Visual Evaluation of Ultrafast MRI in the Assessment of Residual Breast Cancer after Neoadjuvant Systemic Therapy: A Preliminary Study Association with Subtype
Abstract
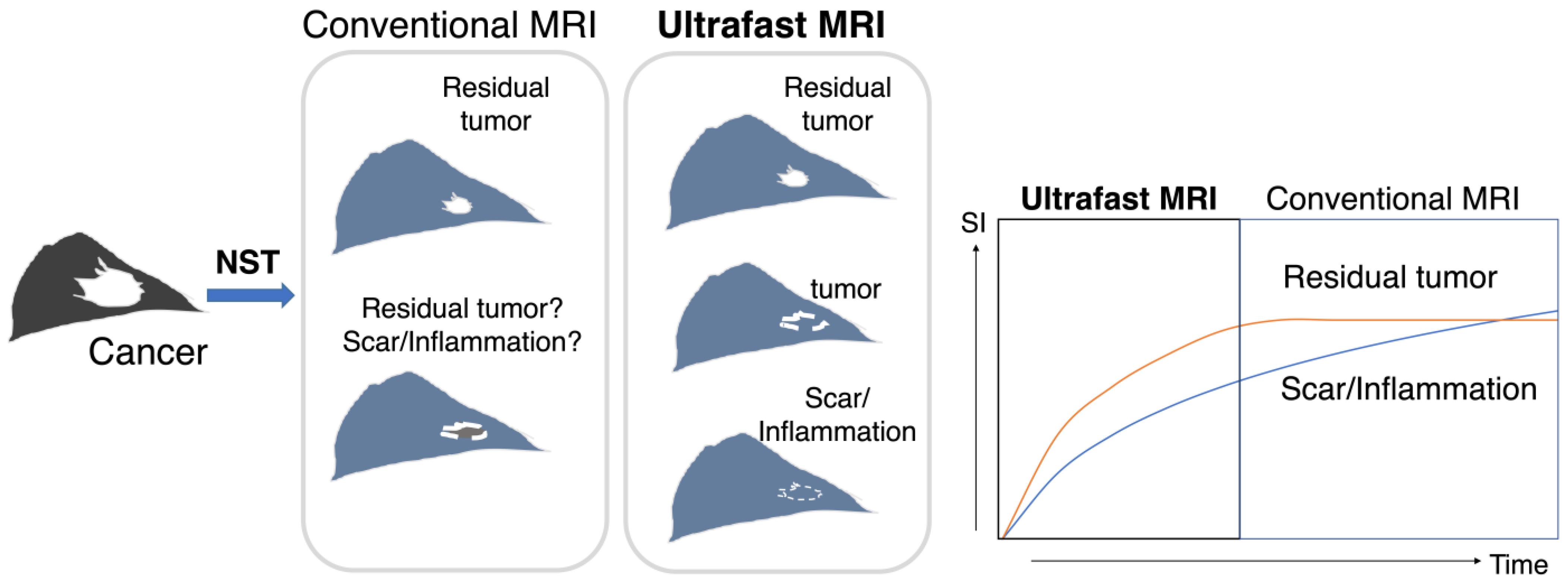
:1. Introduction
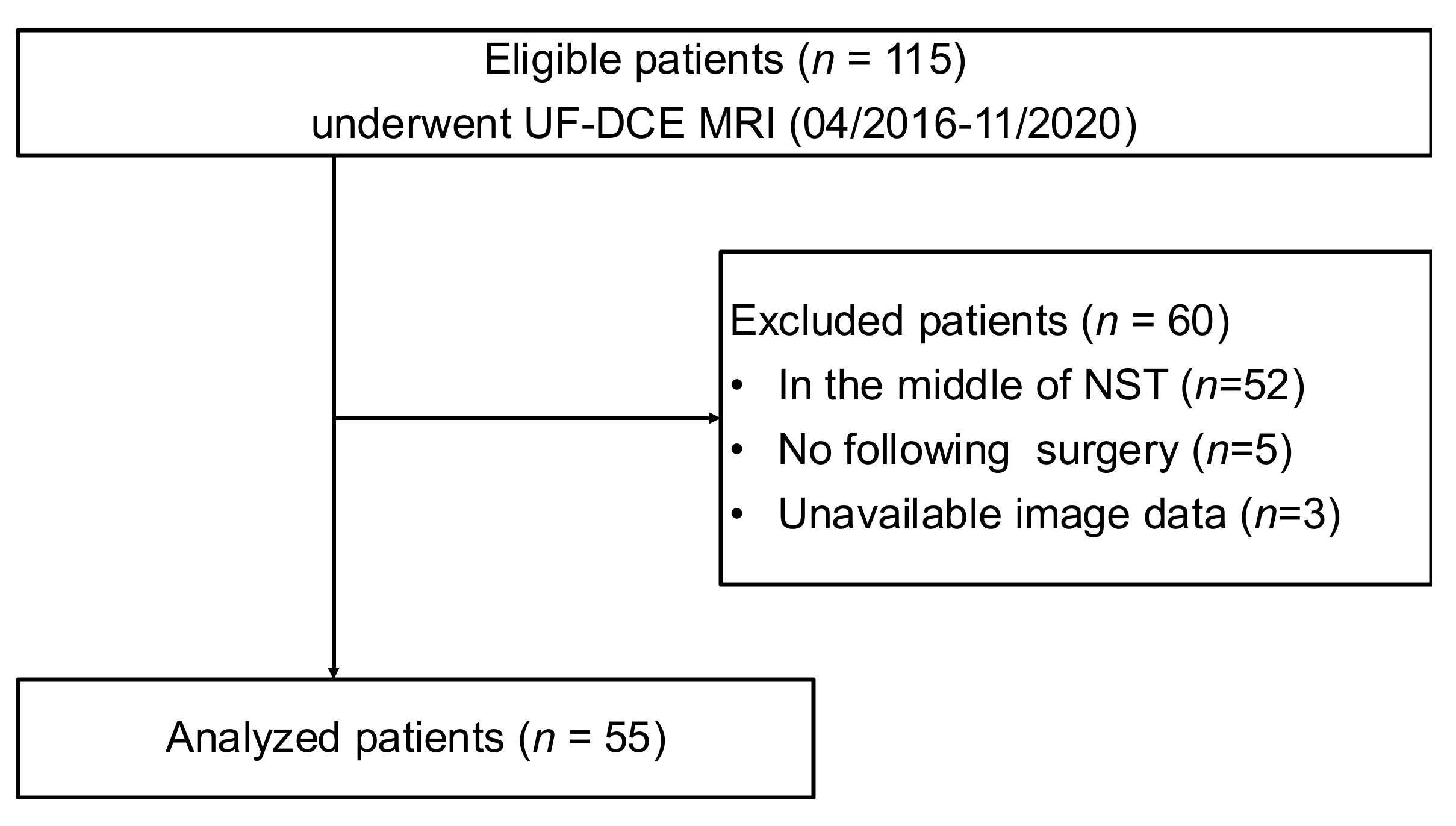
2. Materials and Methods
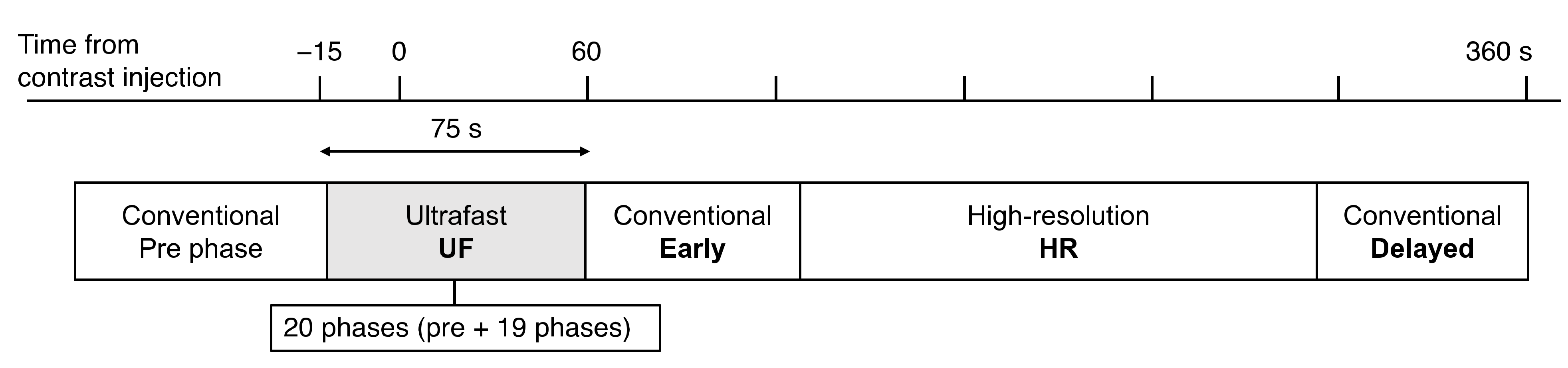
2.1. Patients and MRI Setting
2.2. Image Evaluation
2.3. Histopathological Assessment
2.4. Statistical Analysis
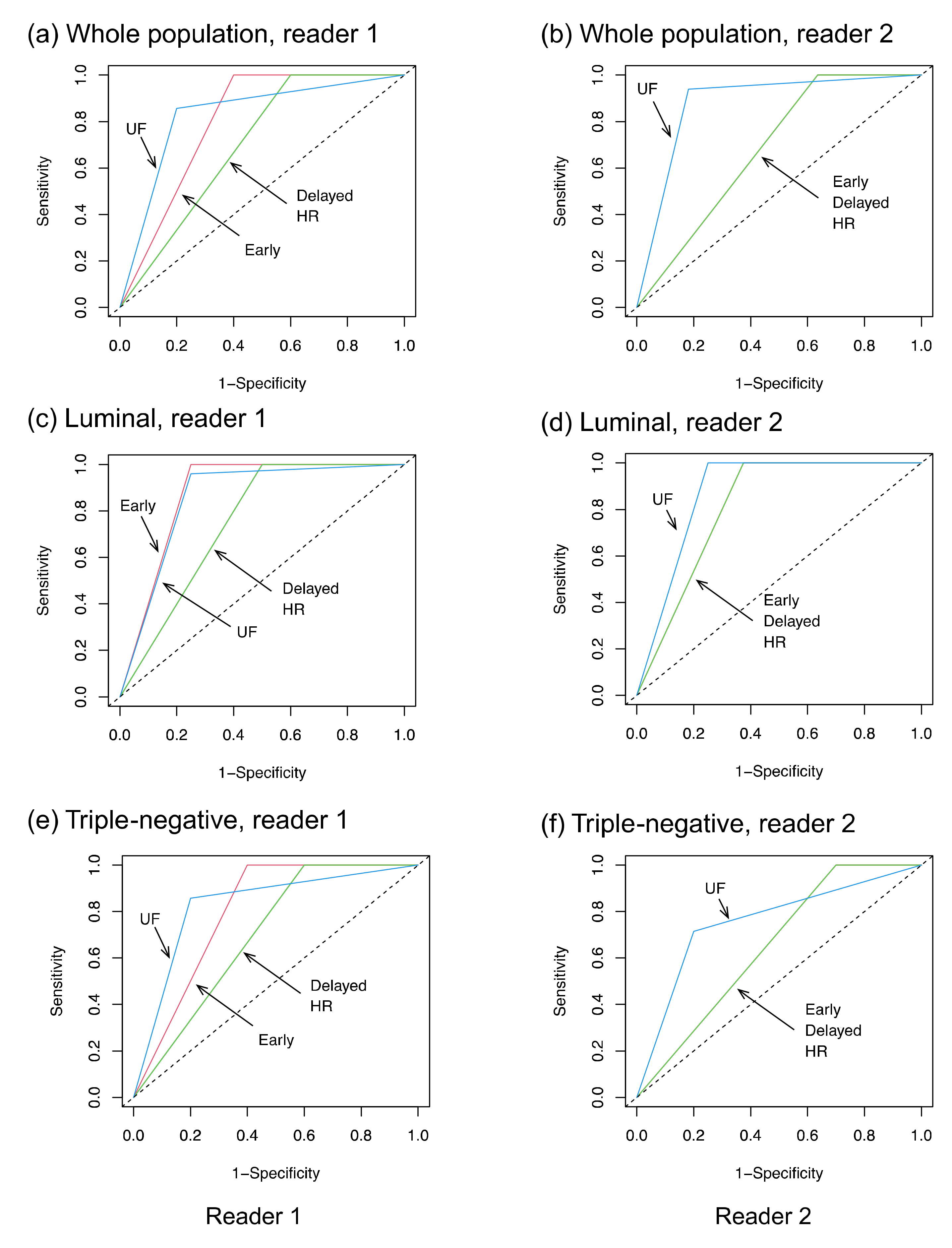
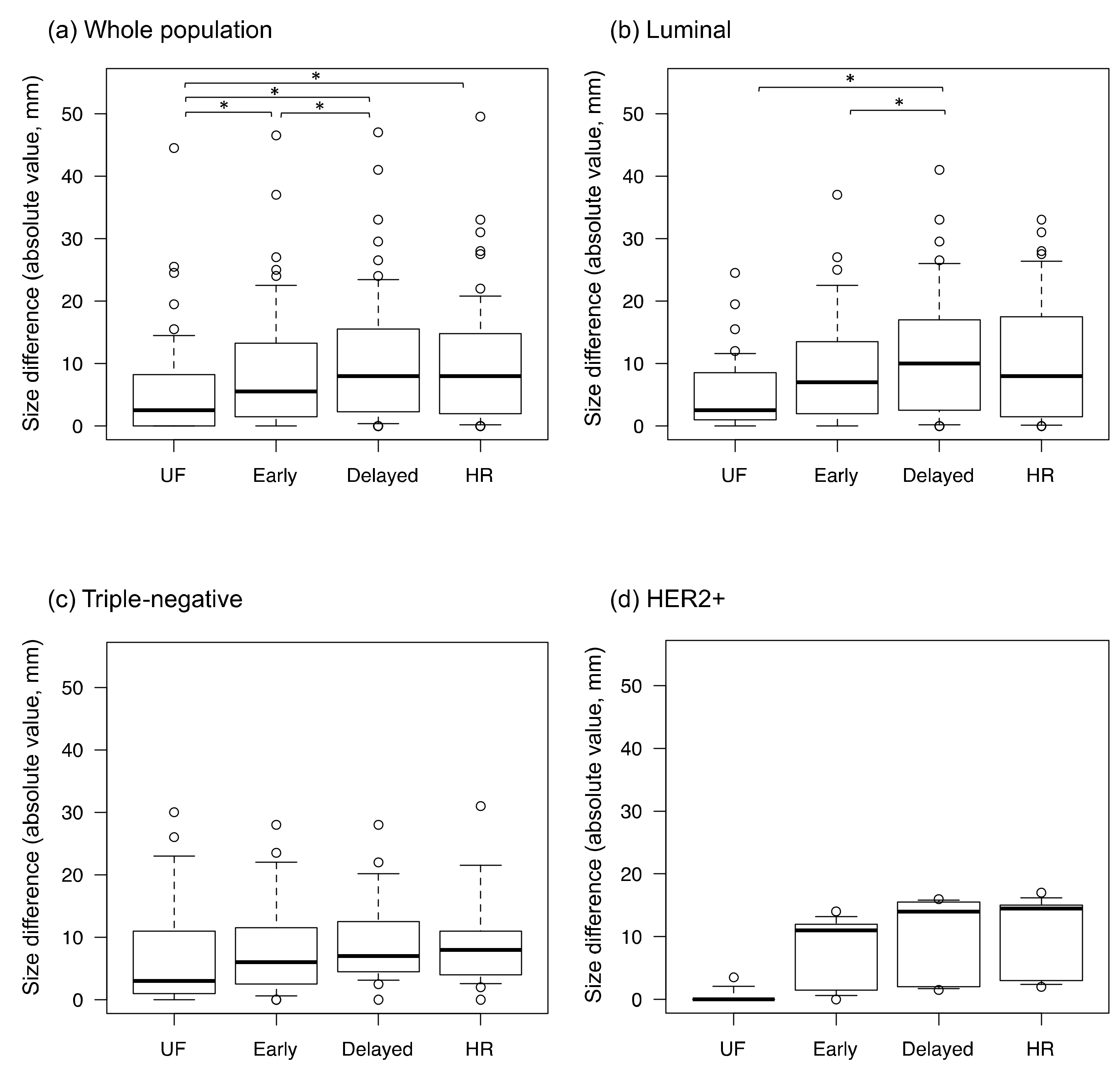
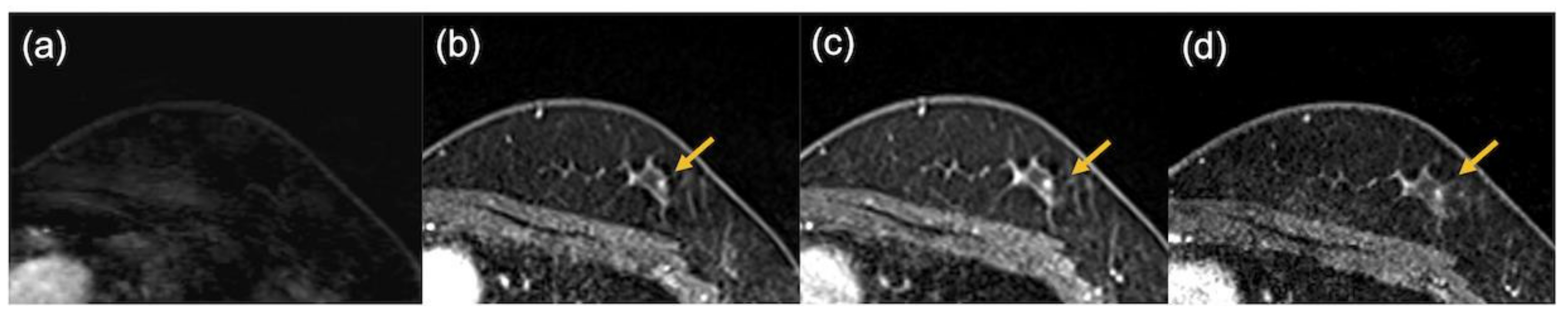
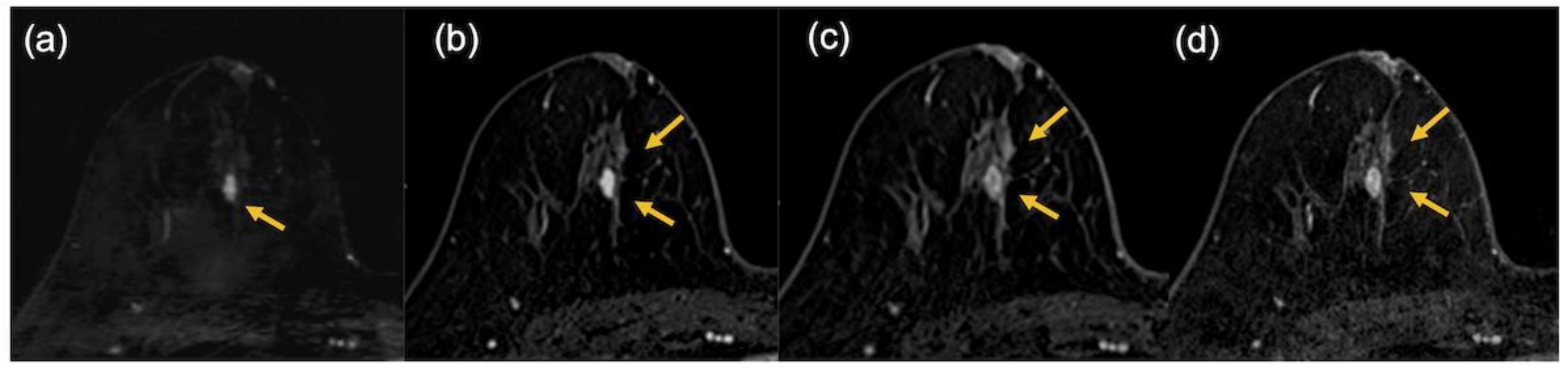
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asaoka, M.; Gandhi, S.; Ishikawa, T.; Takabe, K. Neoadjuvant Chemotherapy for Breast Cancer: Past, Present, and Future. Breast Cancer 2020, 14, 1178223420980377. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Van la Parra, R.F.D.; Kuerer, H.M. Selective elimination of breast cancer surgery in exceptional responders: Historical perspective and current trials. Breast Cancer Res. 2016, 18, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuerer, H.M.; Vrancken Peeters, M.-J.T.F.D.; Rea, D.W.; Basik, M.; De Los Santos, J.; Heil, J. Nonoperative Management for Invasive Breast Cancer After Neoadjuvant Systemic Therapy: Conceptual Basis and Fundamental International Feasibility Clinical Trials. Ann. Surg. Oncol. 2017, 24, 2855–2862. [Google Scholar] [CrossRef]
- Mauriac, L.; MacGrogan, G.; Avril, A.; Durand, M.; Floquet, A.; Debled, M.; Dilhuydy, J.M.; Bonichon, F.; Institut Bergonié Bordeaux Groupe Sein (IBBGS). Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: A unicentre randomized trial with a 124-month median follow-up. Ann. Oncol. 1999, 10, 47–52. [Google Scholar] [CrossRef]
