Diagnostic Accuracy of Chest Digital Tomosynthesis in Patients Recovering after COVID-19 Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Chest X-ray
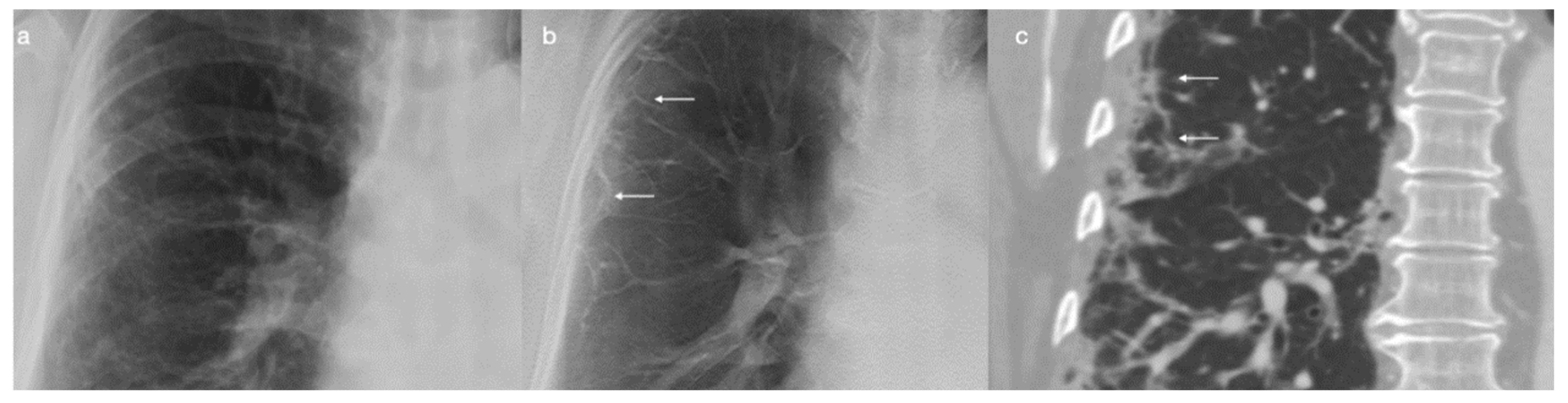
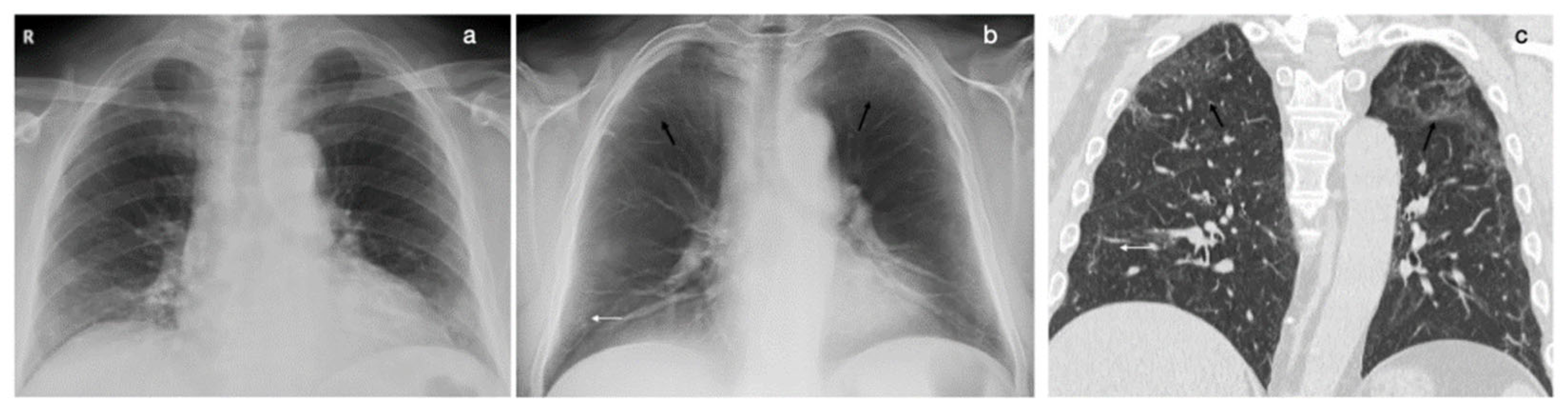
2.2. DTS
2.3. CT Acquisition Protocol
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- McAdams, H.P.; Samei, E.; Dobbins, J., 3rd; Tourassi, G.D.; Ravin, C.E. Recent advances in chest radiography. Radiology 2006, 241, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Crivelli, P.; Marrocchio, C.; Bozzato, A.M.; Vito, A.; Madeddu, G.; Saderi, L.; Confalonieri, M.; Tenaglia, L.; Cova, M.A. Severity of lung involvement on chest X-rays in SARS-coronavirus-2 infected patients as a possible tool to pre-dict clinical progression: An observational retrospective analysis of the relationship between radiological, clinical, and labora-tory data. J. Bras. De Pneumol. Publicacao. Da Soc. Bras. De Pneumol. E Tisilogia 2020, 46, e20200226. [Google Scholar]
- Heitzman, E.R. Thoracic Radiology: The Past 50 Years. Radiology 2000, 214, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E.; et al. Radiological–pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 2139. [Google Scholar] [CrossRef]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.; Martin, I.B.K.; et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pan-demic: A Multinational Consensus Statement From the Fleischner Society. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef]
- Baratella, E.; Ruaro, B.; Marrocchio, C.; Starvaggi, N.; Salton, F.; Giudici, F.; Quaia, E.; Confalonieri, M.; Cova, M.A. Inter-Stitial Lung Disease at High Resolution CT after SARS-CoV-2-Related Acute Respiratory Distress Syndrome According to Pulmonary Segmental Anatomy. J. Clin. Med. 2021, 10, 3985. [Google Scholar] [CrossRef]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Godeau, D.; Petit, A.; Richard, I.; Roquelaure, Y.; Descatha, A. Return-to-work, disabilities and occupational health in the age of COVID-19. Scand. J. Work. Environ. Health 2021, 47, 408–409. [Google Scholar] [CrossRef]
- Calvo, I.; SantaCruz-Calvo, S.; Aranzana, M.G.; Mármol, P.; Luque, J.; Peral, I.; Quijada, E.M.; Gómez, C.; Borrego, C.; Marín, J. Digital Tomosynthesis and COVID-19: An improvement in the assessment of pulmonary opacities. Arch Bronconeumol. 2020, 56, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Baratella, E.; Poillucci, G.; Gennari, A.G.; Cova, M.A. Diagnostic impact of digital tomosynthesis in oncologic patients with suspected pulmonary lesions on chest radiography. Eur. Radiol. 2015, 26, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Kruamak, T.; Edwards, R.; Cheng, S.; Hippe, D.S.; Raghu, G.; Pipavath, S.N.J. Accuracy of Digital Tomosynthesis of the Chest in De-tection of Interstitial Lung Disease Comparison With Digital Chest Radiography. J. Comput. Assist. Tomogr. 