Model-Based Iterative Reconstruction (MBIR) for ASPECT Scoring in Acute Stroke Patients Selection: Comparison to rCBV and Follow-Up Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subsection Participant Selection
2.2. Image Acquisition and Reconstruction
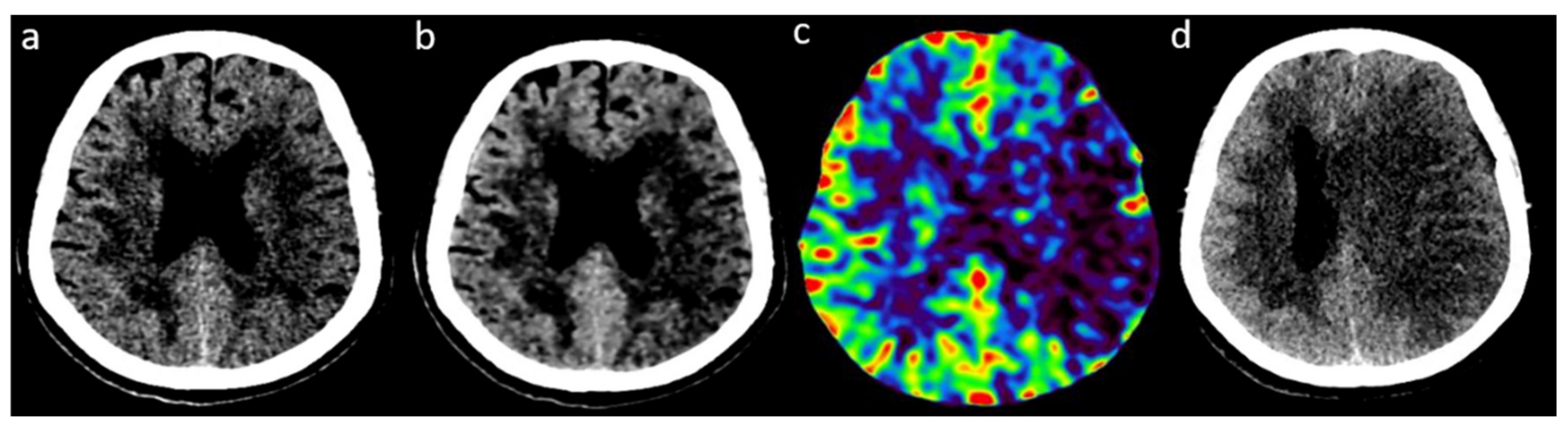
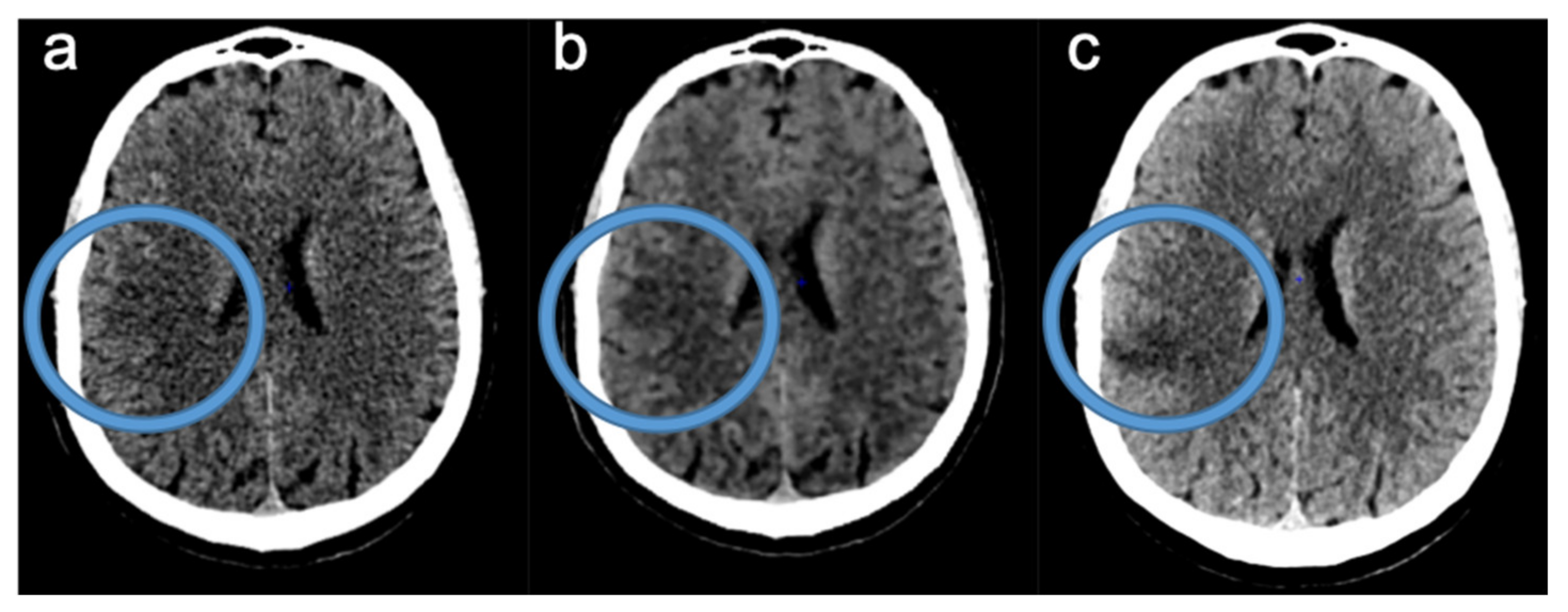
2.3. Readings of FIRST-LCD and AIDR3D ASPECTS on Baseline NCCT
2.4. Consensus Readings of Final and rCBV ASPECTS
2.5. Data Analysis
3. Results
3.1. Participants
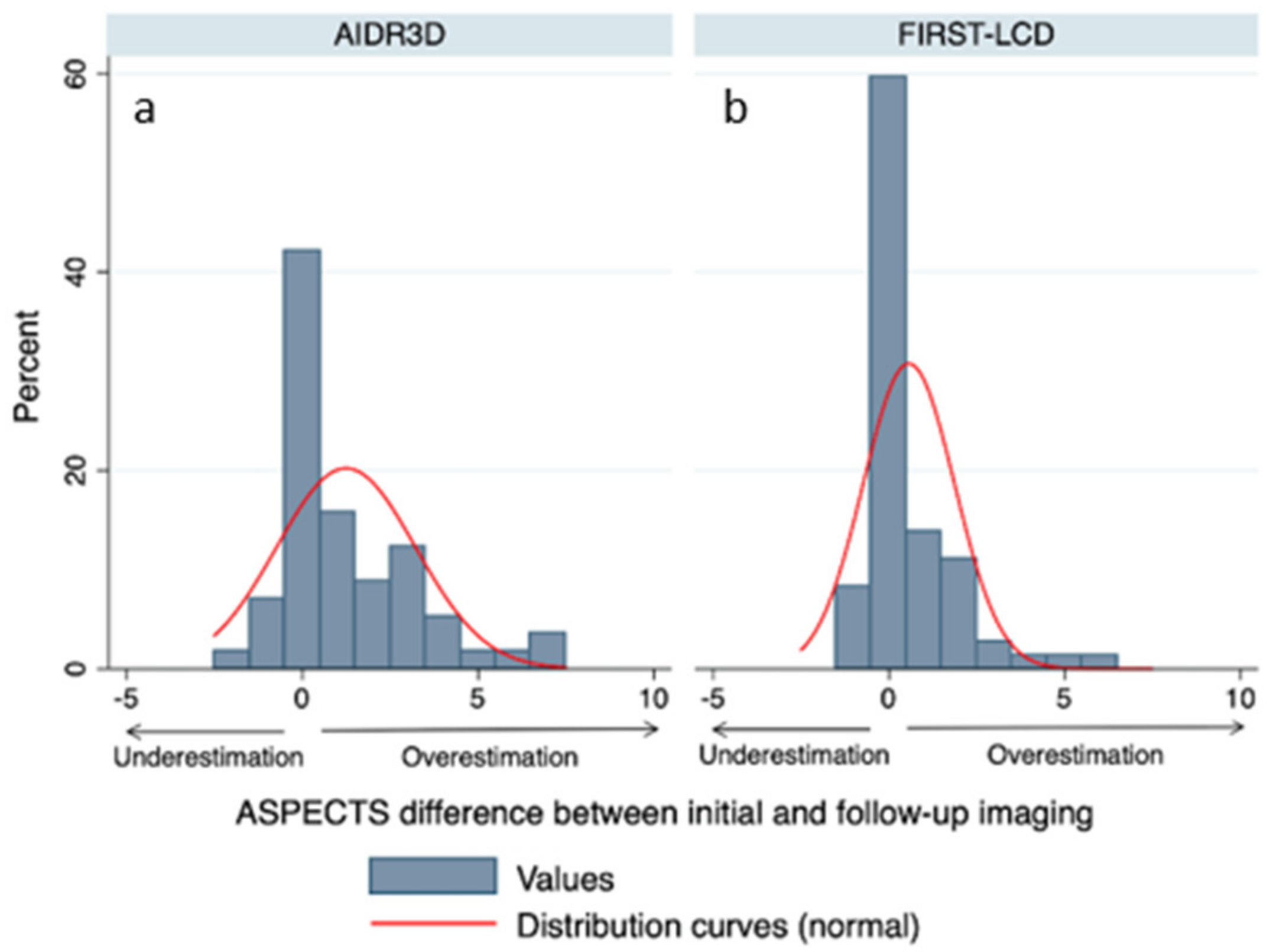
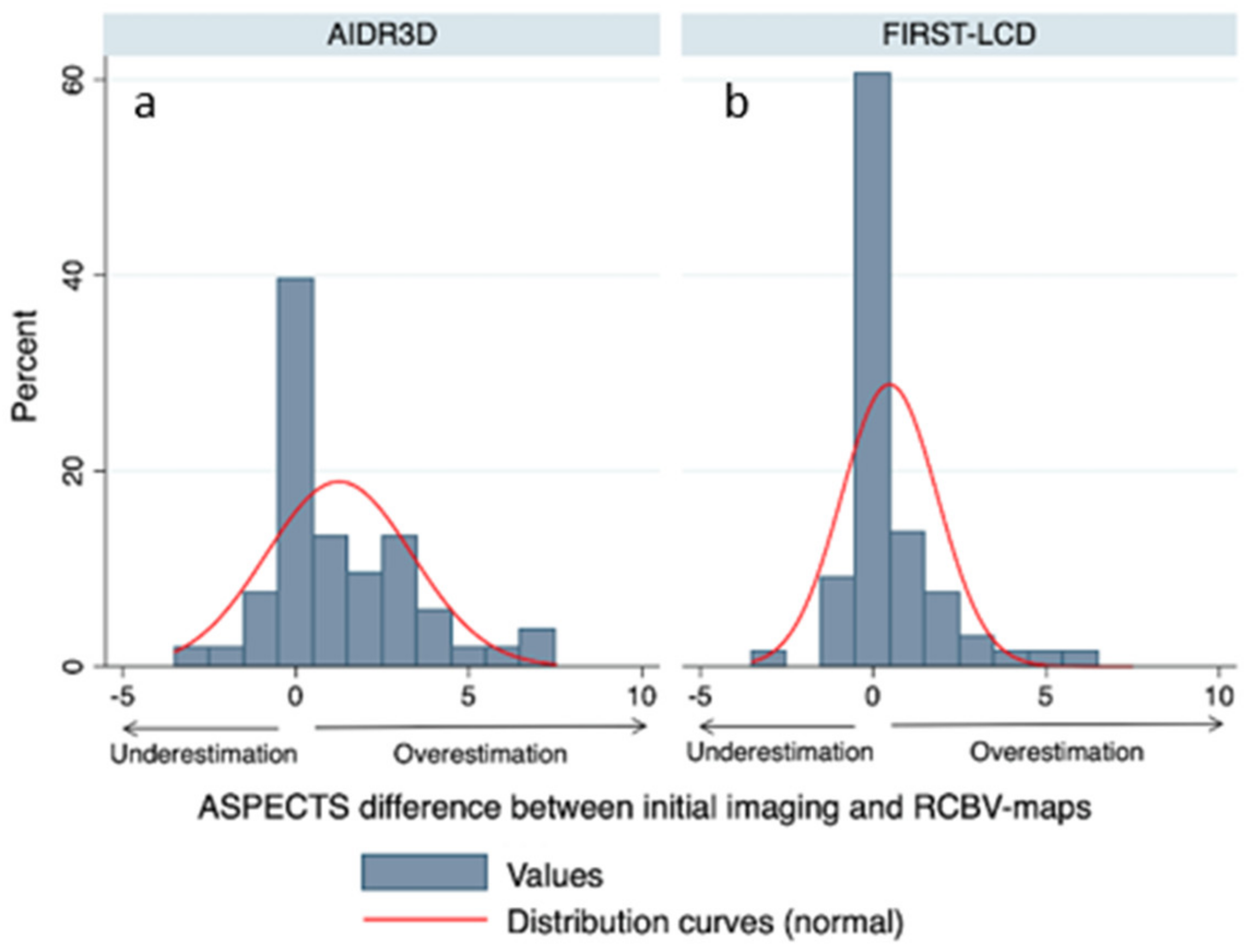
3.2. ASPECTS Distribution
3.3. Comparison of Final and rCBV ASPECTS with FIRST-LCD and AIDR3D ASPECTS
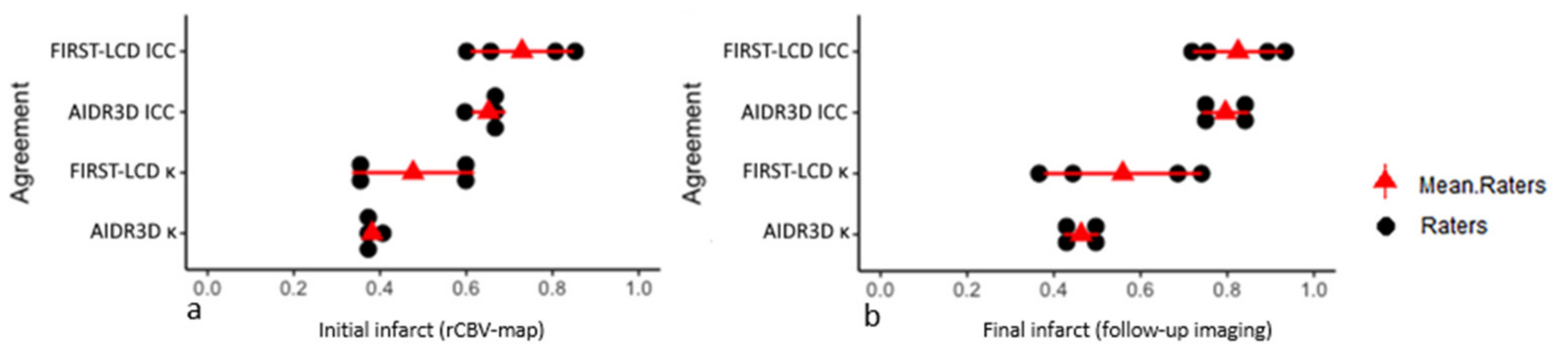
3.4. Agreements between FIRST-LCD and AIDR3D ASPECTS according to Final and rCBV ASPECTS: Readers Analysis
3.5. Inter-Reader Agreement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Sanelli, P.C.; Albers, G.W.; Bello, J.A.; Derdeyn, C.P.; Hetts, S.W.; Johnson, M.H.; Kidwell, C.S.; Lev, M.H.; Liebeskind, D.S.; et al. Imaging Recommendations for Acute Stroke and Transient Ischemic Attack Patients. AJNR 2013, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bier, G.; Bongers, M.N.; Ditt, H.; Bender, B.; Ernemann, U.; Horger, M. Accuracy of Non-Enhanced CT in Detecting Early Ischemic Edema Using Frequency Selective Non-Linear Blending. PLoS ONE 2016, 11, e0147378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Farzin, B.; Fahed, R.; Guilbert, F.; Poppe, A.Y.; Daneault, N.; Durocher, A.P.; Lanthier, S.; Boudjani, H.; Khoury, N.N.; Roy, D.; et