Abstract
Many pathological conditions lead to altered intracellular pH (pHi) disrupting normal cellular functions. The chemical exchange saturation transfer (CEST) method, known as Amine and Amide Concentration Independent Detection (AACID), can produce image contrast that is predominantly dependent on tissue intracellular pHi. The AACID value is linearly related to the ratio of the 3.5 ppm amide CEST effect and the 2.75 ppm amine CEST effect in the physiological range. However, the amine CEST effect at 2 ppm is often more clearly defined in vivo, and may provide greater sensitivity to pH changes. The purpose of the current study was to compare AACID measurement precision utilizing the 2.0 and 2.75 ppm amine CEST effects. We hypothesized that the 2.0 ppm amine CEST resonance would produce measurements with greater sensitivity to pH changes. In the current study, we compare the range of the AACID values obtained in 24 mice with brain tumors and in normal tissue using the 2 ppm and 2.75 ppm amine resonances. All CEST data were acquired on a 9.4T MRI scanner. The AACID measurement range increased by 39% when using the 2 ppm amine resonance compared to the 2.75 ppm resonance, with decreased measurement variability across the brain. These data indicate that in vivo pH measurements made using AACID CEST can be enhanced by incorporating the 2 ppm amine resonance. This approach should be considered for pH measurements made over short intervals when no changes are expected in the concentration of metabolites that contribute to the 2 ppm amine resonance.
1. Introduction
Intracellular pH plays an important role in many physiological processes, including apoptosis, cell proliferation, and protein interactions, and it is altered in several disease states. In cancer, altered intracellular and extracellular pH gradients can lead to drug resistance [1,2]. Chemical exchange saturation transfer (CEST) can produce image contrast that is dependent on tissue pH [3,4,5,6], and can be used to non-invasively study cellular pH under various conditions. More specifically, amide proton transfer (APT) efficiency varies with pH, providing sensitivity to this physiological parameter. Although the measurement of the APT CEST effect depends on several factors including the amide proton concentration, water concentration, and the relaxivity (R1) of bulk water [6], this contrast has been successfully used to identify ischemic tissue following acute stroke [7,8,9,10,11,12] and to study cancer [13,14,15,16,17].
In previous work [18] using 9.4T MRI, we have demonstrated that the ratio of the 3.5 ppm amide CEST effect to the 2.75 ppm amine CEST effect varies linearly with pH in the physiological range, and is largely independent of protein concentration and temperature [18]. This ratiometric CEST method called Amine and Amide Concentration Independent Detection (AACID) [18] is sensitive to the acidification of the brain following stroke, and to the increased intracellular pH in brain tumors [19,20]. The method has also been used to measure the magnitude of acute tumor acidification using the pharmacologic agents lonidamine, topiramate, dichloroacetate, cariporide, and quercetin [19,20,21,22,23].
However, due to the fast exchange rate of amine protons in vivo, the 2.75 amine CEST effect provides only a small response to changes in pH when using the low amplitude, long duration saturation schemes typically used for amide proton detection. In contrast, the 2.0 ppm amine peak is better defined and produces a greater in vivo pH response [24,25]. The CEST effect at 2.0 ppm is largely associated with creatine [24,25] and guanidinium [26], and has been shown to correlate with the creatine concentration measured by 1H-MRS [25]. A recent study demonstrated that reduced creatine CEST measured at 2 ppm could help differentiate aggressive from non-aggressive gliomas [26]. Given that the 2.0 ppm amine peak is more easily detected in vivo, the use of this peak could increase the sensitivity of short-interval in vivo AACID ratiometric measurements compared to the use of the 2.75 ppm amine peak, particularly for the rapid measurement of pH changes after administration of pharmacologic agents, where short term changes in creatine and guanidinium are not expected.
The purpose of the current study was to investigate whether AACID CEST measurements incorporating the 2 ppm amine resonance would have greater sensitivity compared to measurements made using the 2.75 ppm resonance in brain tumors and contralateral tissue. Protamine phantoms were first used to determine whether there was a linear relationship between the AACID value measured using the 2.0 ppm amine resonance and pH within the physiological pH range, followed by a re-examination of AACID CEST data previously acquired in mice with brain tumors.
2. Experimental
2.1. Phantom Preparation
To validate the use of the 2 ppm amine resonance in the calculation of the AACID ratio, we examined whether the AACID response was linear over the physiological pH range. A series of protamine (EMD Millipore, Oakville, ON, Canada) phantoms were created in 5 mm diameter tubes with pH values ranging from 6–8: 6.12, 6.32, 6.56, 6.78, 7.12, 7.44, 7.71, and 8.03. Protamine produces a 2 ppm amine CEST peak analogous to that observed in the human brain [27,28]. The phantoms contained protamine at a concentration of 12 mg/mL dissolved in phosphate buffered saline. All phantoms were scanned simultaneously at 37 °C.