2019, 43, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chou SH, S.; Kicska, G.A.; Pipavath, S.N.; Reddy, G.P. Digital Tomosyn-thesis of the Chest: Current and Emerging Applications. Radiographics 2014, 34, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Baratella, E.; Cernic, S.; Lorusso, A.; Casagrande, F.; Cioffi, V.; Cova, M.A. Analysis of the impact of digital tomosynthesis on the radiological investigation of pa-tients with suspected pulmonary lesions on chest radiography. Eur. Radiol. 2012, 22, 1912–1922. [Google Scholar] [CrossRef]
- Dobbins, J.T., 3rd; Godfrey, D.J. Overview of two years of clinical experience of chest tomosynthesis at Sahlgren-ska University Hospital. Radiat. Prot. Dosim. 2010, 139, 124–129. [Google Scholar]
- Dobbins, J.T., 3rd; Godfrey, D.J. Digital x-ray tomosynthesis: Current state of the art and clinical potential. Phys. Med. Biol. 2003, 48, R65–R106. [Google Scholar] [CrossRef]
- Dobbins, J.T., 3rd; McAdams, H.P.; Godfrey, D.J.; Li, C.M. Digital tomosynthesis of the chest. J. Thorac. Imaging 2008, 23, 86–92. [Google Scholar] [CrossRef]
- Yamada, Y.; Jinzaki, M.; Hasegawa, I.; Shiomi, E.; Sugiura, H.; Abe, T.; Sato, Y.; Kuribayashi, S.; Ogawa, K. Fast scanning tomosynthesis for the detection of pulmonary nodules: Diagnostic performance compared with chest radiography, using multidetector-row computed tomography as the reference. Investig. Radiol. 2011, 46, 471–477. [Google Scholar] [CrossRef]
- Vult von Steyern, K.; Björkman-Burtscher, I.; Geijer, M. Tomosynthesis in pulmonary cystic fibrosis with comparison to radiog-raphy and computed tomography: A pictorial review. Insights Imaging 2012, 3, 81–89. [Google Scholar] [CrossRef]
- Gomi, T.; Nakajima, M.; Fujiwara, H.; Takeda, T.; Saito, K.; Umeda, T.; Sakaguchi, K. Comparison between chest digital tomosynthesis and CT as a screening method to detect artificial pulmonary nodules: A phantom study. Br. J. Radiol. 2012, 85, e622–e629. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, C.; Fagman, E.; Vikgren, J.; Molnar, D.; Borna, E.; Beni, M.M.; Brandberg, J.; Bergman, B.; Båth, M.; Johnsson, A. Surveillance of small, solid pulmonary nodules at digital chest tomosynthesis: Data from a cohort of the pilot Swedish CArdioPulmonary bioImage Study (SCAPIS). Acta Radiol. 2020, 62, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Grisi, G.; Baratella, E.; Cuttin, R.; Poillucci, G.; Kus, S.; Cova, M.A. Diagnostic imaging costs before and after digital tomosynthesis implementation in patient management after detection of suspected thoracic lesions on chest radiog-raphy. Insights Imaging 2014, 5, 147–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martini, K.; Larici, A.R.; Revel, M.P.; Ghaye, B.; Sverzellati, N.; Parkar, A.P.; Snoeckx, A.; Screaton, N.; Biederer, J.; Prosch, H.; et al. COVID-19 pneumonia imaging follow-up: When and how? A proposition from ESTI and ESR. Eur. Radiol. 2021, 32, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef]
