AI Lung Segmentation and Perfusion Analysis of Dual-Energy CT Can Help to Distinguish COVID-19 Infiltrates from Visually Similar Immunotherapy-Related Pneumonitis Findings and Can Optimize Radiological Workflows
Abstract
:1. Introduction
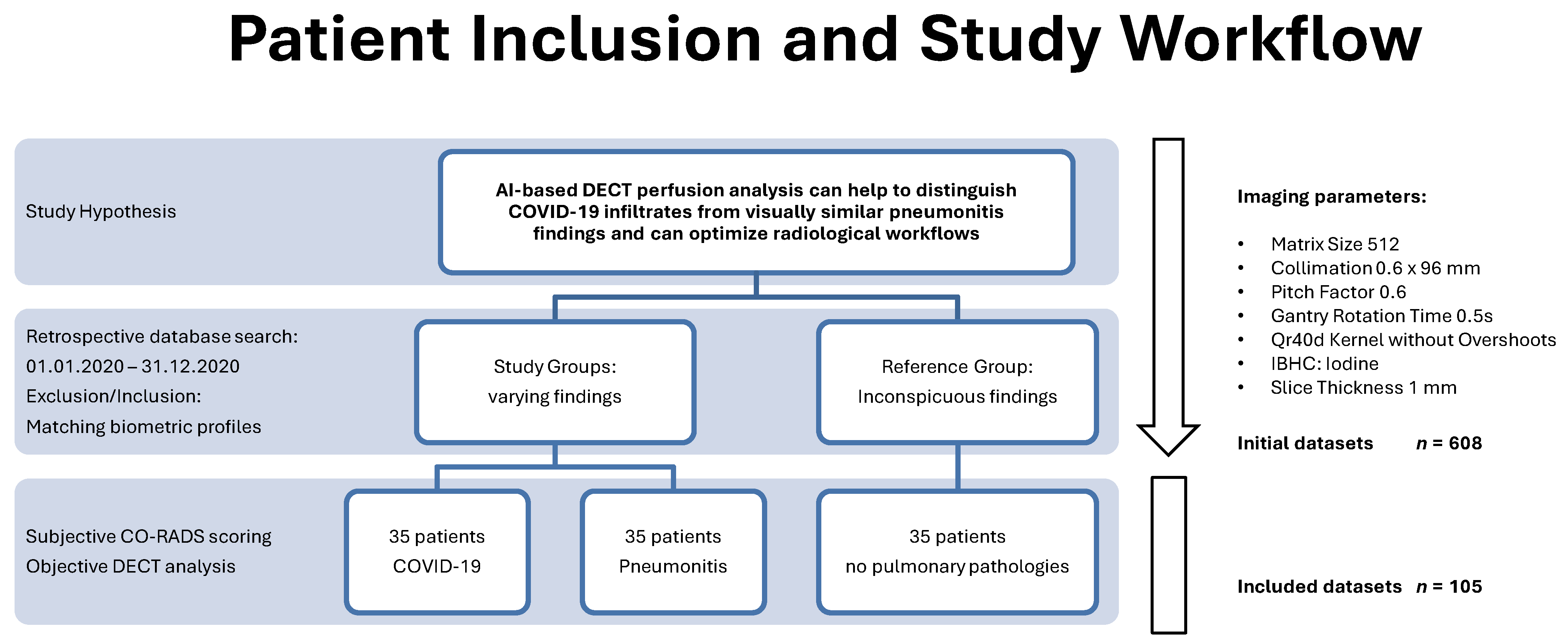
2. Materials and Methods
2.1. Study Design and Population
2.2. Image Acquisition and Reconstruction Parameters
2.3. Subjective Reading and CO-RADS Scoring
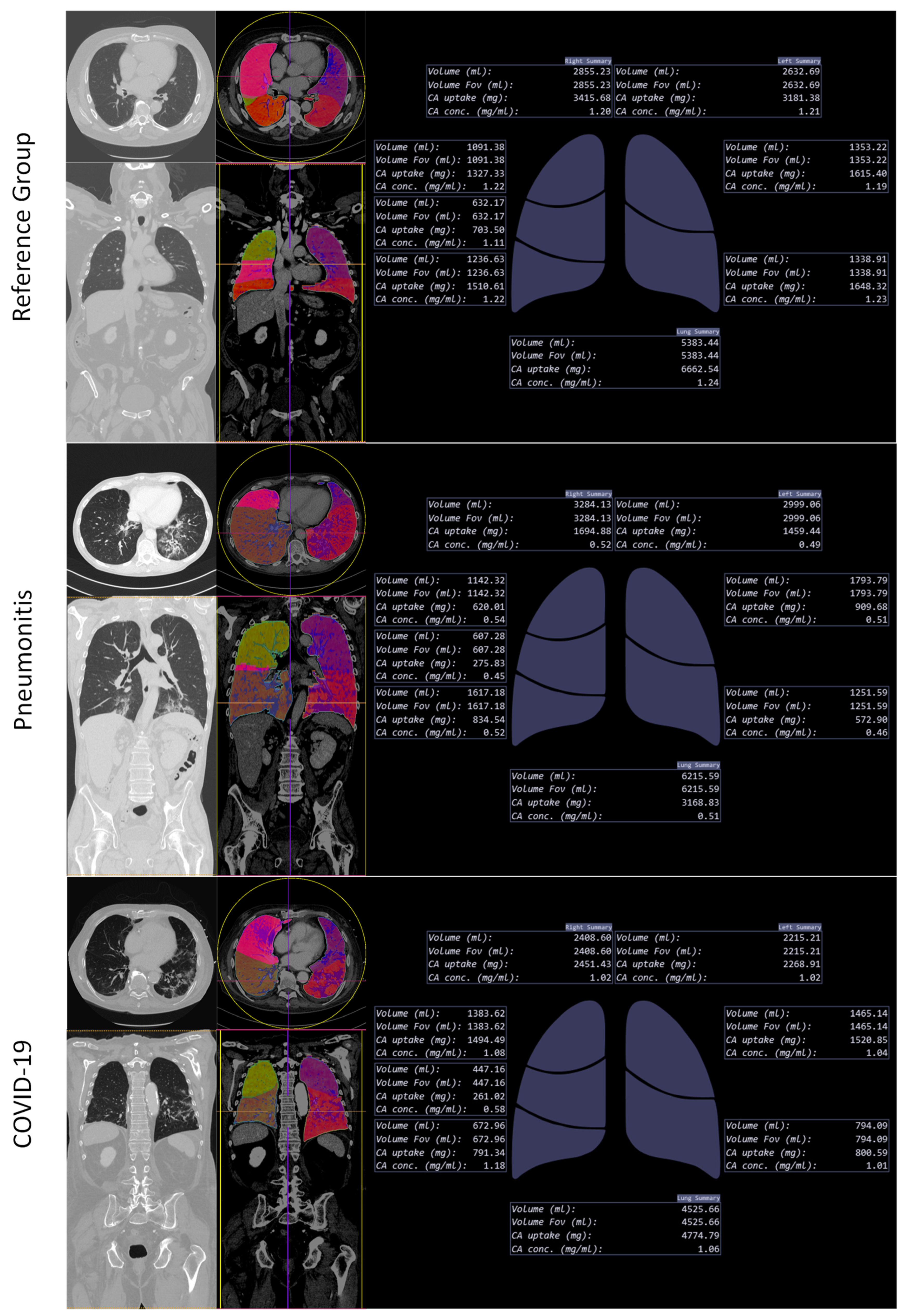
2.4. Lung Segmentation and Perfusion Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Population and CO-RADS Score
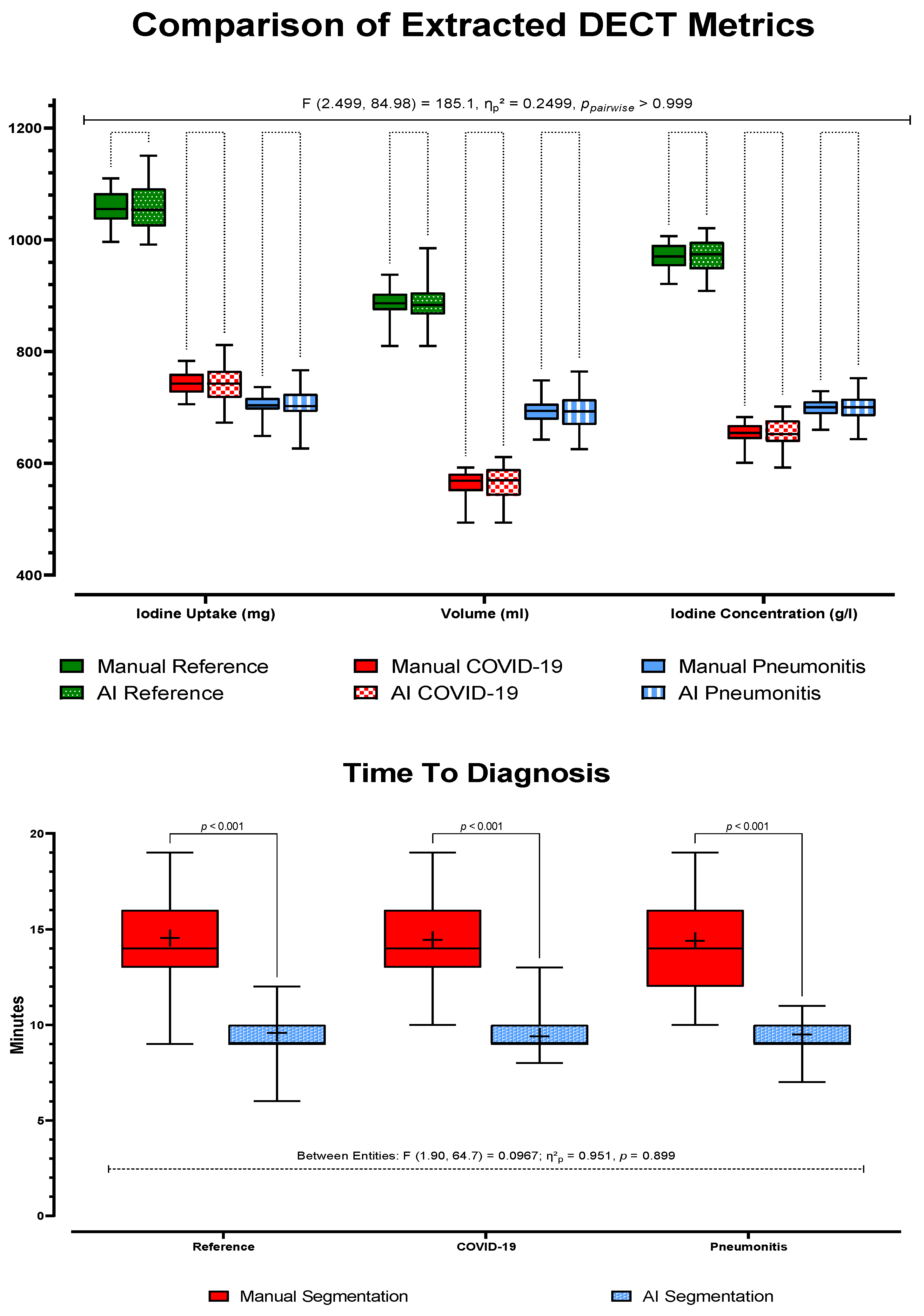
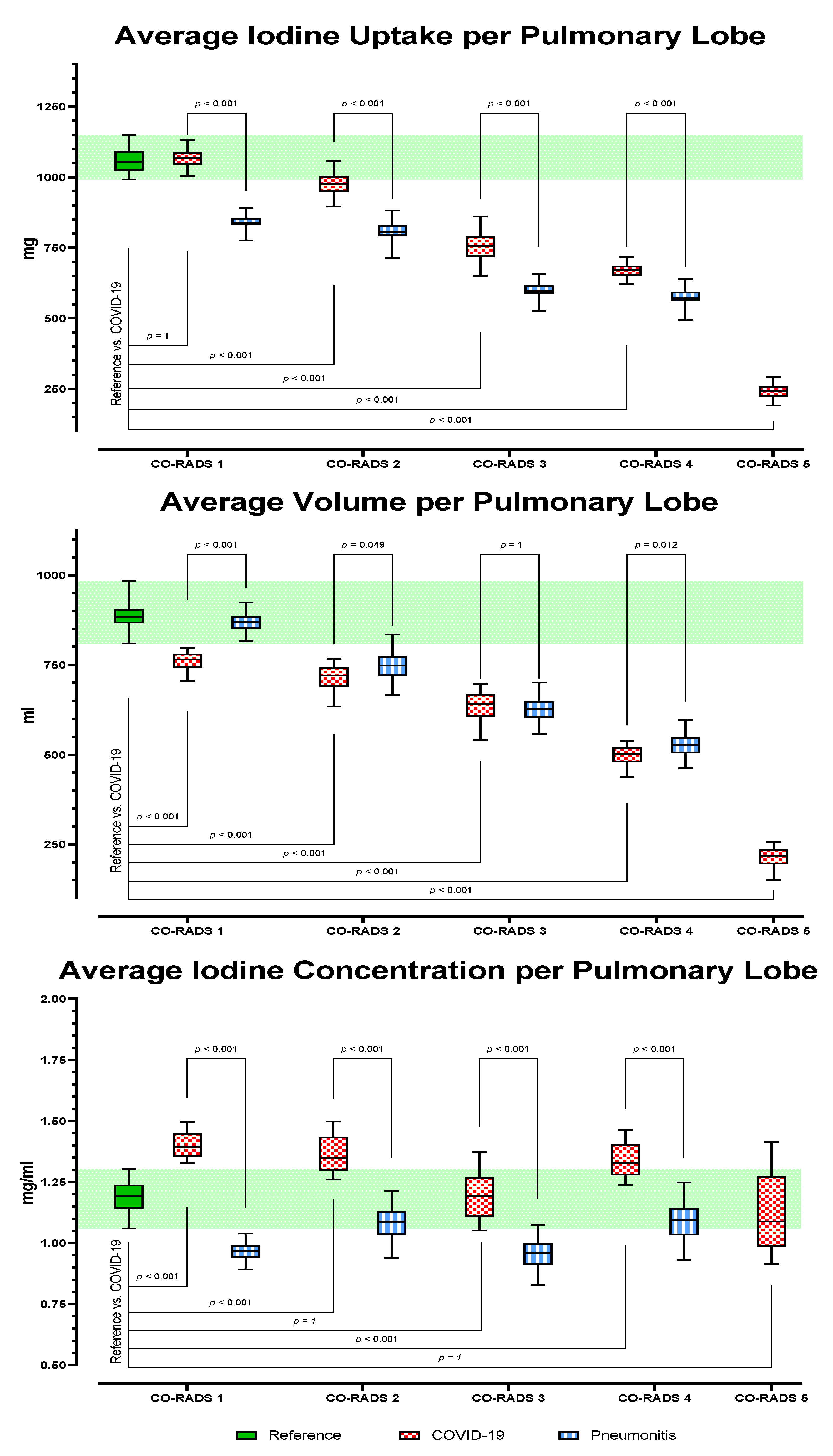
