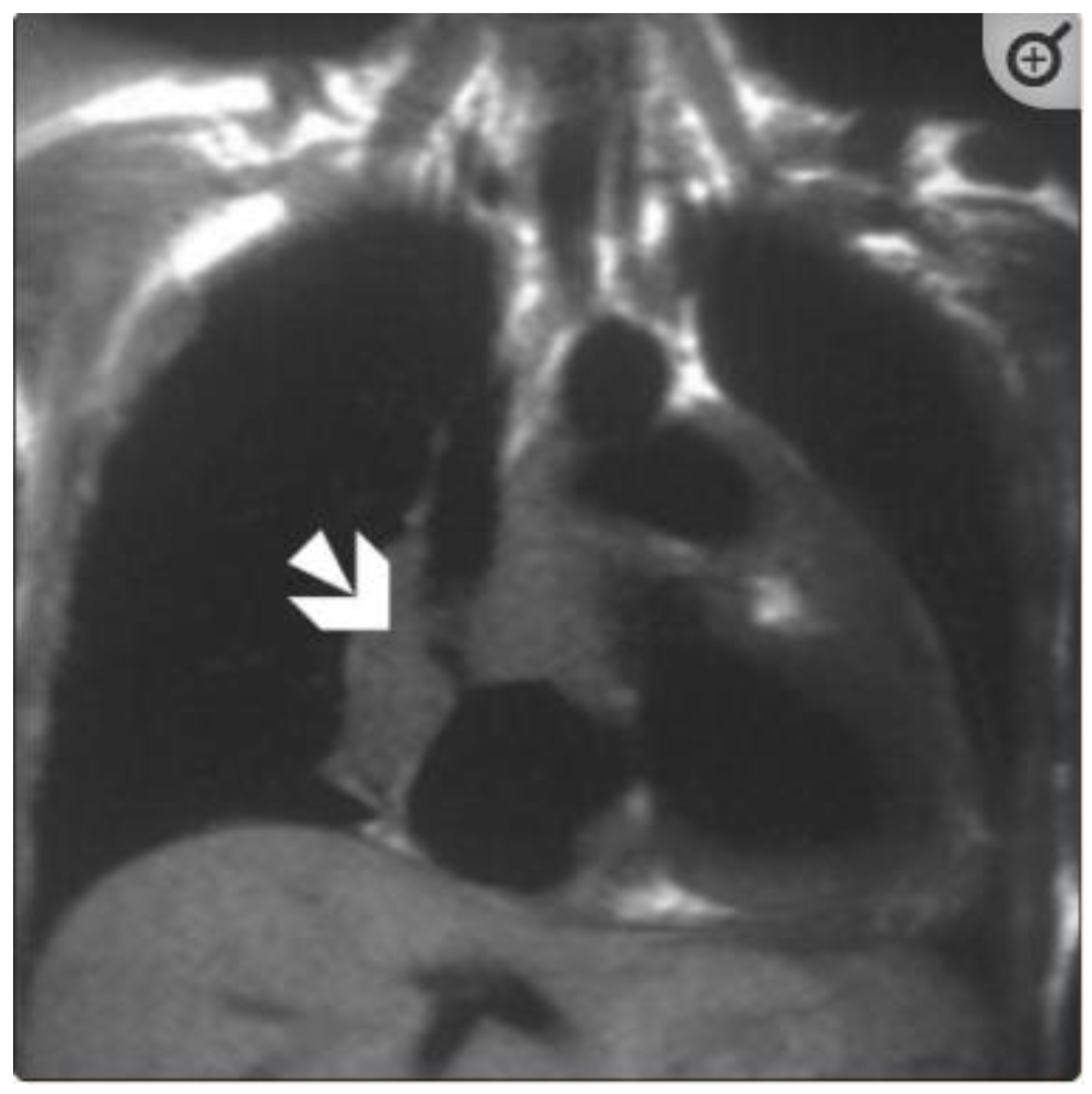
5.1. TB pericarditis and Myopericarditis
5.2. TB Myocarditis
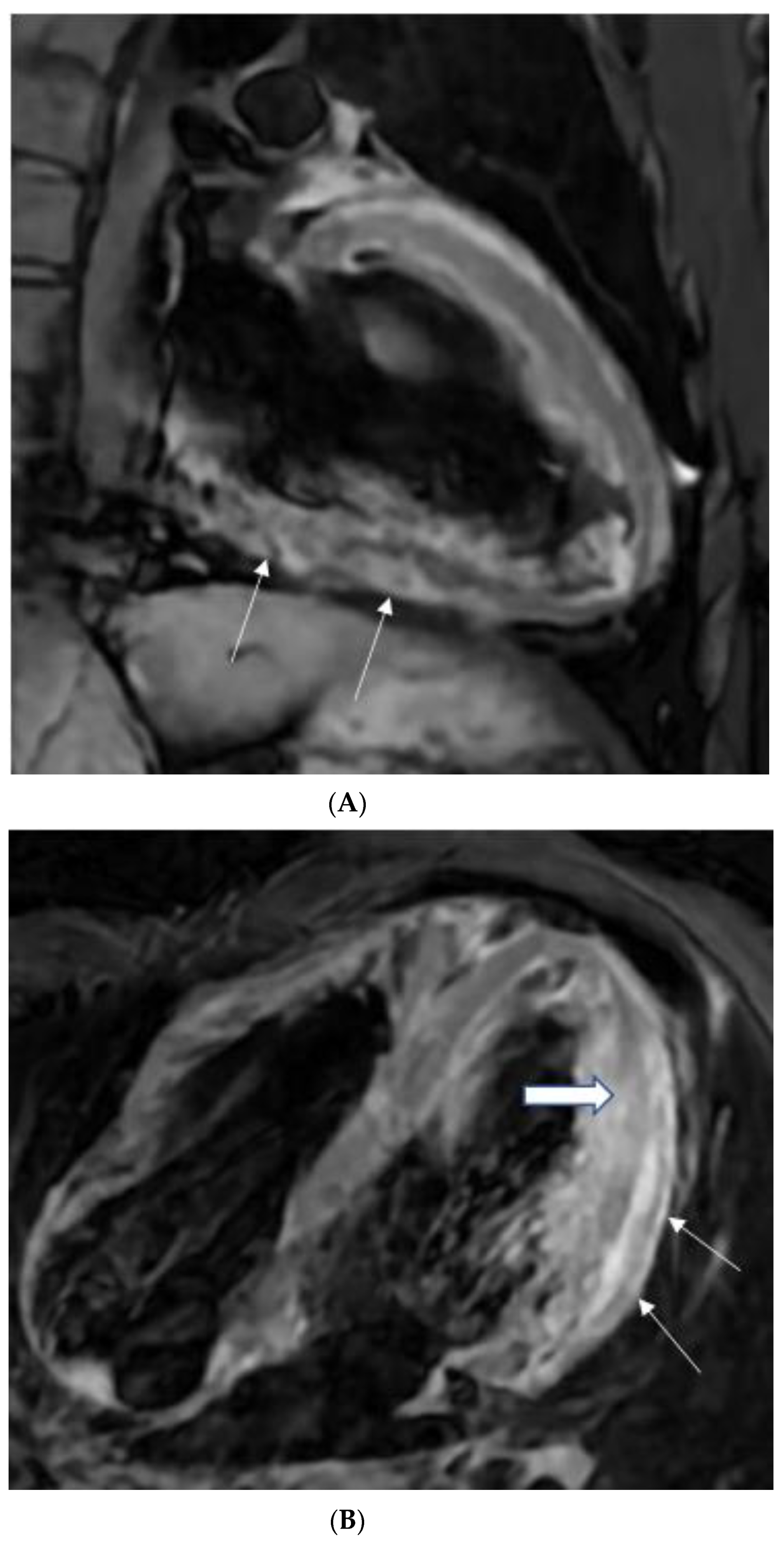
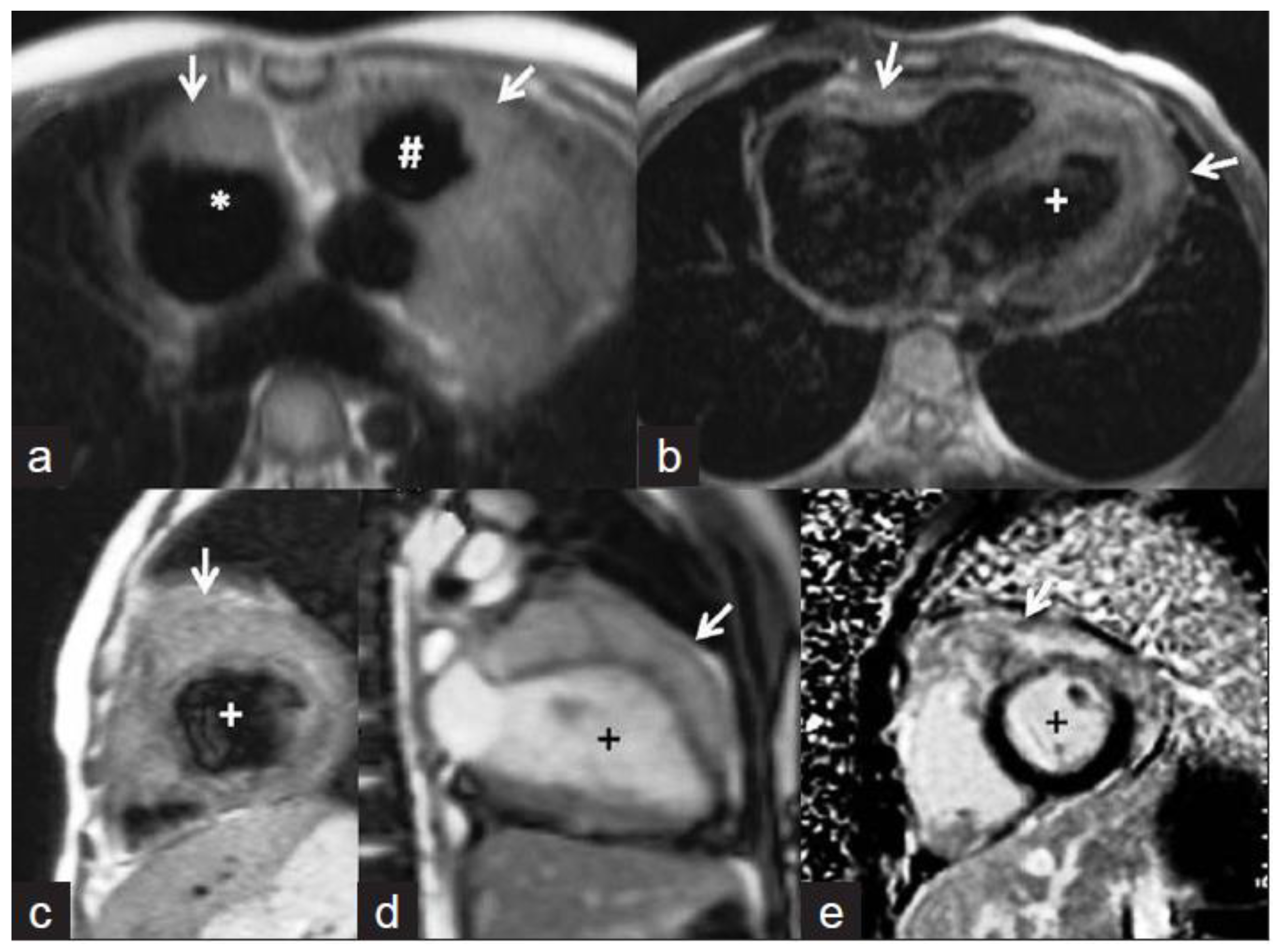
5.3. Papillary Muscle Enlargement
5.4. Intracardiac Tuberculoma
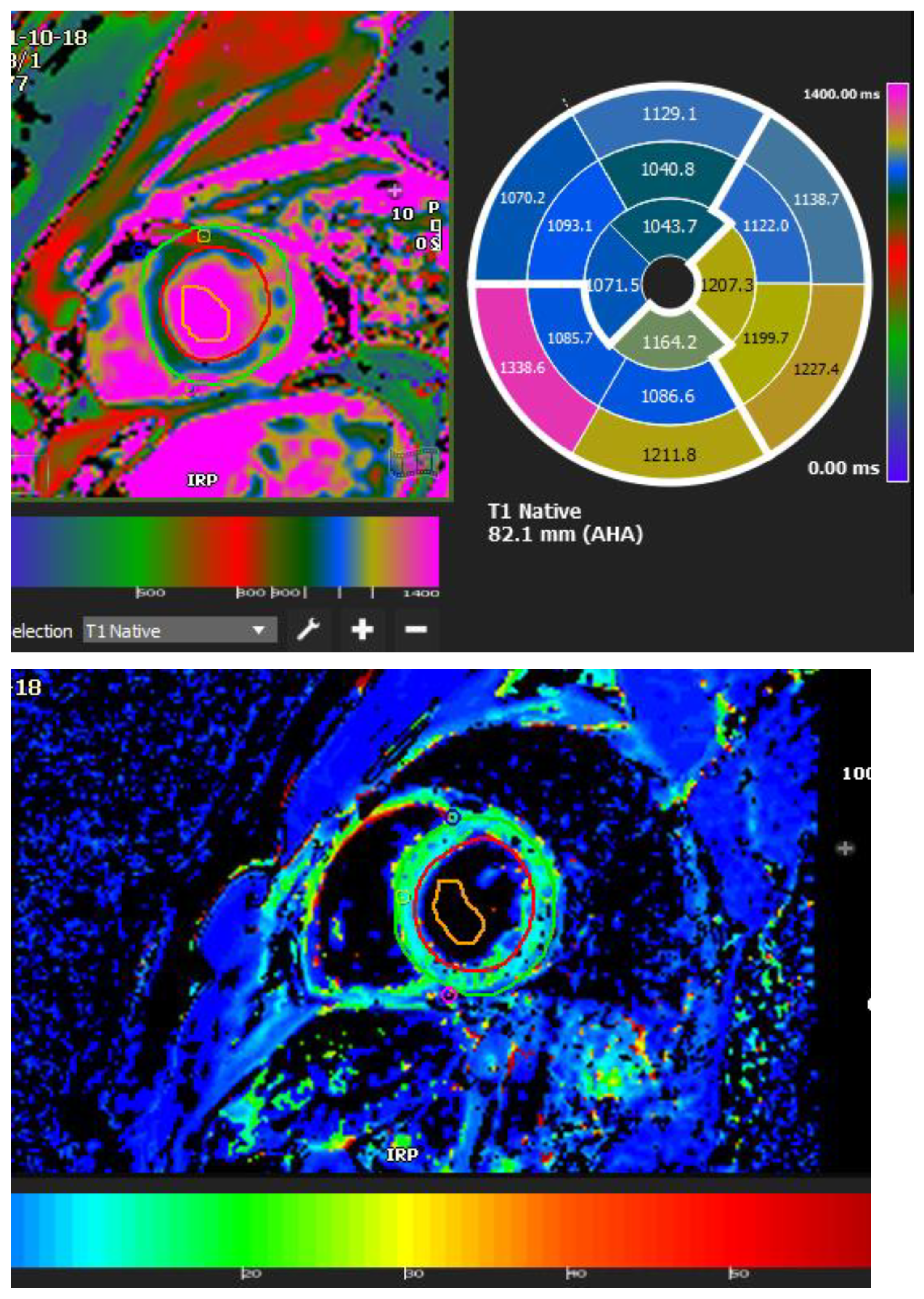
5.5. CMR Feature Tracking
5.6. Advantages of CMR with Echocardiography
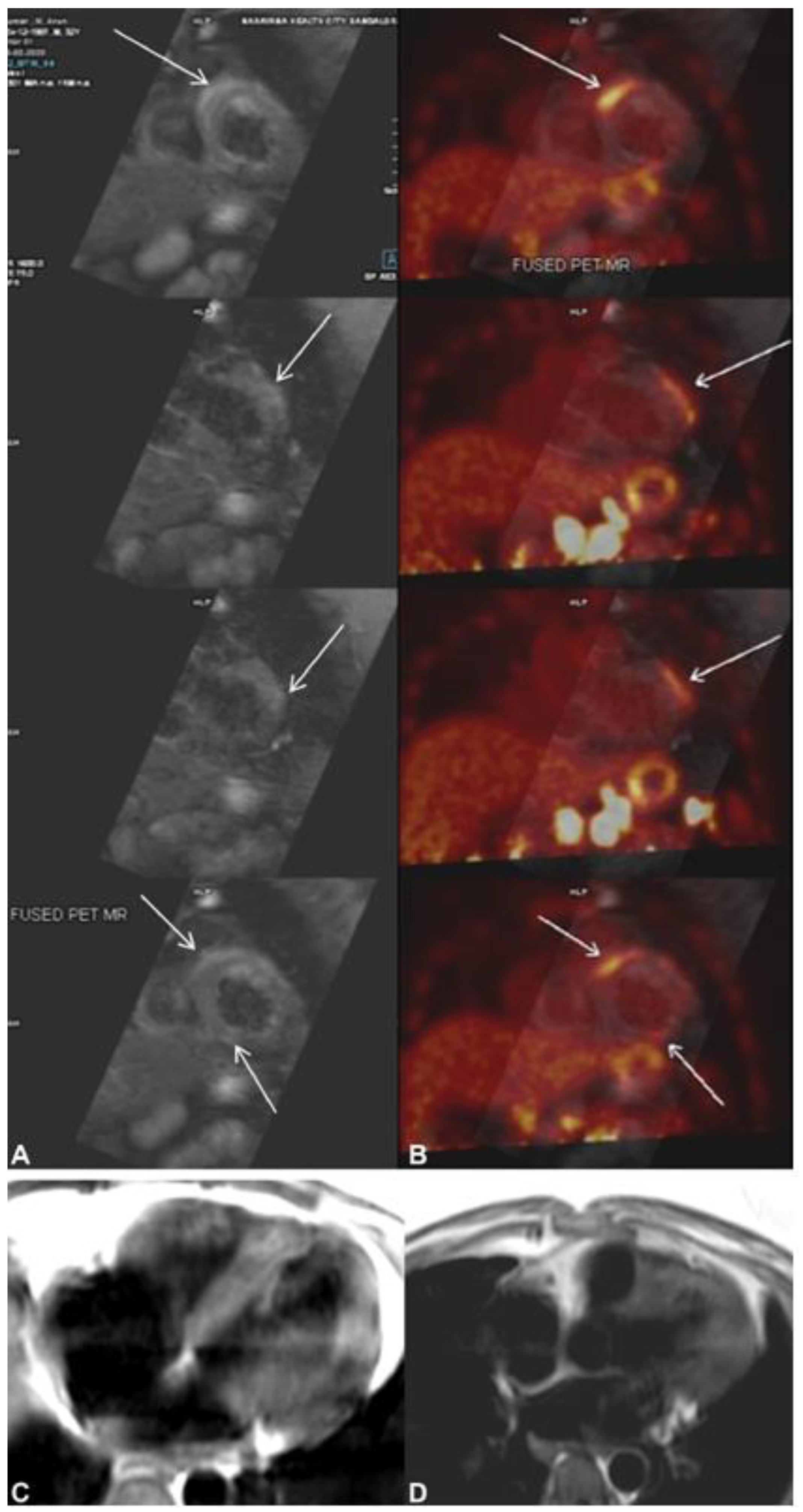
6. Role of Tc-99m Sestamibi and SPECT
7. Role of Cardiac 18F-FDG PET
8. Endomyocardial Biopsy
9. Advantage and Disadvantages of Imaging Modalities (CMR, CT, PET)
10. Remaining Challenges
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Frieden, T.R.; Brudney, K.F.; Harries, A.D. Global tuberculosis: Perspectives, prospects, and priorities. JAMA 2014, 312, 1393–1394. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.F.; Ntsekhe, M.; Gumedze, F.; Badri, M.; Mayosi, B.M. Myopericarditis in tuberculous pericardial effusion: Prevalence, predictors and outcome. Heart 2014, 100, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.; Saphir, O. The Involvement of the Myocardium in Tuberculosis, A Review of the Literature and Report of 3 Cases. Am. Rev. Tuberc. 1935, 32, 492–506. [Google Scholar]
- Fowler, N.O. Tuberculous pericarditis. JAMA 1991, 266, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Njovane, X. Intramyocardial tuberculosis: A rare underdiagnosed entity. SAMJ S. Afr. Med. J. 2009, 99, 152–153. [Google Scholar]
- Anders, J.M. Tuberculosis of the Myocardium. J. Am. Med. Assoc. 1902, 39, 1081–1086. [Google Scholar] [CrossRef][Green Version]
- Rose, A.G. Cardiac tuberculosis. A study of 19 patients. Arch. Pathol. Lab. Med. 1987, 111, 422–426. [Google Scholar]
- Mayosi, B.M. Tuberculous Pericarditis and Myocarditis in Adults and. Tuberculosis E-Book: A Comprehensive Clinical Reference; Elsevier: Amsterdam, The Netherlands, 2009; p. 351. [Google Scholar]
- López-López, J.P.; Posada-Martínez, E.L.; Saldarriaga, C.; Wyss, F.; Ponte-Negretti, C.I.; Alexander, B.; Miranda-Arboleda, A.F.; Martínez-Sellés, M.; Baranchuk, A.; the Neglected Tropical Diseases, Other Infectious Diseases Affecting the Heart (the NET-Heart Project). Tuberculosis and the Heart. J. Am. Heart Assoc. 2021, 10, e019435. [Google Scholar] [CrossRef]