al. Early CT changes in patients admitted for thrombectomy. Neurology 2016, 87, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemink, M.J.; de Jong, P.A.; Leiner, T.; de Heer, L.M.; Nievelstein, R.A.J.; Budde, R.P.J.; Schilham, A.M.R. Iterative reconstruction techniques for computed tomography Part 1: Technical principles. Eur. Radiol. 2013, 23, 1623–1631. [Google Scholar] [CrossRef]
- Willemink, M.J.; Leiner, T.; de Jong, P.A.; de Heer, L.M.; Nievelstein, R.A.J.; Schilham, A.M.R.; Budde, R.P.J. Iterative reconstruction techniques for computed tomography part 2: Initial results in dose reduction and image quality. Eur. Radiol. 2013, 23, 1632–1642. [Google Scholar] [CrossRef]
- Stiller, W. Basics of iterative reconstruction methods in computed tomography: A vendor-independent overview. Eur. J. Radiol. 2018, 109, 147–154. [Google Scholar] [CrossRef]
- Den Harder, A.M.; Willemink, M.J.; Budde, R.P.J.; Schilham, A.M.R.; Leiner, T.; de Jong, P.A. Hybrid and model-based iterative reconstruction techniques for pediatric CT. Am. J. Roentgenol. 2015, 204, 645–653. [Google Scholar] [CrossRef]
- Nishizawa, M.; Tanaka, H.; Watanabe, Y.; Kunitomi, Y.; Tsukabe, A.; Tomiyama, N. Model-based iterative reconstruction for detection of subtle hypoattenuation in early cerebral infarction: A phantom study. Jpn. J. Radiol. 