To characterize the magnitude of the CEST effect at 2 ppm as a function of creatine concentration at constant pH, a series of creatine (Sigma Aldrich, Oakville, ON, Canada) and bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO, USA) phantoms were used. These phantoms were created in 5 mm diameter tubes with creatine concentrations ranging from 0–20 mM, mixed in 10% bovine serum albumin (BSA) at pH 7. Creatine contributes to the 2 ppm amine CEST peak observed in vivo [24,25]. All phantoms were scanned simultaneously at 37 °C.
2.2. Animal Tumor Preparation
All data utilized in this work were obtained from previous studies where animal procedures were approved by the Animal Care Committee at Western University. The animal GBM model used in these studies was previously described [19,21,22,23] but is provided here for completeness. GBM brain tumors were induced in all twenty-four (N = 24), Crl:Nu-Foxn1Nu (NU/NU) mice (22–27 g, Charles River Laboratories, Saint-Constant, QC, Canada) included in this study. Briefly, U87MG glioma cells established from a human GBM (ATCC; Rockville, MD, USA) [29] were grown at 37 °C using Dulbecco’s modified Eagles’ medium supplemented with 10% fetal bovine serum (Wisent Inc., St-Jean-Baptiste, QC, Canada). Cells were grown in a humidified incubator with 5% CO2 and passaged twice a week. The U87MG cells were washed and dissociated with versene solution (PBS plus 0.5 mM EDTA). The cells were then washed twice with PBS, counted, and re-suspended to produce a final concentration of 1 × 105 cells in 2 mL PBS prior to injection. The mice were anesthetized using 4% inhaled isoflurane and then maintained under anesthesia using 1.5% isoflurane. A stereotactic head frame (Stoelting instruments, Wood Dale, IL, USA) was used to guide cell injection. After exposing the bregma, a 1 mm diameter hole was drilled 1 mm anterior and 2 mm lateral to the bregma. A Hamilton syringe (Reno, NV, USA) with a 27-gauge needle attached was used to inject 2 µL of U87MG cells 3 mm deep from the bregma at a rate of 0.5 μL/min, into the right frontal lobe [19,21,22,23].
2.3. Preparation of Mice for In Vivo Imaging
CEST data from all mice included in this study have been previously used to produce AACID maps using the 2.75 ppm resonance [19,21,22,23]. In the current study, we re-examined the data from these mice to compare the AACID CEST measurements using the 2.0 ppm and 2.75 ppm amine resonances. The imaging protocol that was used is summarized as follows. The mouse head was placed within a 30 mm millipede volume coil in a dedicated small animal MRI scanner (9.4T, Agilent, Palo Alto, CA, USA) approximately fifteen (15 ± 1) days after the injection of cancer cell. Mice were anesthetized initially using 4% inhaled isoflurane in oxygen and then maintained using 1.5%–2.5% isoflurane in oxygen. A custom-built MRI-compatible stage was used to secure each mouse, and a bite bar was used to further reduce motion in the head [20]. Respiratory motion was also reduced using surgical tape. A rectal temperature probe was used to monitor temperature, and a respiratory sensor pad connected to a pressure transducer placed on the thoracic region was used to monitor breathing. Warm air blown over the animal using a model 1025 small-animal monitoring and gating system (SA Instruments Inc., Stony Brook, NY, USA) maintained body temperature between 36.9–37.1 °C during imaging. Animals were sacrificed immediately after MR imaging. Data from a single (NU/NU) mouse (Charles River Laboratories, Saint-Constant, QC, Canada) without tumor was also included for comparison.