- Scheel, J.R.; Kim, E.; Partridge, S.C.; Lehman, C.D.; Rosen, M.A.; Bernreuter, W.K.; Pisano, E.D.; Marques, H.S.; Morris, E.A.; Weatherall, P.T.; et al. MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 trial. Am. J. Roentgenol. 2018, 210, 1376–1385. [Google Scholar] [CrossRef]
- Heil, J.; Kuerer, H.M.; Pfob, A.; Rauch, G.; Sinn, H.P.; Golatta, M.; Liefers, G.J.; Peeters, M.J.V. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: Current evidence and future challenges. Ann. Oncol. 2020, 31, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.F.D.L.; Cantor, A.; Amos, K.D.; Forero, A.; Golshan, M.; Horton, J.K.; Hudis, C.A.; Hylton, N.M.; McGuire, K.; Meric-Bernstam, F.; et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer 2013, 119, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Sener, S.F.; Sargent, R.E.; Lee, C.; Manchandia, T.; Do, V.L.; Olimpiadi, Y.; Zaremba, N.; Alabd, A.; Nelson, M.; Lang, J.E. MRI does not predict pathologic complete response after neoadjuvant chemotherapy for breast cancer. J. Surg. Oncol. 2019, 120, 903–910. [Google Scholar] [CrossRef]
- Mann, R.M.; van Zelst, J.C.M.; Vreemann, S.; Mus, R.D.M. Is Ultrafast or Abbreviated Breast MRI Ready for Prime Time? Curr. Breast Cancer Rep. 2019, 11, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Pineda, F.D.; Medved, M.; Wang, S.; Fan, X.; Schacht, D.V.; Sennett, C.; Oto, A.; Newstead, G.M.; Abe, H.; Karczmar, G.S. Ultrafast Bilateral DCE-MRI of the Breast with Conventional Fourier Sampling: Preliminary Evaluation of Semi-quantitative Analysis. Acad. Radiol. 2016, 23, 1137–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, M.; Sakai, K.; Yokota, H.; Kiba, M.; Yoshida, M.; Imai, H.; Weiland, E.; Yokota, I.; Yamada, K. Diagnostic performance of initial enhancement analysis using ultra-fast dynamic contrast-enhanced MRI for breast lesions. Eur. Radiol. 