2014, 33, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Sugisawa, K.; Minamishima, K.; Yamazaki, A.; Watanabe, T. Evaluation of Low-contrast Detectability of Iterative Reconstruction Algorithm in High-speed CT Technology for Head. Jpn. J. Radiol. Technol. 2019, 75, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitani, H.; Tatsugami, F.; Higaki, T.; Kaichi, Y.; Nakamura, Y.; Smit, E.; Prokop, M.; Ono, C.; Ono, K.; Korogi, Y.; et al. Accuracy of thin-slice model-based iterative reconstruction designed for brain CT to diagnose acute ischemic stroke in the middle cerebral artery territory: A multicenter study. Neuroradiology 2021, 63, 2013–2021. [Google Scholar] [CrossRef]
- Inoue, T.; Nakaura, T.; Yoshida, M.; Yokoyama, K.; Uetani, H.; Oda, S.; Utsunomiya, D.; Kitajima, M.; Harada, K.; Yamashita, Y. Brain computed tomography using iterative reconstruction to diagnose acute middle cerebral artery stroke: Usefulness in combination of narrow window setting and thin slice reconstruction. Neuroradiology 2018, 60, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ognard, J.; Dissaux, B.; Haioun, K.; Nonent, M.; Gentric, J.-C.; Ben Salem, D. A “one-stop-shop” 4D CTA protocol using 320-row CT for advanced imaging in acute ischemic stroke: A technical note. Eur. Radiol. 2019, 29, 4930–4936. [Google Scholar] [CrossRef]
- Sundaram, V.; Goldstein, J.; Wheelwright, D.; Aggarwal, A.; Pawha, P.; Doshi, A.; Fifi, J.; De Leacy, R.; Mocco, J.; Puig, J.; et al. Automated ASPECTS in Acute Ischemic Stroke: A Comparative Analysis with CT Perfusion. Am. J. Neuroradiol. 2019, 40, 2033–2038. [Google Scholar] [CrossRef]