2.4. Magnetic Resonance Imaging
All phantoms (protamine and creatine phantoms) were scanned using the same 30 mm millipede volume coil used to scan the mice. This study reanalyzed the in vivo data from previously published studies that used the following imaging protocol [19,21,22]. T2-weighted images were acquired to visualize the phantoms or the tumor in mice using a fast spin-echo pulse sequence (FSE) with the following parameters: slice thickness = 1 mm, matrix size = 128 × 128, FOV = 25.6 × 25.6 mm2, ETL = 4, effective TE = 40 ms, and TR/TE = 3000/10 ms. These T2-weighted images were used to position the CEST slab (4 mm thickness) for maximum phantom or tumor coverage. An FSE pulse sequence preceded by a continuous wave radiofrequency (RF) pulse (1.5 µT amplitude and 4 s duration) was used to acquire CEST images (slice thickness = 4 mm, matrix size = 64 × 64, FOV = 25.6 × 25.6 mm2, ETL = 32, effective TE = 7 ms, TR = 7000). A series of 49 CEST images was acquired at sampling saturation frequencies from 1.2 to 4.5 (∆ = 0.1) ppm, from 5.4 to 6.6 (∆ = 0.1) ppm, and at −1000 and 1000 ppm as references. Two series of CEST images were acquired to increase the signal-to-noise ratio. The water saturation shift referencing (WASSR) technique was used for correction of the B0 shifts [30]. A series of 37 WASSR CEST images were acquired with saturation frequencies linearly spaced between −0.6−0.6 ppm. The same pulse sequence as the AACID CEST acquisition was used for WASSR, except it was preceded by a low amplitude (0.2 µT) short duration (100 ms) RF saturation pulse.
2.5. CEST Data Processing
Custom MATLAB (Mathworks, Natick, MA, USA) code was used to analyze all CEST data on a pixel-by-pixel basis as previously described [19,21,22,23]. The WASSR and CEST spectra associated with each pixel were interpolated to 1-Hz resolution. The “smooth” algorithm in the MATLAB curve fitting toolbox was used to smooth all the CEST spectra. To correct the shifts induced by B0 field variations within the sample, the CEST spectrum was frequency shifted for each pixel so that the water signal was centered at 0 ppm, using the corresponding WASSR spectrum. This correction ensured that B0 variations were corrected prior to summing the spectra from pixels within defined regions of interest. After the B0 corrections, the CEST spectra from both acquisitions were added to increase the signal-to-noise ratio. No B1 correction was applied [19] because we have previously shown that the B1 variation in the CEST slice was not appreciable [19].
2.5.1. Calculation of AACID Values
The ratio of the CEST effects at 2.75 ppm (amine protons) and at 3.50 ppm (amide protons), normalized by the CEST effect at 6.0 ppm, gives the AACID2.75 value, as shown in Equation (1) [18].
The AACID2.0 value (Equation (2)) was calculated by substituting the CEST effect at 2.0 ppm for the CEST effect at 2.75 ppm in Equation (1).
The mouse brain AACID2.0 maps were normalized to the average AACID2.0 value measured in the contralateral ROIs of all 24 mice. The same approach was used to normalize the mouse brain AACID2.75 maps using the average AACID2.75 value. This approach simplified the visual assessment of the quantitative AACID maps.
2.5.2. Statistical Analysis
Contralateral tissue and tumor tissue regions of interest (ROIs) were manually drawn in each mouse brain by M.A., guided by the T2-weighted images. The ROIs were drawn using the MATLAB “roipoly” function and the average AACID values within each ROI were calculated. A paired Student’s t-test was used to identify differences in average AACID values between the U87MG tumors and the contralateral tissue in the 24 mice when using the 2.75 ppm and 2.0 ppm amine resonances (GraphPad Prism Version 9.3.1.471 for Windows, GraphPad Software, San Diego, CA, USA). In all comparisons, p < 0.05 was considered statistically significant.
3. Results
A sample image of the arrangement of the protamine phantoms in the MRI scanner is provided in (Figure 1a). In the protamine solutions, the AACID2.0 value calculated using Equation (2) varied nonlinearly as a function of pH (Figure 1b) over the large range of pH values tested, showing greater pH sensitivity at a low pH (below 6.6) and little sensitivity above pH 7.4. The pH response in the range from pH 6.6–7.4 could be approximated as a linear function (indicated by the superimposed line).
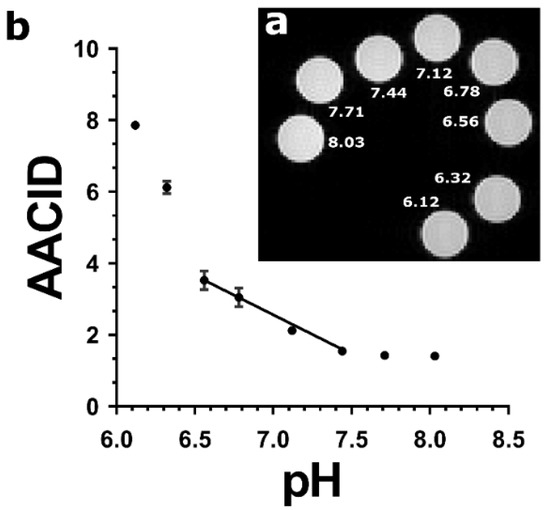
Figure 1.