- Behr, G.; Palin, H.C.; Temperly, J.M. Myocardial tuberculosis. Br. Med. J. 1977, 1, 951. [Google Scholar] [CrossRef][Green Version]
- Kapoor, O.P.; Mascarenhas, E.; Rananaware, M.M.; Gadgil, R.K. Tuberculoma of the heart: Report of 9 cases. Am. Heart J. 1973, 86, 334–340. [Google Scholar] [CrossRef]
- Jeilan, M.; Schmitt, M.; McCann, G.; Davies, J.; Leverment, J.; Chin, D. Cardiac tuberculoma. Circulation 2008, 117, 984–986. [Google Scholar] [CrossRef]
- Mayosi, B.M.; Wiysonge, C.S.; Ntsekhe, M.; Volmink, J.A.; Gumedze, F.; Maartens, G.; Aje, A.; Thomas, B.M.; Thomas, K.M.; Awotedu, A.A. Clinical characteristics and initial management of patients with tuberculous pericarditis in the HIV era: The Investigation of the Management of Pericarditis in Africa (IMPI Africa) registry. BMC Infect. Dis. 2006, 6, 2. [Google Scholar] [CrossRef]
- Mayosi, B.M.; Ntsekhe, M.; Bosch, J.; Pandie, S.; Jung, H.; Gumedze, F.; Pogue, J.; Thabane, L.; Smieja, M.; Francis, V. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N. Engl. J. Med. 2014, 371, 1121–1130. [Google Scholar] [CrossRef]
- Gooi, H.C.; Smith, J.M. Tuberculous pericarditis in Birmingham. Thorax 1978, 33, 94–96. [Google Scholar] [CrossRef]
- Ntsekhe, M.; Matthews, K.; Syed, F.F.; Deffur, A.; Badri, M.; Commerford, P.J.; Gersh, B.J.; Wilkinson, K.A.; Wilkinson, R.J.; Mayosi, B.M. Prevalence, hemodynamics, and cytokine profile of effusive-constrictive pericarditis in patients with tuberculous pericardial effusion. PLoS ONE 2013, 8, e77532. [Google Scholar] [CrossRef]
- Strang, J.I.G. Tuberculous pericarditis in Transkei. Clin. Cardiol. 1984, 7, 667–670. [Google Scholar] [CrossRef]
- Strang, J.I.G.; Gibson, D.G.; Mitchison, D.A.; Girling, D.J.; Kakaza, H.H.S.; Allen, B.W.; Evans, D.J.; Nunn, A.J.; Fox, W. Controlled clinical trial of complete open surgical drainage and of prednisolone in treatment of tuberculous pericardial effusion in Transkei. Lancet 1988, 332, 759–764. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lee, M.-H.; Liu, J.-W.; Leu, H.-S. Diagnosis of tuberculous pericarditis and treatment without corticosteroids at a tertiary teaching hospital in Taiwan: A 14-year experience. J. Microbiol. Immunol. Infect. = Wei Mian Yu Gan Ran Za Zhi 2005, 38, 47–52. [Google Scholar]