3.2. Dual-Energy CT Metric Comparison
3.2.1. Method Validation and Time to Diagnosis
3.2.2. Analysis of AI-Based Lung Segmentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. Corrigendum to “World Health Organization declares Global Emergency: A review of the 2019 Novel Coronavirus (COVID-19)”. Int. J. Surg. 2020, 77, 217. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, J.; Moss, B.; Kahn, J.; Rutkow, L. The Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/ (accessed on 17 November 2021).
- Li, Y.; Xia, L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. AJR Am. J. Roentgenol. 2020, 214, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Sun, D.; Liu, Y.; Fan, Y.; Zhao, L.; Li, X.; Zhu, W. Clinical and High-Resolution CT Features of the COVID-19 Infection: Comparison of the Initial and Follow-up Changes. Investig. Radiol. 2020, 55, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295, 200463. [Google Scholar] [CrossRef] [Green Version]
- Prokop, M.; Van Everdingen, W.; van Rees Vellinga, T.; Quarles van Ufford, H.; Stöger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C. CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19—Definition and evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef] [PubMed]
- Duzgun, S.A.; Durhan, G.; Demirkazik, F.B.; Akpinar, M.G.; Ariyurek, O.M. COVID-19 pneumonia: The great radiological mimicker. Insights Imaging 2020, 11, 118. [Google Scholar] [CrossRef]
- Kalisz, K.R.; Ramaiya, N.H.; Laukamp, K.R.; Gupta, A. Immune Checkpoint Inhibitor Therapy-related Pneumonitis: Patterns and Management. Radiographics 2019, 39, 1923–1937. [Google Scholar] [CrossRef]
- Rossi, E.; Schinzari, G.; Tortora, G. Pneumonitis from immune checkpoint inhibitors and COVID-19: Current concern in cancer treatment. J. Immunother. Cancer 2020, 8, e000952. [Google Scholar] [CrossRef]
- Smith, T.; Bushek, J.; LeClaire, A.; Prosser, T. COVID-19 Drug Therapy. 2020. Available online: https://www.elsevier.com/__data/assets/pdf_file/0007/988648/COVID-19-Drug-Therapy_Mar-2020.pdf (accessed on 10 November 2021).