(a) T2-weighted FSE image showing cross sections of eight protamine samples at pH values ranging from 6.12 to 8.03 in NMR tubes scanned at 9.4T and at 37 °C. (b) AACID values calculated using the 2 ppm amine resonance as a function of pH in protamine samples. The relationship between AACID and pH can be approximated as linear between pH 6.6–7.4. The AACID value is most sensitive to change at low pH (6.1–6.6) and does not change appreciably above pH 7.4. Error bars represent the standard error of the mean within each NMR tube.
The creatine CEST effect was concentration-dependent and easily detected when using the 1.5 µT and 4 s duration saturation pulse used in the AACID acquisition scheme. The CEST peak observed at 2 ppm showed a greater CEST effect as the creatine concentration increased (Figure 2a). Consequently, the AACID values increase linearly as expected as the creatine concentration increased (Figure 2b).
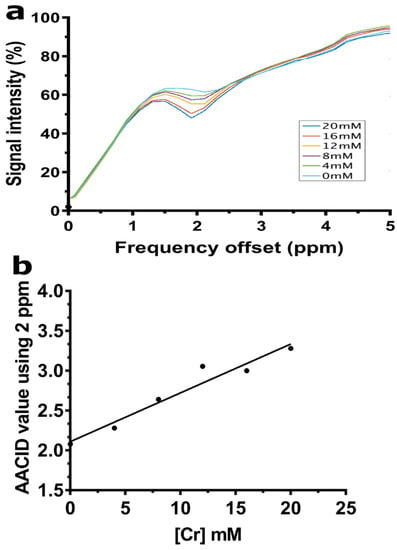
Figure 2.
(a) CEST spectra acquired at 9.4T are shown for samples with Cr concentrations ranging from 0–20 mM mixed in 10% bovine serum albumin (BSA) at 37 °C and pH 7. (b) The AACID value shows a linear increase proportional to the increase in creatine concentration when using the 2 ppm resonance at constant pH.
The average AACID2.75 value in U87MG tumors was 1.15 ± 0.05, while the average AACID2.75 value in contralateral tissue was 1.27 ± 0.041. The average AACID2.0 value in U87MG tumors was 1.93 ± 0.11 while the average AACID2.0 value in contralateral tissue was 2.12 ± 0.10. In both cases, the AACID value in the tumor was significantly lower (p < 0.05) than the contralateral side, indicating a more basic pH in the tumor. The average difference in AACID values between U87MG tumors and contralateral tissue in the 24 mice studied was 39% greater when using the 2 ppm amine resonance compared to the 2.75 ppm resonance (Figure 3). Therefore, use of the 2 ppm amine resonance provides a greater range for the AACID measurement.
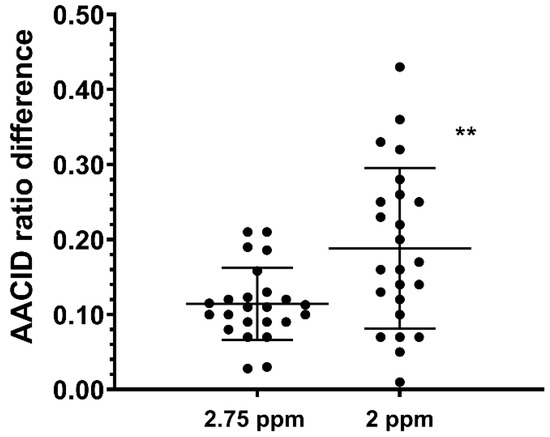
Figure 3.
Average difference in AACID values between U87MG tumors and contralateral tissue in 24 mice when using the 2.75 ppm and 2.0 ppm amine resonances. ** indicates p < 0.001 in a repeated measures two-tailed t-test. Error bars represent the standard error of the mean.
Example AACID CEST maps from a single mouse are provided in Figure 4 when using the 2 ppm amine resonance (Figure 4a) and the 2.75 ppm amine resonance (Figure 4b). These normalized AACID maps appear more symmetric when using the 2 ppm amine resonance (Figure 4a) compared to the 2.75 ppm amine resonance (Figure 4b). Similarly, AACID CEST maps from a single mouse with a brain tumor are shown when using the 2 ppm amine resonance (Figure 4c) and the 2.75 ppm amine resonance (Figure 4d). The dark circular region of low AACID value (elevated pHi) represents the tumor region. The tumor was more easily identified in the AACID maps when using the 2 ppm amine resonance (Figure 4c) compared to the 2.75 ppm amine resonance (Figure 4d).