- Reuter, H.; Burgess, L.J.; Louw, V.J.; Doubell, A.F. The management of tuberculous pericardial effusion: Experience in 233 consecutive patients. Cardiovasc. J. S. Afr. 2007, 18, 20–25. [Google Scholar] [PubMed]
- Bali, H.K.; Wahi, S.; Sharma, B.K.; Anand, I.S.; Datta, B.N.; Wahi, P.L. Myocardial tuberculosis presenting as restrictive cardiomyopathy. Am. Heart J. 1990, 120, 703–706. [Google Scholar] [CrossRef]
- Sogabe, O.; Ohya, T. A case of tuberculous endocarditis with acute aortic valve insufficiency and annular subvalvular left ventricular aneurysm. Gen. Thorac. Cardiovasc. Surg. 2007, 55, 61–64. [Google Scholar] [CrossRef]
- Cope, A.P.; Heber, M.; Wilkins, E.G.L. Valvular tuberculous endocarditis: A case report and review of the literature. J. Infect. 1990, 21, 293–296. [Google Scholar] [CrossRef]
- Kinare, S.G.; Bhatia, B.I. Tuberculous coronary arteritis with aneurysm of the ventricular septum. Chest 1971, 60, 613–616. [Google Scholar] [CrossRef]
- Wilbur, E.L. Myocardial Tuberculosis: A Cause of Congestive Heart Failure. Am. Rev. Tuberc. 1938, 38, 769–776. [Google Scholar]
- Michira, B.N.; Alkizim, F.O.; Matheka, D.M. Patterns and clinical manifestations of tuberculous myocarditis: A systematic review of cases. Pan Afr. Med. J. 2015, 21, 118. [Google Scholar] [CrossRef]
- Liu, A.; Nicol, E.; Hu, Y.; Coates, A. Tuberculous endocarditis. Int. J. Cardiol. 2013, 167, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Brucato, A.; Barbieri, A.; Ferroni, F.; Maestroni, S.; Ligabue, G.; Chinaglia, A.; Cumetti, D.; Casa, G.D.; Bonomi, F. Good prognosis for pericarditis with and without myocardial involvement: Results from a multicenter, prospective cohort study. Circulation 2013, 128, 42–49. [Google Scholar] [CrossRef]
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef]
- Liao, C.-H.; Chou, C.-H.; Lai, C.-C.; Huang, Y.T.; Tan, C.K.; Hsu, H.-L.; Hsueh, P.R. Diagnostic performance of an enzyme-linked immunospot assay for interferon-γ in extrapulmonary tuberculosis varies between different sites of disease. J. Infect. 2009, 59, 402–408. [Google Scholar] [CrossRef]
- Parkhurst, G.F.; Decker, J.P. Bacterial aortitis and mycotic aneurysm of the aorta: A report of twelve cases. Am. J. Pathol. 1955, 31, 821. [Google Scholar]