| CXR | GGO | Consolidations | Reticulations | Linear Consolidations | ||||
|---|---|---|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| Sensitivity (%) | 1/11 (9) | 2/11 (18) | 4/8 (50) | 3/8 (37) | 4/7 (57) | 4/7 (57) | 6/20 (30) | 7/20 (35) |
| Specificity (%) | 14/14(100) | 13/14 (93) | 16/17 (94) | 13/17(76) | 18/18(100) | 14/18 (78) | 5/5 (100) | 5/5 (100) |
| PPV (%) | 1/1 (100) | 2/3 (67) | 4/5 (80) | 3/7 (43) | 4/4 (100) | 4/8 (50) | 6/6 (100) | 7/7 (100) |
| NPV (%) | 14/24 (58) | 13/22 (59) | 16/20 (80) | 13/18(72) | 18/21 (86) | 14/17 (82) | 5/19 (26) | 5/18 (28) |
| Accuracy (%) | 15/25 (60) | 15/25 (60) | 20/25 (80) | 16/25 (64) | 22/25 (88) | 18/25 (72) | 11/25 (44) | 12/25 (48) |
| DTS | GGO | Consolidations | Reticulations | Linear Consolidations | ||||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| Sensitivity (%) | 5/11 (45) | 4/11 (36) | 6/8 (75) | 7/8 (87) | 7/7 (100) | 7/7 (100) | 19/20 (95) | 17/20 (85) |
| Specificity (%) | 12/14 (86) | 11/14 (79) | 17/17(100) | 16/17 (94) | 17/18 (94) | 16/18 (89) | 5/5 (100) | 5/5 (100) |
| PPV (%) | 5/7 (71) | 4/7 (57) | 6/6 (100) | 7/8 (87) | 7/8 (87) | 7/9 (78) | 19/19 (100) | 17/17 (100) |
| NPV (%) | 12/18 (67) | 7/18 (61) | 17/19 (89) | 16/17 (94) | 17/17 (100) | 16/16 (100) | 5/6 (96) | 5/8 (62) |
| Accuracy (%) | 17/25 (68) | 15/25 (60) | 23/25 (92) | 23/25 (92) | 24/25 (96) | 23/25 (92) | 24/25 (96) | 22/25 (88) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratella, E.; Ruaro, B.; Marrocchio, C.; Poillucci, G.; Pigato, C.; Bozzato, A.M.; Salton, F.; Confalonieri, P.; Crimi, F.; Wade, B.; et al. Diagnostic Accuracy of Chest Digital Tomosynthesis in Patients Recovering after COVID-19 Pneumonia. Tomography 2022, 8, 1221-1227. https://doi.org/10.3390/tomography8030100
Baratella E, Ruaro B, Marrocchio C, Poillucci G, Pigato C, Bozzato AM, Salton F, Confalonieri P, Crimi F, Wade B, et al. Diagnostic Accuracy of Chest Digital Tomosynthesis in Patients Recovering after COVID-19 Pneumonia. Tomography. 2022; 8(3):1221-1227. https://doi.org/10.3390/tomography8030100
Chicago/Turabian StyleBaratella, Elisa, Barbara Ruaro, Cristina Marrocchio, Gabriele Poillucci, Caterina Pigato, Alessandro Marco Bozzato, Francesco Salton, Paola Confalonieri, Filippo Crimi, Barbara Wade, and et al. 2022. "Diagnostic Accuracy of Chest Digital Tomosynthesis in Patients Recovering after COVID-19 Pneumonia" Tomography 8, no. 3: 1221-1227. https://doi.org/10.3390/tomography8030100
APA StyleBaratella, E., Ruaro, B., Marrocchio, C., Poillucci, G., Pigato, C., Bozzato, A. M., Salton, F., Confalonieri, P., Crimi, F., Wade, B., Quaia, E., & Cova, M. A. (2022). Diagnostic Accuracy of Chest Digital Tomosynthesis in Patients Recovering after COVID-19 Pneumonia. Tomography, 8(3), 1221-1227. https://doi.org/10.3390/tomography8030100