- Iyama, Y.; Nakaura, T.; Oda, S.; Kidoh, M.; Utsunomiya, D.; Yoshida, M.; Yuki, H.; Hirata, K.; Funama, Y.; Harada, K.; et al. Iterative Reconstruction Designed for Brain CT. J. Comput. Assist. Tomogr. 2017, 41, 884–890. [Google Scholar] [CrossRef]
- Nicholson, P.; Hilditch, C.A.; Neuhaus, A.; Seyedsaadat, S.M.; Benson, J.C.; Mark, I.; Tsang, C.O.A.; Schaafsma, J.; Kallmes, D.F.; Krings, T.; et al. Per-region interobserver agreement of Alberta Stroke Program Early CT Scores (ASPECTS). J. NeuroInterv. Surg. 2020, 12, 1069–1071. [Google Scholar] [CrossRef]
- Maegerlein, C.; Fischer, J.; Mönch, S.; Berndt, M.; Wunderlich, S.; Seifert, C.L.; Lehm, M.; Boeckh-Behrens, T.; Zimmer, C.; Friedrich, B. Automated Calculation of the Alberta Stroke Program Early CT Score: Feasibility and Reliability. Radiology 2019, 291, 141–148. [Google Scholar] [CrossRef]
- Finlayson, O.; John, V.; Yeung, R.; Dowlatshahi, D.; Howard, P.; Zhang, L.; Swartz, R.; Aviv, R.I. Interobserver Agreement of ASPECT Score Distribution for Noncontrast CT, CT Angiography, and CT Perfusion in Acute Stroke. Stroke 2013, 44, 234–236. [Google Scholar] [CrossRef] [Green Version]
- Rapalino, O.; Kamalian, S.; Kamalian, S.; Payabvash, S.; Souza, L.; Zhang, D.; Mukta, J.; Sahani, D.; Lev, M.; Pomerantz, S. Cranial CT with Adaptive Statistical Iterative Reconstruction: Improved Image Quality with Concomitant Radiation Dose Reduction. Am. J. Neuroradiol. 