- Bogaert, J.; Francone, M. Cardiovascular magnetic resonance in pericardial diseases. J. Cardiovasc. Magn. Reson. 2009, 11, 14. [Google Scholar] [CrossRef]
- Taylor, A.M.; Dymarkowski, S.; Verbeken, E.K.; Bogaert, J. Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: Initial results. Eur. Radiol. 2006, 16, 569–574. [Google Scholar] [CrossRef]
- Yelgec, N.S.; Dymarkowski, S.; Ganame, J.; Bogaert, J. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur. Radiol. 2007, 17, 2211–2217. [Google Scholar] [CrossRef]
- Troughton, R.W.; Asher, C.R.; Klein, A.L. Pericarditis. Lancet 2004, 363, 717–727. [Google Scholar] [CrossRef]
- Ling, L.H.; Oh, J.K.; Schaff, H.V.; Danielson, G.K.; Mahoney, D.W.; Seward, J.B.; Tajik, A.J. Constrictive pericarditis in the modern era: Evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999, 100, 1380–1386. [Google Scholar] [CrossRef]
- Myers, R.B.H.; Spodick, D.H. Constrictive pericarditis: Clinical and pathophysiologic characteristics. Am. Heart J. 1999, 138, 219–232. [Google Scholar] [CrossRef]
- Nishimura, R.A. Constrictive pericarditis in the modern era: A diagnostic dilemma. Heart 2001, 86, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Little, W.C.; Freeman, G.L. Pericardial disease. Circulation 2006, 113, 1622–1632. [Google Scholar] [CrossRef]
- Oh, K.Y.; Shimizu, M.; Edwards, W.D.; Tazelaar, H.D.; Danielson, G.K. Surgical pathology of the parietal pericardium: A study of 344 cases (1993–1999). Cardiovasc. Pathol. 2001, 10, 157–168. [Google Scholar] [CrossRef]
- Talreja, D.R.; Edwards, W.D.; Danielson, G.K.; Schaff, H.V.; Tajik, A.J.; Tazelaar, H.D.; Breen, J.F.; Oh, J.K. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation 2003, 108, 1852–1857. [Google Scholar] [CrossRef]
- Feng, D.; Glockner, J.; Kim, K.; Martinez, M.; Syed, I.S.; Araoz, P.; Breen, J.; Espinosa, R.E.; Sundt, T.; Schaff, H.V. Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy: A pilot study. Circulation 2011, 124, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M. Comparative evaluation of left and right ventricular endomyocardial biopsy: Differences in complication rate and diagnostic performance. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Fulton, N.L.; Bolen, M. Magnetic resonance imaging of the papillary muscles of the left ventricle: Normal anatomy, variants, and abnormalities. Insights Imaging 2019, 10, 83. [Google Scholar]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2017, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Cohen, L.S. Left ventricular papillary muscles: Description of the normal and a survey of conditions causing them to be abnormal. Circulation 1972, 46, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.S.; Kothari, S.S. Diffuse infiltrative cardiac tuberculosis. Ann. Pediatric Cardiol. 2011, 4, 87. [Google Scholar]
- Cantinotti, M.; De Gaudio, M.; De Martino, M.; Assanta, N.; Moschetti, R.; Veneruso, G.; Crocetti, M.; Murzi, B.; Chiappini, E.; Galli, L. Intracardiac left atrial tuberculoma in an eleven-month-old infant: Case report. BMC Infect. Dis. 2011, 11, 359. [Google Scholar]
- Licht, J.; Diefenbach, C.; Stang, A.; Hartmann, V.; Bolte, J.; Kirsten, D. Tuberculoma of the myocardium: A rare case of intra-vitam diagnosis. Clin. Res. Cardiol. 2009, 98, 331–333. [Google Scholar] [CrossRef]
- Chang, B.-C.; Ha, J.-W.; Kim, J.-T.; Chung, N.; Cho, S.-H. Intracardiac tuberculoma. Ann. Thorac. Surg. 1999, 67, 226–228. [Google Scholar] [CrossRef]
- O’Neill, P.G.; Rokey, R.; Greenberg, S.; Pacifico, A. Resolution of ventricular tachycardia and endocardial tuberculoma following antituberculosis therapy. Chest 1991, 100, 1467–1469. [Google Scholar] [CrossRef]
- Borrhomée, S.; Vergnat, M.; Roussin, R.; Hascoët, S. A rare case of left ventricular pseudoaneurysm due to tuberculosis in a 13-year-old boy. World J. Pediatric Congenit. Heart Surg. 2019, 10, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Chowdhury, V.; Singh, S. Case report: Myocardial tuberculosis-MRI. Indian J. Radiol. Imaging 2009, 19, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Obrist, S.J.; Erne, S.A.; Stark, A.W.; Marggraf, M.; Kaneko, K.; Guensch, D.P.; Huber, A.T.; Greulich, S.; Aghayev, A. Feature tracking myocardial strain incrementally improves prognostication in myocarditis beyond traditional CMR imaging features. Cardiovasc. Imaging 2020, 13, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.F.; Aje, A.; Ntsekhe, M.; Mayosi, B.M.; Moosa, S.; Tshifularo, M.; Smedema, J.P. Resolution of nodular myocardial tuberculosis demonstrated by contrast-enhanced magnetic resonance imaging. Cardiovasc. J. Afr. 2008, 19, 198–199. [Google Scholar] [PubMed]