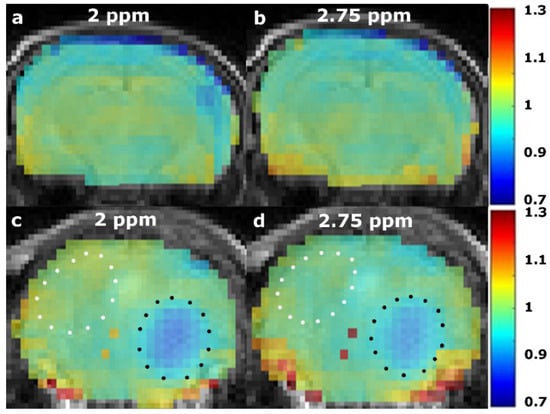
Figure 4.
Normalized AACID maps obtained in a single healthy NU/NU mouse brain using the 2 ppm amine resonance (a), compared to the 2.75 ppm (b) amine resonance. Normalized AACID map of a NU/NU mouse with a brain tumor using the 2 ppm amine resonance (c), compared to the 2.75 ppm (d) amine resonance. Typical manually defined ROIs are shown on the contralateral side (white dotted line), and over the tumor (black dotted line).
4. Discussion
The objective of this work was to determine whether the use of the 2 ppm amine resonance could increase the sensitivity of the AACID ratiometric measurement of intracellular pH. In protamine phantoms, it was shown that the AACID2.0 value obtained using the 2 ppm resonance could be approximated by a linear function between pH 6.6 and 7.4 at 37 °C. Comparing the in vivo AACID2.0 measurements in tumor and in contralateral tissue to the AACID2.75 measurements, the 2 ppm amine resonance increased the AACID range by 39% compared to the 2.75 ppm resonance. This greater range suggests that using the 2 ppm amine resonance could improve AACID-based pH measurement in vivo. The main drawback of using the 2 ppm amine resonance is that it includes contributions from metabolites such as creatine (guanidinium protons), which can fluctuate in concentration in different tissue types. Therefore, the 2 ppm amine resonance should only be used in conditions where creatine concentration can be approximated as stable throughout the measurement.
Protamine is a small, arginine-rich protein that was used in the current study to model CEST contrast because it contains both exchangeable amine and amide protons that can be used in the AACID measurement. The protamine CEST spectra showed the expected peaks at 2.0 ppm from amine protons and at 3.5 ppm from amide protons. The approximately linear relationship observed between the AACID2.0 value and pH in the protamine phantoms within the physiological pH range (6.6–7.4) is not required, but ensures that changes in the AACID value can be easily related to changes in pH. Such a linear response was previously shown for the AACID value obtained when using the 2.75 ppm amine resonance in bovine serum albumin [18]. The previously observed pHi dependent contrast was also insensitive to macromolecule concentration, tissue temperature, and bulk water T1 relaxation [18]. In vivo, the 2 ppm resonance is mostly produced by creatine, although there are also contributions from other metabolites that have chemical shifts near 2 ppm, such as phosphocreatine, adenosine triphosphate, guanidinium and adenosine diphosphate. However, these metabolites have a slower amine proton exchange rate at physiological pH compared to creatine [31]. Therefore, the in vivo 2 ppm CEST signal can be mostly attributed to creatine [26,31,32].
To estimate the absolute pH change indicated by a change to the AACID2.0 measurement, the AACID2.0 values can be calibrated using the literature values of pH in tumors and healthy tissue. For example, the average AACID2.0 measurement in tumors from all 24 mice can be equated to 7.3, the average pH previously measured in brain tumors [33,34,35,36,37,38]. Similarly, the average AACID2.0 measurement on the contralateral side can be equated to 7.0, the average pH previously found in normal brain tissue [33,34,35,36,37,38]. Since AACID2.0 is linearly-dependent on pHi over this narrow range (Figure 1b), this approach to calibration can be used to estimate the absolute pH change in healthy tissue or a tumor following an intervention. However, as described above, the 2 ppm CEST signal is also dependent on creatine concentration [26,31]. Creatine concentration is known to be different in cancerous tissue and to change with tumor progression. Specifically, glioma had significantly lower Cr CEST, a feature that could help to differentiate gliomas with different aggressiveness [26]. Moreover, creatine concentration depends on the tissue cell type and cellular composition. Therefore, to avoid potential errors caused by differences in the tissue Cr concentration, the use of AACID-based pH measurement in vivo with the 2 ppm amine resonance is best suited only to measure the change in pH after drug treatments. The 2 ppm resonance should not be used to compare tissues or in conditions that could potentially involve changes in Cr concentration associated with disease progression [25,26].