2011, 33, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Q.; Dewan, S.K.; Li, M.; Li, J.; Mao, D.; Wang, Z.; Hua, Y. Comparison of adaptive statistical iterative and filtered back projection reconstruction techniques in brain CT. Eur. J. Radiol. 2012, 81, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Yu, L.; Kofler, J.M.; Leng, S.; Zhang, Y.; Li, Z.; Carter, R.E. Degradation of CT Low-Contrast Spatial Resolution Due to the Use of Iterative Reconstruction and Reduced Dose Levels. Radiology 2015, 276, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Schindera, S.T.; Odedra, D.; Raza, S.A.; Kim, T.K.; Jang, H.-J.; Szucs-Farkas, Z.; Rogalla, P. Iterative Reconstruction Algorithm for CT: Can Radiation Dose Be Decreased While Low-Contrast Detectability Is Preserved? Radiology 2013, 269, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nakaura, T.; Yoshida, M.; Yokoyama, K.; Hirata, K.; Kidoh, M.; Oda, S.; Utsunomiya, D.; Harada, K.; Yamashita, Y. Diagnosis of small posterior fossa stroke on brain CT: Effect of iterative reconstruction designed for brain CT on detection performance. Eur. Radiol. 2017, 27, 3710–3715. [Google Scholar] [CrossRef] [PubMed]
| AIDR3D | MBIR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kappa | 95%CI | Correlation | Kappa | 95%CI | Correlation | |||||
| NCCT or DWI Control | ASPECTS * | All | 0.59 | 0.52 | 0.65 | 0.74 | 0.81 | 0.78 | 0.83 | 0.92 |
| ASPECTS < 10 | 0.42 | 0.33 | 0.52 | 0.67 | 0.63 | 0.54 | 0.73 | 0.75 | ||
| SUBITEMS ** | Caudate | 0.49 | 0.28 | 0.71 | 0.55 | 0.65 | 0.46 | 0.83 | 0.66 | |
| Insula | 0.72 | 0.61 | 0.84 | 0.73 | 0.85 | 0.77 | 0.94 | 0.86 | ||