- Immer, F.F.; Pirovino, M.; Saner, H. Isolated tuberculosis of the heart: A clinical and echocardiography follow-up. Zeitschrift fur Kardiologie 1997, 86, 15–19. [Google Scholar] [CrossRef]
- Breton, G.; Leclerc, S.; Longuet, P.; Leport, C.; Vildé, J.-L.; Laissy, J.-P. Myocardial localisation of tuberculosis: The diagnostic value of cardiac MRI. Presse Med. 2005, 34, 293–296. [Google Scholar] [CrossRef]
- Salem, R.; Boucher, L.; Laflamme, L. Dual Tc-99m sestamibi and Gallium-67 SPECT localize a myocardial abscess around a bioprosthetic aortic valve. Clin. Nucl. Med. 2004, 29, 799–800. [Google Scholar] [CrossRef]
- Cornel, J.H.; Arnese, M.; Forster, T.; Postma-Tjoa, J.; Reijs, A.E.; Fioretti, P.M. Potential and limitations of Tc-99m sestamibi scintigraphy for the diagnosis of myocardial viability. Herz 1994, 19, 19–27. [Google Scholar]
- Sundaraiya, S.; Sulaiman, A.; Rajendran, A. Cardiac Tuberculosis on 18F-FDG PET Imaging—A Great Masquerader of Cardiac Sarcoidosis. Indian J. Radiol. Imaging 2021, 31, 1002–1007. [Google Scholar] [CrossRef]
- Bokhari, S.; Sheikh, T. Cardiac sarcoidosis: Advantages and limitations of advanced cardiac imaging. J. Nucl. Cardiol. 2021, 1–4. [Google Scholar] [CrossRef]
- Shields, R.C.; Tazelaar, H.D.; Berry, G.J.; Cooper, L.T., Jr. The role of right ventricular endomyocardial biopsy for idiopathic giant cell myocarditis. J. Card. Fail. 2002, 8, 74–78. [Google Scholar] [CrossRef]
- Yasuda, T.; Palacios, I.F.; Dec, G.W.; Fallon, J.T.; Gold, H.K.; Leinbach, R.C.; Strauss, H.W.; Khaw, B.A.; Haber, E. Indium 111-monoclonal antimyosin antibody imaging in the diagnosis of acute myocarditis. Circulation 1987, 76, 306–311. [Google Scholar] [CrossRef]
- Camargo, P.R.; Mazzieri, R.; Snitcowsky, R.; de Lourdes Higuchi, M.; Meneghetti, J.C.; Soares, J., Jr.; Fiorelli, A.; Ebaid, M.; Pileggi, F. Correlation between gallium-67 imaging and endomyocardial biopsy in children with severe dilated cardiomyopathy. Int. J. Cardiol. 1990, 28, 293–297. [Google Scholar] [CrossRef]
- Biesbroek, P.S.; Beek, A.M.; Germans, T.; Niessen, H.W.M.; van Rossum, A.C. Diagnosis of myocarditis: Current state and future perspectives. Int. J. Cardiol. 2015, 191, 211–219. [Google Scholar] [CrossRef]