Several limitations must be considered in this study. First, the scope of testing was limited to only one tumor model (U87 glioma). It is expected that different tumor types will produce different changes in the AACID measurement, potentially due to a different concentration of creatine within the tumors. Future studies that incorporate regional 1H magnetic resonance spectroscopy measurements of creatine within the tumors could be used to assess the dependency of the 2 ppm AACID CEST measurement on creatine concentrations. Similarly, our in vitro work was limited to protamine. Other protein solutions containing a different ratio of amine to amide group concentrations would be expected to show a different response of the AACID value to pH.
5. Conclusions
AACID measurements sensitive to tissue intracellular pH can be made with the 2.75 ppm or 2.0 ppm amine resonance. Use of the 2 ppm amine resonance can provide a greater measurement range compared to the 2.75 ppm amine resonance. However, this method includes contributions from metabolites such as creatine that can fluctuate in concentration in different tissue types. Therefore, use of the 2 ppm amine resonance can be advantageous, but it should be considered only when measuring pH changes under conditions where the metabolites that contribute to the 2 ppm amine signal are stable for the duration of the measurement.
Author Contributions
Conceptualization, M.A. and R.B.; methodology, M.A., S.M.; formal analysis, M.A.; resources, S.M. and R.B.; data curation, M.A.; writing—original draft preparation, M.A.; writing—review and editing, M.A., S.M. and R.B.; supervision, R.B.; project administration, R.B.; funding acquisition, R.B. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study was provided by the Ontario Institute of Cancer Research (OICR) Smarter Imaging Program and the Natural Science and Engineering Research Council (NSERC) of Canada.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Committee at Western University (AUP# 2019-010 approved on 17 October 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical considerations.
Acknowledgments
Thanks to Misan University-Ministry of Higher Education and Scientific Research, Iraq.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| GBM | glioblastoma multiforme |
| pHi | intracellular pH |
| Cr | creatine |
| CEST | chemical exchange saturation transfer |
| RF | radiofrequency |
| MTRasym | asymmetric magnetization transfer ratio |
| MT | magnetization transfer |
| AACID | amine and amide concentration-independent detection |
| FSE | fast spin-echo |
| WASSR | water saturation shift referencing |
| AFI | actual flip-angle imaging |
| ROI | region of interest |
References
- Tavares-Valente, D.; Baltazar, F.; Moreira, R.; Queiros, O. Cancer cell bioenergetics and pH regulation influence breast cancer cell resistance to paclitaxel and doxorubicin. J. Bioenerg. Biomembr. 2013, 45, 467–475. [Google Scholar] [CrossRef]
- Alfarouk, K.O. Tumor metabolism, cancer cell transporters, and microenvironmental resistance. J. Enzym. Inhib. Med. Chem. 2016, 31, 859–866. [Google Scholar] [CrossRef]
- Ward, K.M.; Balaban, R.S. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST). Magn. Reson. Med. 2000, 44, 799–802. [Google Scholar] [CrossRef]
- Sun, P.Z.; Benner, T.; Kumar, A.; Sorensen, A.G. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn. Reson. Med. 2008, 60, 834–841. [Google Scholar] [CrossRef]
- Sun, P.Z.; Wang, E.; Cheung, J.S.; Zhang, X.; Benner, T.; Sorensen, A.G. Simulation and optimization of pulsed radio frequency irradiation scheme for chemical exchange saturation transfer (CEST) MRI-demonstration of pH-weighted pulsed-amide proton CEST MRI in an animal model of acute cerebral ischemia. Magn. Reson. Med. 2011, 66, 1042–1048. [Google Scholar] [CrossRef]