2019, 29, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Nakazono, T.; Egashira, R.; Fukui, S.; Baba, K.; Hamamoto, T.; Irie, H. Maximum slope of ultrafast dynamic contrast-enhanced MRI of the breast: Comparisons with prognostic factors of breast cancer. Jpn J. Radiol. 2021, 39, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Onishi, N.; Sadinski, M.; Gibbs, P.; Gallagher, K.M.; Hughes, M.C.; Ko, E.S.; Dashevsky, B.Z.; Shanbhag, D.D.; Fung, M.M.; Hunt, T.M.; et al. Differentiation between subcentimeter carcinomas and benign lesions using kinetic parameters derived from ultrafast dynamic contrast-enhanced breast MRI. Eur. Radiol. 2020, 30, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Honda, M.; Ohashi, A.; Yamaguchi, K.; Mori, N.; Goto, M.; Fujioka, T.; Mori, M.; Kato, Y.; Satake, H.; et al. Ultrafast Dynamic Contrast-enhanced MRI of the Breast: How Is It Used? Magn. Reason. Med. Sci. 2022, 21, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, V.Y.; Shin, H.J.; Kim, M.J.; Yoon, J.H. Ultrafast dynamic contrast-enhanced breast MRI: Association with pathologic complete response in neoadjuvant treatment of breast cancer. Eur. Radiol. 2022, 1–11. [Google Scholar] [CrossRef]
- Kato, E.; Mori, N.; Mugikura, S.; Sato, S.; Ishida, T.; Takase, K. Value of ultrafast and standard dynamic contrast-enhanced magnetic resonance imaging in the evaluation of the presence and extension of residual disease after neoadjuvant chemotherapy in breast cancer. Jpn J. Radiol. 2021, 39, 791–801. [Google Scholar] [CrossRef]
- Fukuda, I. Pattern of Tumor shrinkage during neoadjuvant chemotherapy is associated with Prognosis in low-grade luminal early. Breast Cancer 2018, 286, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Goksuluk, D.; Korkmaz, S.; Zararsiz, G.A.; Karaagaoglu, E. easyROC: An