- Ling, L.H.; Oh, J.K.; Tei, C.; Click, R.L.; Breen, J.F.; Seward, J.B.; Tajik, A.J. Pericardial thickness measured with transesophageal echocardiography: Feasibility and potential clinical usefulness. J. Am. Coll. Cardiol. 1997, 29, 1317–1323. [Google Scholar] [CrossRef]
- Houang, M.T.; Arozena, X.; Shaw, D.G. Demonstration of the pericardium and pericardial effusion by computed tomography. J. Comput. Assist. Tomogr. 1979, 3, 601–603. [Google Scholar] [CrossRef]
- Giorgi, B.; Mollet, N.R.A.; Dymarkowski, S.; Rademakers, F.E.; Bogaert, J. Clinically suspected constrictive pericarditis: MR imaging assessment of ventricular septal motion and configuration in patients and healthy subjects. Radiology 2003, 228, 417–424. [Google Scholar] [CrossRef]
- Francone, M.; Dymarkowski, S.; Kalantzi, M.; Bogaert, J. Real-time cine MRI of ventricular septal motion: A novel approach to assess ventricular coupling. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2005, 21, 305–309. [Google Scholar] [CrossRef]
- Zurick, A.O.; Bolen, M.A.; Kwon, D.H.; Tan, C.D.; Popovic, Z.B.; Rajeswaran, J.; Rodriguez, E.R.; Flamm, S.D.; Klein, A.L. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy: A case series with histopathological correlation. JACC Cardiovasc. Imaging 2011, 4, 1180–1191. [Google Scholar] [CrossRef]
- Kojima, S.; Yamada, N.; Goto, Y. Diagnosis of constrictive pericarditis by tagged cine magnetic resonance imaging. N. Engl. J. Med. 1999, 341, 373–374. [Google Scholar] [CrossRef]
- Byrne, J.G.; Karavas, A.N.; Colson, Y.L.; Bueno, R.; Richards, W.G.; Sugarbaker, D.J.; Goldhaber, S.Z. Cardiac decortication (epicardiectomy) for occult constrictive cardiac physiology after left extrapleural pneumonectomy. Chest 2002, 122, 2256–2259. [Google Scholar] [CrossRef]
- Maksimović, R.; Seferović, P.M.; Ristić, A.D.; Vujisić-Tešić, B.; Simeunović, D.S.; Radovanović, G.; Matucci-Cerinic, M.; Maisch, B. Cardiac imaging in rheumatic diseases. Rheumatology 2006, 45, iv26–iv31. [Google Scholar] [CrossRef]
- Bogaert, J.; Francone, M. Pericardial disease: Value of CT and MR imaging. Radiology 2013, 267, 340–356. [Google Scholar] [CrossRef]