| Internal Capsule | 0.36 | 0.07 | 0.66 | 0.47 | 0.34 | 0.05 | 0.63 | 0.40 | ||
| Lenticular | 0.60 | 0.43 | 0.76 | 0.63 | 0.79 | 0.67 | 0.91 | 0.79 | ||
| M1 | 0.68 | 0.51 | 0.85 | 0.68 | 0.76 | 0.62 | 0.91 | 0.77 | ||
| M2 | 0.64 | 0.48 | 0.79 | 0.65 | 0.82 | 0.70 | 0.93 | 0.82 | ||
| M3 | 0.43 | 0.15 | 0.72 | 0.53 | 0.68 | 0.46 | 0.90 | 0.70 | ||
| M4 | 0.58 | 0.37 | 0.79 | 0.60 | 0.71 | 0.53 | 0.89 | 0.72 | ||
| M5 | 0.51 | 0.37 | 0.64 | 0.54 | 0.85 | 0.76 | 0.93 | 0.85 | ||
| M6 | 0.31 | 0.06 | 0.56 | 0.33 | 0.69 | 0.51 | 0.87 | 0.69 | ||
| rCBV Acute CTP | ASPECTS * | All | 0.55 | 0.53 | 0.57 | 0.68 | 0.72 | 0.67 | 0.79 | 0.85 |
| ASPECTS < 10 | 0.34 | 0.30 | 0.42 | 0.51 | 0.51 | 0.48 | 0.63 | 0.57 | ||
| SUBITEMS ** | Caudate | 0.50 | 0.25 | 0.76 | 0.52 | 0.55 | 0.32 | 0.78 | 0.55 | |
| Insula | 0.52 | 0.37 | 0.68 | 0.53 | 0.62 | 0.48 | 0.76 | 0.63 | ||
| Internal Capsule | 0.49 | 0.07 | 0.92 | 0.51 | 0.66 | 0.30 | 1,00 | 0.67 | ||
| Lenticular | 0.55 | 0.35 | 0.75 | 0.55 | 0.65 | 0.49 | 0.82 | 0.66 | ||
| M1 | 0.62 | 0.45 | 0.80 | 0.63 | 0.76 | 0.62 | 0.89 | 0.76 | ||
| M2 | 0.46 | 0.27 | 0.65 | 0.46 | 0.65 | 0.49 | 0.81 | 0.65 | ||
| M3 | 0.36 | 0.06 | 0.66 | 0.42 | 0.66 | 0.42 | 0.89 | 0.66 | ||
| M4 | 0.49 | 0.27 | 0.71 | 0.52 | 0.44 | 0.22 | 0.65 | 0.45 | ||
| M5 | 0.50 | 0.36 | 0.64 | 0.52 | 0.81 | 0.72 | 0.90 | 0.81 | ||
| M6 | 0.31 | 0.03 | 0.60 | 0.31 | 0.55 | 0.33 | 0.78 | 0.58 | ||
| Agreement Parameter | Items | AIDR3D | FIRST-LCD |
|---|---|---|---|
| Kappa * | All | 0.31 | 0.26 |