Interactive Web-tool for ROC Curve Analysis Using R Language Environment. R J. 2016, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaría, G.; Bargalló, X.; Fernández, P.L.; Farrús, B.; Caparrós, X.; Velasco, M. Neoadjuvant Systemic Therapy in Breast Cancer: Association of Contrast-enhanced MR Imaging Findings, Diffusion-weighted Imaging Findings, and Tumor Subtype with Tumor Response. Radiology 2017, 283, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Lakhani, S.R.; Ring, A.E.; Ashley, S.; Walsh, G.; Smith, I.E. Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br. J. Cancer 2006, 94, 358–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, A.; Parker, S.; Ghersi, D.; Wilcken, N. Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst. Rev. 2013, CD000563. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-Y.; Cho, N.; Park, I.-A.; Kwon, B.R.; Shin, S.U.; Lee, S.H.; Chang, J.M.; Moon, W.K. Dynamic contrast-enhanced breast MRI for evaluating residual tumor size after neoadjuvant chemotherapy. Radiology 2018, 289, 327–334. [Google Scholar] [CrossRef]
- Ms, B.C.M.; Abdelhafez, A.H.; Adrada, B.E.; Candelaria, R.P.; Mohamed, R.M.; Boge, M.; Le-Petross, H.; Arribas, E.; Lane, D.L.; Spak, D.A.; et al. Functional Tumor Volume by Fast Dynamic Contrast-Enhanced MRI for Predicting Neoadjuvant Systemic Therapy Response in Triple-Negative Breast Cancer. J. Magn. Reason. Imaging 2021, 54, 251–260. [Google Scholar] [CrossRef]
- Gonzalez, V.; Sandelin, K.; Karlsson, A.; Åberg, W.; Löfgren, L.; Iliescu, G.; Eriksson, S.; Arver, B. Preoperative MRI of the breast (POMB) influences primary treatment in breast cancer: A prospective, randomized, multicenter study. World J. Surg. 2014, 38, 1685–1693. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Macaskill, P.; Irwig, L.; Sardanelli, F.; Mamounas, E.; Von Minckwitz, G.; Guarneri, V.; Partridge, S.C.; Wright, F.C.; Choi, J.H.; et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: Individual patient data meta-analysis. BMC Cancer 2015, 15, 662. [Google Scholar] [CrossRef] [Green Version]