- Zhou, J.; Payen, J.F.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef]
- Huang, D.; Li, S.; Dai, Z.; Shen, Z.; Yan, G.; Wu, R. Novel gradient echo sequencebased amide proton transfer magnetic resonance imaging in hyperacute cerebral infarction. Mol. Med. Rep. 2015, 11, 3279–3284. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Zu, Z.; Zaiss, M.; Khan, I.S.; Singer, R.J.; Gochberg, D.F.; Bachert, P.; Gore, J.C.; Xu, J. Imaging of amide proton transfer and nuclear Overhauser enhancement in ischemic stroke with corrections for competing effects. NMR Biomed. 2015, 28, 200–209. [Google Scholar] [CrossRef]
- Sun, P.Z.; Murata, Y.; Lu, J.; Wang, X.; Lo, E.H.; Sorensen, A.G. Relaxation-compensated fast multislice amide proton transfer (APT) imaging of acute ischemic stroke. Magn. Reson. Med. 2008, 59, 1175–1182. [Google Scholar] [CrossRef]
- Tee, Y.K.; Harston, G.W.; Blockley, N.; Okell, T.W.; Levman, J.; Sheerin, F.; Cellerini, M.; Jezzard, P.; Kennedy, J.; Payne, S.J.; et al. Comparing different analysis methods for quantifying the MRI amide proton transfer (APT) effect in hyperacute stroke patients. NMR Biomed. 2014, 27, 1019–1029. [Google Scholar] [CrossRef]
- Tietze, A.; Blicher, J.; Mikkelsen, I.K.; Ostergaard, L.; Strother, M.K.; Smith, S.A.; Donahue, M.J. Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI. NMR Biomed. 2014, 27, 163–174. [Google Scholar] [CrossRef]
- Wang, M.; Hong, X.; Chang, C.F.; Li, Q.; Ma, B.; Zhang, H.; Xiang, S.; Heo, H.Y.; Zhang, Y.; Lee, D.H.; et al. Simultaneous detection and separation of hyperacute intracerebral hemorrhage and cerebral ischemia using amide proton transfer MRI. Magn. Reson. Med. 2015, 74, 42–50. [Google Scholar] [CrossRef]
- Togao, O.; Kessinger, C.W.; Huang, G.; Soesbe, T.C.; Sagiyama, K.; Dimitrov, I.; Sherry, A.D.; Gao, J.; Takahashi, M. Characterization of lung cancer by amide proton transfer (APT) imaging: An in-vivo study in an orthotopic mouse model. PLoS ONE 2013, 8, e77019. [Google Scholar] [CrossRef]
- Togao, O.; Yoshiura, T.; Keupp, J.; Hiwatashi, A.; Yamashita, K.; Kikuchi, K.; Suzuki, Y.; Suzuki, S.O.; Iwaki, T.; Hata, N.; et al. Amide proton transfer imaging of adult diffuse gliomas: Correlation with histopathological grades. Neuro-Oncology 2014, 16, 441–448. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, S.; King, A.D.; Zhou, J.; Bhatia, K.S.; Zhang, Q.; Yeung, D.K.; Wei, J.; Mok, G.S.; Wang, Y.X. Amide proton transfer-weighted imaging of the head and neck at 3 T: A feasibility study on healthy human subjects and patients with head and neck cancer. NMR Biomed. 2014, 27, 1239–1247. [Google Scholar] [CrossRef]
- Zhou, J.; Blakeley, J.O.; Hua, J.; Kim, M.; Laterra, J.; Pomper, M.G.; van Zijl, P.C. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn. Reson. Med. 2008, 60, 842–849. [Google Scholar] [CrossRef]
- Zhou, J.; Hong, X.; Zhao, X.; Gao, J.H.; Yuan, J. APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn. Reson. Med. 2013, 70, 320–327. [Google Scholar] [CrossRef]
- McVicar, N.; Li, A.X.; Goncalves, D.F.; Bellyou, M.; Meakin, S.O.; Prado, M.A.; Bartha, R. Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J. Cereb. Blood Flow Metab. 2014, 34, 690–698. [Google Scholar] [CrossRef]
- McVicar, N.; Li, A.X.; Meakin, S.O.; Bartha, R. Imaging chemical exchange saturation transfer (CEST) effects following tumor-selective acidification using lonidamine. NMR Biomed. 2015, 28, 566–575. [Google Scholar] [CrossRef]
- Marathe, K.; McVicar, N.; Li, A.; Bellyou, M.; Meakin, S.; Bartha, R. Topiramate induces acute intracellular acidification in glioblastoma. J. Neurooncol. 2016, 130, 465–472. [Google Scholar] [CrossRef]