| ICC | All | 0.93 | 0.92 |
| Kappa * | Caudate | 0.38 | 0.25 |
| Insula | 0.63 | 0.69 | |
| Internal Capsule | 0.44 | 0.31 | |
| Lenticular | 0.53 | 0.38 | |
| M1 | 0.62 | 0.57 | |
| M2 | 0.53 | 0.58 | |
| M3 | 0.40 | 0.48 | |
| M4 | 0.60 | 0.65 | |
| M5 | 0.53 | 0.40 | |
| M6 | 0.47 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dissaux, B.; Cheddad El Aouni, M.; Ognard, J.; Gentric, J.-C. Model-Based Iterative Reconstruction (MBIR) for ASPECT Scoring in Acute Stroke Patients Selection: Comparison to rCBV and Follow-Up Imaging. Tomography 2022, 8, 1260-1269. https://doi.org/10.3390/tomography8030104
Dissaux B, Cheddad El Aouni M, Ognard J, Gentric J-C. Model-Based Iterative Reconstruction (MBIR) for ASPECT Scoring in Acute Stroke Patients Selection: Comparison to rCBV and Follow-Up Imaging. Tomography. 2022; 8(3):1260-1269. https://doi.org/10.3390/tomography8030104
Chicago/Turabian StyleDissaux, Brieg, Mourad Cheddad El Aouni, Julien Ognard, and Jean-Christophe Gentric. 2022. "Model-Based Iterative Reconstruction (MBIR) for ASPECT Scoring in Acute Stroke Patients Selection: Comparison to rCBV and Follow-Up Imaging" Tomography 8, no. 3: 1260-1269. https://doi.org/10.3390/tomography8030104
APA StyleDissaux, B., Cheddad El Aouni, M., Ognard, J., & Gentric, J.-C. (2022). Model-Based Iterative Reconstruction (MBIR) for ASPECT Scoring in Acute Stroke Patients Selection: Comparison to rCBV and Follow-Up Imaging. Tomography, 8(3), 1260-1269. https://doi.org/10.3390/tomography8030104