- McGuire, K.P.; Toro-Burguete, J.; Dang, H.; Young, J.; Soran, A.; Zuley, M.; Bhargava, R.; Bonaventura, M.; Johnson, R.; Ahrendt, G. MRI staging after neoadjuvant chemotherapy for breast cancer: Does tumor biology affect accuracy? Ann. Surg. Oncol. 2011, 18, 3149–3154. [Google Scholar] [CrossRef]
- Apte, A.; Marsh, S.; Chandrasekharan, S.; Chakravorty, A. Avoiding breast cancer surgery in a select cohort of complete responders to neoadjuvant chemotherapy: The long-term outcomes. Ann. Med. Surg. 2021, 66, 102380. [Google Scholar] [CrossRef]
- Figueiredo, M.I.; Cullen, J.; Hwang, Y.-T.; Rowland, J.H.; Mandelblatt, J.S. Breast cancer treatment in older women: Does getting what you want improve your long-term body image and mental health? J. Clin. Oncol. 2004, 22, 4002–4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hylton, N.M. Residual Disease after Neoadjuvant Therapy for Breast Cancer: Can MRI Help? Radiology 2018, 289, 335–336. [Google Scholar] [CrossRef] [PubMed]

| UF | Early, Delayed | HR | |
|---|---|---|---|
| Orientation | Axial | Axial | Coronal |
| Sequence | VIBE without FS | VIBE with FS | VIBE with FS |
| TR, ms | 4.80 | 3.84 | 4.61 |
| TE, ms | 2.46 | 1.43 | 1.80 |
| FOV, mm2 | 360 × 360 | 330 × 330 | 330 × 330 |
| Matrix | 384 × 269 | 384 × 346 | 512 × 461 |
| Thickness, mm | 2.5 | 1 | 0.8 |
| Slices | 60 | 144 | 176 |
| Temporal resolution, s | 3.65 | 60 | 146 |
| Variables | n | Overall, n = 55 1 | Non-pCR, n = 22 1 | pCR, n = 33 1 | p-Value 2 |
|---|---|---|---|---|---|
| Age (years) | 55 | 49.7 ± 11.7 | 48.6 ± 9.7 | 50.5 ± 12.9 | 0.8 |