- Albatany, M.; Li, A.; Meakin, S.; Bartha, R. Dichloroacetate induced intracellular acidification in glioblastoma: In vivo detection using AACID-CEST MRI at 9.4 Tesla. J. Neuro-Oncol. 2017, 136, 255–262. [Google Scholar] [CrossRef]
- Albatany, M.; Li, A.; Meakin, S.; Bartha, R. In vivo detection of acute intracellular acidification in glioblastoma multiforme following a single dose of cariporide. Int. J. Clin. Oncol. 2018, 23, 812–819. [Google Scholar] [CrossRef]
- Albatany, M.; Meakin, S.; Bartha, R. The Monocarboxylate transporter inhibitor Quercetin induces intracellular acidification in a mouse model of Glioblastoma Multiforme: In-vivo detection using magnetic resonance imaging. Investig. New Drugs 2018, 37, 595–601. [Google Scholar] [CrossRef]
- Haris, M.; Singh, A.; Cai, K.; Kogan, F.; McGarvey, J.; Debrosse, C.; Zsido, G.A.; Witschey, W.R.; Koomalsingh, K.; Pilla, J.J.; et al. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat. Med. 2014, 20, 209–214. [Google Scholar] [CrossRef]
- Cai, K.; Singh, A.; Poptani, H.; Li, W.; Yang, S.; Lu, Y.; Hariharan, H.; Zhou, X.J.; Reddy, R. CEST signal at 2ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed. 2015, 28, 1–8. [Google Scholar] [CrossRef]
- Cai, K.; Tain, R.W.; Zhou, X.J.; Damen, F.C.; Scotti, A.M.; Hariharan, H.; Poptani, H.; Reddy, R. Creatine CEST MRI for Differentiating Gliomas with Different Degrees of Aggressiveness. Mol. Imaging Biol. 2017, 19, 225–232. [Google Scholar] [CrossRef]
- Bar-Shir, A.; Liu, G.; Chan, K.W.; Oskolkov, N.; Song, X.; Yadav, N.N.; Walczak, P.; McMahon, M.T.; van Zijl, P.C.; Bulte, J.W.; et al. Human protamine-1 as an MRI reporter gene based on chemical exchange. ACS Chem. Biol. 2014, 9, 134–138. [Google Scholar] [CrossRef]
- Oskolkov, N.; Bar-Shir, A.; Chan, K.W.; Song, X.; van Zijl, P.C.; Bulte, J.W.; Gilad, A.A.; McMahon, M.T. Biophysical Characterization of Human Protamine-1 as a Responsive CEST MR Contrast Agent. ACS Macro. Lett. 2015, 4, 34–38. [Google Scholar] [CrossRef]
- Li, A.X.; Suchy, M.; Li, C.; Gati, J.S.; Meakin, S.; Hudson, R.H.; Menon, R.S.; Bartha, R. In vivo detection of MRI-PARACEST agents in mouse brain tumors at 9.4 T. Magn. Reson. Med. 2011, 66, 67–72. [Google Scholar] [CrossRef]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef]
- Haris, M.; Nanga, R.P.; Singh, A.; Cai, K.; Kogan, F.; Hariharan, H.; Reddy, R. Exchange rates of creatine kinase metabolites: Feasibility of imaging creatine by chemical exchange saturation transfer MRI. NMR Biomed. 2012, 25, 1305–1309. [Google Scholar] [CrossRef]
- Arus, C.; Barany, M.; Westler, W.M.; Markley, J.L. 1H NMR of intact muscle at 11 T. FEBS Lett. 1984, 165, 231–237. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar]
- Stubbs, M.; Bhujwalla, Z.M.; Tozer, G.M.; Rodrigues, L.M.; Maxwell, R.J.; Morgan, R.; Howe, F.A.; Griffiths, J.R. An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed. 1992, 5, 351–359. [Google Scholar] [CrossRef]
- Ha, D.H.; Choi, S.; Oh, J.Y.; Yoon, S.K.; Kang, M.J.; Kim, K.U. Application of 31P MR spectroscopy to the brain tumors. Korean J. Radiol. 2013, 14, 477–486. [Google Scholar] [CrossRef]
- Cichocka, M.; Kozub, J.; Urbanik, A. PH Measurements of the Brain Using Phosphorus Magnetic Resonance Spectroscopy (31PMRS) in Healthy Men—Comparison of Two Analysis Methods. Pol. J. Radiol. 2015, 80, 509. [Google Scholar] [CrossRef]
- Oberhaensli, R.D.; Galloway, G.J.; Hilton-Jones, D.; Bore, P.J.; Styles, P.; Rajagopalan, B.; Taylor, D.J.; Radda, G.K. The study of human organs by phosphorus-31 topical magnetic resonance spectroscopy. Br. J. Radiol. 1987, 60, 367–373. [Google Scholar] [CrossRef]
- Maintz, D.; Heindel, W.; Kugel, H.; Jaeger, R.; Lackner, K.J. Phosphorus-31 MR spectroscopy of normal adult human brain and brain tumours. NMR Biomed. 2002, 15, 18–27. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).