- Wang, Z.J.; Reddy, G.P.; Gotway, M.B.; Yeh, B.M.; Hetts, S.W.; Higgins, C.B. CT and MR imaging of pericardial disease. Radiographics 2003, 23, S167–S180. [Google Scholar] [CrossRef]
- Alter, P.; Figiel, J.H.; Rupp, T.P.; Bachmann, G.F.; Maisch, B.; Rominger, M.B. MR, CT, and PET imaging in pericardial disease. Heart Fail. Rev. 2013, 18, 289–306. [Google Scholar] [CrossRef]
- Brett, N.J.; Strugnell, W.E.; Slaughter, R.E. Acute myocarditis demonstrated on CT coronary angiography with MRI correlation. Circ. Cardiovasc. Imaging 2011, 4, e5–e6. [Google Scholar] [CrossRef]
- Akaike, G.; Itani, M.; Shah, H.; Ahuja, J.; Yilmaz Gunes, B.; Assaker, R.; Behnia, F. PET/CT in the diagnosis and workup of sarcoidosis: Focus on atypical manifestations. Radiographics 2018, 38, 1536–1549. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular disease in the developing world: Prevalences, patterns, and the potential of early disease detection. J. Am. Coll. Cardiol. 2012, 60, 1207–1216. [Google Scholar] [CrossRef]
- Gaziano, T.A. Cardiovascular disease in the developing world and its cost-effective management. Circulation 2005, 112, 3547–3553. [Google Scholar] [CrossRef]
- Sippel, S.; Muruganandan, K.; Levine, A.; Shah, S. Use of ultrasound in the developing world. Int. J. Emerg. Med. 2011, 4, 72. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, K.M.; Mansoori, T.A.; Alattar, Y.H.; Gorkom, K.V.; Shamisi, A.; Melethil, A.P.; Alkoteesh, J.A. Tuberculosis of the Heart: A Diagnostic Challenge. Tomography 2022, 8, 1649-1665. https://doi.org/10.3390/tomography8040137
Das KM, Mansoori TA, Alattar YH, Gorkom KV, Shamisi A, Melethil AP, Alkoteesh JA. Tuberculosis of the Heart: A Diagnostic Challenge. Tomography. 2022; 8(4):1649-1665. https://doi.org/10.3390/tomography8040137
Chicago/Turabian StyleDas, Karuna M., Taleb Al Mansoori, Yousef Habeeb Alattar, Klaus V. Gorkom, Ali Shamisi, Anisha Pulinchani Melethil, and Jamal Aldeen Alkoteesh. 2022. "Tuberculosis of the Heart: A Diagnostic Challenge" Tomography 8, no. 4: 1649-1665. https://doi.org/10.3390/tomography8040137
APA StyleDas, K. M., Mansoori, T. A., Alattar, Y. H., Gorkom, K. V., Shamisi, A., Melethil, A. P., & Alkoteesh, J. A. (2022). Tuberculosis of the Heart: A Diagnostic Challenge. Tomography, 8(4), 1649-1665. https://doi.org/10.3390/tomography8040137