| Subtype | 55 | 0.007 | |||
| Luminal | 33 (60%) | 8 (36%) | 25 (76%) | ||
| HER2+ | 5 (9.1%) | 4 (18%) | 1 (3.0%) | ||
| TN | 17 (31%) | 10 (45%) | 7 (21%) | ||
| pre-NST size (mm) | 54 3 | 40.5 ± 23.7 | 28.9 ± 19.8 | 48.5 ± 23.0 | <0.001 |
| Morphology | 54 3 | 0.5 | |||
| mass | 43 (80%) | 19 (86%) | 24 (75%) | ||
| NME | 11 (20%) | 3 (14%) | 8 (25%) | ||
| Shrink pattern | 54 3 | 0.004 | |||
| CR | 5 (9.3%) | 5 (23%) | 0 (0%) | ||
| CS | 24 (44%) | 12 (55%) | 12 (38%) | ||
| non-CS | 17 (31%) | 4 (18%) | 13 (41%) | ||
| SD | 8 (15%) | 1 (4.5%) | 7 (22%) |
| Population | Protocol | Reader | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| All | UF | 1 | 0.86 (0.77–0.96) | 0.91 (0.76–0.98) | 0.82 (0.60–0.95) | 0.88 (0.71–0.98) | 0.86 (0.65–0.96) |
| 2 | 0.88 (0.79–0.97) | 0.94 (0.80–0.99) | 0.82 (0.60–0.95) | 0.89 (0.72–0.99) | 0.90 (0.70–0.97) | ||
| Early | 1 | 0.80 (0.69–0.90) | 1.00 (0.89–NA) | 0.59 (0.36–0.79) | 0.79 (0.59–NA) | 1.00 (0.77–1.00) | |
| 2 | 0.68 (0.58–0.78) | 1.00 (0.89–NA) | 0.36 (0.17–0.59) | 0.70 (0.46–NA) | 1.00 (0.67–1.00) | ||
| Delayed | 1 | 0.70 (0.60–0.81) | 1.00 (0.89–NA) | 0.49 (0.21–0.64) | 0.72 (0.49–NA) | 1.00 (0.70–1.00) | |
| 2 | 0.68 (0.58–0.78) | 1.00 (0.89–NA) | 0.36 (0.17–0.59) | 0.70 (0.46–NA) | 1.00 (0.67–1.00) | ||
| HR | 1 | 0.70 (0.60–0.81) | 1.00 (0.89–NA) | 0.41 (0.21–0.64) | 0.72 (0.49–NA) | 1.00 (0.70–1.00) | |
| 2 | 0.68 (0.58–0.78) | 1.00 (0.89–NA) | 0.36 (0.17–0.59) | 0.70 (0.46–NA) | 1.00 (0.67–1.00) | ||
| Luminal | UF | 1 | 0.86 (0.69–1.02) | 0.96 (0.80–1.00) | 0.75 (0.35–0.97) | 0.92 (0.68–1.00) | 0.86 (0.50–0.98) |
| 2 | 0.88 (0.71–1.03) | 1.00 (0.86-NA) | 0.75 (0.35–0.97) | 0.93 (0.69–NA) | 1.00 (0.60–NA) | ||
| Early | 1 | 0.88 (0.71–1.04) | 1.00 (0.86-NA) | 0.75 (0.35–0.97) | 0.93 (0.61–NA) | 1.00 (0.60–1.00) | |
| 2 | 0.81 (0.63–0.99) | 1.00 (0.86-NA) | 0.63 (0.25–0.92) | 0.89 (0.62–NA) | 1.00 (0.56–1.00) | ||
| Delayed | 1 | 0.75 (0.56–0.94) | 1.00 (0.86-NA) | 0.50 (0.16–0.84) | 0.86 (0.54–NA) | 1.00 (0.50–1.00) | |
| 2 | 0.81 (0.63–0.99) | 1.00 (0.86-NA) | 0.63 (0.25–0.92) | 0.89 (0.62–NA) | 1.00 (0.56–1.00) | ||
| HR | 1 | 0.75 (0.56–0.94) | 1.00 (0.86–NA) | 0.50 (0.16–0.84) | 0.86 (0.54–NA) | 1.00 (0.50–1.00) | |
| 2 | 0.81 (0.63–0.99) | 1.00 (0.86-NA) | 0.63 (0.25–0.92) | 0.89 (0.62–NA) | 1.00 (0.56–1.00) | ||
| TN | UF | 1 | 0.83 (0.64–1.02) | 0.86 (0.42–1.00) | 0.80 (0.44–0.98) | 0.75 (0.37–0.99) | 0.89 (0.49–0.99) |
| 2 | 0.76 (0.53–0.98) | 0.71 (0.29–0.96) | 0.80 (0.44–0.98) | 0.71 (0.33–0.96) | 0.80 (0.40–0.98) | ||
| Early | 1 | 0.80 (0.64–0.96) | 1.00 (0.59–NA) | 0.60 (0.26–0.88) | 0.63 (0.29–NA) | 1.00 (0.55–1.00) | |
| 2 | 0.65 (0.50–0.80) | 1.00 (0.59–NA) | 0.30 (0.07–0.65) | 0.50 (0.14–NA) | 1.00 (0.38–1.00) | ||
| Delayed | 1 | 0.82 (0.54–0.86) | 1.00 (0.59–NA) | 0.40 (0.12–0.74) | 0.54 (0.20–NA) | 1.00 (0.45–1.00) | |
| 2 | 0.65 (0.50–0.80) | 1.00 (0.59–NA) | 0.30 (0.07–0.65) | 0.50 (0.14–NA) | 1.00 (0.38–1.00) | ||
| HR | 1 | 0.82 (0.54–0.86) | 1.00 (0.59–NA) | 0.40 (0.12–0.74) | 0.54 (0.20–NA) | 1.00 (0.45–1.00) | |
| 2 | 0.65 (0.50–0.80) | 1.00 (0.59–NA) | 0.30 (0.07–0.65) | 0.50 (0.14–NA) | 1.00 (0.38–1.00) | ||
| HER2+ | UF | 1 | 0.50 | 1.00 (0.03–NA) | 0.00 (NA–0.60) | 0.20 (NA) | NA (0.00–1.00) |
| 2 | 1.00 | 1.00 (0.03–NA) | 1.00 (0.40–NA) | 1.00 (0.14–NA) | 1.00 (0.09–NA) | ||
| Early | 1 | 0.63 | 1.00 (0.03–NA) | 0.25 (0.01–0.81) | 0.25 (0.01–NA) | 1.00 (0.03–1.00) | |
| 2 | 0.63 | 1.00 (0.03–NA) | 0.25 (0.01–0.81) | 0.25 (0.01–NA) | 1.00 (0.03–1.00) | ||
| Delayed | 1 | 0.63 | 1.00 (0.03–NA) | 0.25 (0.01–0.81) | 0.25 (0.01–NA) | 1.00 (0.03–1.00) | |
| 2 | 0.50 | 1.00 (0.03–NA) | 0.00 (NA–0.60) | 0.20 (NA) | NA (0.00–1.00) | ||
| HR | 1 | 0.63 | 1.00 (0.03–NA) | 0.25 (0.01–0.81) | 0.25 (0.01–NA) | 1.00 (0.03–1.00) | |
| 2 | 0.50 | 1.00 (0.03–NA) | 0.00 (NA–0.60) | 0.20 (NA) | NA (0.00–1.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, M.; Kataoka, M.; Iima, M.; Ota, R.; Ohashi, A.; Kishimoto, A.O.; Miyake, K.K.; Nickel, M.D.; Yamada, Y.; Toi, M.; et al. Visual Evaluation of Ultrafast MRI in the Assessment of Residual Breast Cancer after Neoadjuvant Systemic Therapy: A Preliminary Study Association with Subtype. Tomography 2022, 8, 1522-1533. https://doi.org/10.3390/tomography8030125
Honda M, Kataoka M, Iima M, Ota R, Ohashi A, Kishimoto AO, Miyake KK, Nickel MD, Yamada Y, Toi M, et al. Visual Evaluation of Ultrafast MRI in the Assessment of Residual Breast Cancer after Neoadjuvant Systemic Therapy: A Preliminary Study Association with Subtype. Tomography. 2022; 8(3):1522-1533. https://doi.org/10.3390/tomography8030125
Chicago/Turabian StyleHonda, Maya, Masako Kataoka, Mami Iima, Rie Ota, Akane Ohashi, Ayami Ohno Kishimoto, Kanae Kawai Miyake, Marcel Dominik Nickel, Yosuke Yamada, Masakazu Toi, and et al. 2022. "Visual Evaluation of Ultrafast MRI in the Assessment of Residual Breast Cancer after Neoadjuvant Systemic Therapy: A Preliminary Study Association with Subtype" Tomography 8, no. 3: 1522-1533. https://doi.org/10.3390/tomography8030125
APA StyleHonda, M., Kataoka, M., Iima, M., Ota, R., Ohashi, A., Kishimoto, A. O., Miyake, K. K., Nickel, M. D., Yamada, Y., Toi, M., & Nakamoto, Y. (2022). Visual Evaluation of Ultrafast MRI in the Assessment of Residual Breast Cancer after Neoadjuvant Systemic Therapy: A Preliminary Study Association with Subtype. Tomography, 8(3), 1522-1533. https://doi.org/10.3390/tomography8030125