- Woloshin, S.; Patel, N.; Kesselheim, A.S. False Negative Tests for SARS-CoV-2 Infection—Challenges and Implications. N. Engl. J. Med. 2020, 383, e38. [Google Scholar] [CrossRef]
- Wang, B.; Jin, S.; Yan, Q.; Xu, H.; Luo, C.; Wei, L.; Zhao, W.; Hou, X.; Ma, W.; Xu, Z.; et al. AI-assisted CT imaging analysis for COVID-19 screening: Building and deploying a medical AI system. Appl. Soft Comput. 2021, 98, 106897. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.J.; Park, C.M. Clinical Implementation of Deep Learning in Thoracic Radiology: Potential Applications and Challenges. Korean J. Radiol. 2020, 21, 511–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, D.L. Artificial Intelligence in Imaging: The Radiologist’s Role. J. Am. Coll. Radiol. 2019, 16, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R. Dual-energy CT: General principles. AJR Am. J. Roentgenol. 2012, 199, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Afat, S.; Othman, A.E.; Nikolaou, K.; Gassenmaier, S. Dual-Energy Computed Tomography of the Lung in COVID-19 Patients: Mismatch of Perfusion Defects and Pulmonary Opacities. Diagnostics 2020, 10, 870. [Google Scholar] [CrossRef]
- Novak, C.L.; Kiraly, A.P.; Wang, J. Trained Generative Network for Lung Segmentation in Medical Imaging. Available online: https://www.freepatentsonline.com/20190220701.pdf (accessed on 18 December 2021).
- Kalligeros, M.; Shehadeh, F.; Mylona, E.K.; Benitez, G.; Beckwith, C.G.; Chan, P.A.; Mylonakis, E. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity 2020, 28, 1200–1204. [Google Scholar] [CrossRef]
- Singh, R.; Nie, R.Z.; Homayounieh, F.; Schmidt, B.; Flohr, T.; Kalra, M.K. Quantitative lobar pulmonary perfusion assessment on dual-energy CT pulmonary angiography: Applications in pulmonary embolism. Eur. Radiol. 2020, 30, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Bobak, C.A.; Barr, P.J.; O’Malley, A.J. Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Med. Res. Methodol. 2018, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Kromrey, J.D.; Rendina-Gobioff, G. An empirical comparison of regression analysis strategies with discrete ordinal variables. Mult. Linear Regres. Viewp. 2002, 28, 30–43. [Google Scholar]
- McCullagh, P. Regression models for ordinal data. J. R. Stat.Soc. Ser. B 1980, 42, 109–127. [Google Scholar] [CrossRef]
- Bai, H.X.; Hsieh, B.; Xiong, Z.; Halsey, K.; Choi, J.W.; Tran, T.M.L.; Pan, I.; Shi, L.B.; Wang, D.C.; Mei, J.; et al. Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest CT. Radiology 2020, 296, E46–E54. [Google Scholar] [CrossRef]
- Oudkerk, M.; Buller, H.R.; Kuijpers, D.; van Es, N.; Oudkerk, S.F.; McLoud, T.; Gommers, D.; van Dissel, J.; Ten Cate, H.; van Beek, E.J.R. Diagnosis, Prevention, and Treatment of Thromboembolic Complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology 2020, 297, E216–E222. [Google Scholar] [CrossRef]
- Grillet, F.; Busse-Cote, A.; Calame, P.; Behr, J.; Delabrousse, E.; Aubry, S. COVID-19 pneumonia: Microvascular disease revealed on pulmonary dual-energy computed tomography angiography. Quant. Imaging Med. Surg. 2020, 10, 1852–1862. [Google Scholar] [CrossRef]
- Si-Mohamed, S.; Chebib, N.; Sigovan, M.; Zumbihl, L.; Turquier, S.; Boccalini, S.; Boussel, L.; Mornex, J.F.; Cottin, V.; Douek, P. In vivo demonstration of pulmonary microvascular involvement in COVID-19 using dual-energy computed tomography. Eur. Respir. J. 2020, 56, 2002608. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef]
- Lang, M.; Som, A.; Mendoza, D.P.; Flores, E.J.; Reid, N.; Carey, D.; Li, M.D.; Witkin, A.; Rodriguez-Lopez, J.M.; Shepard, J.O.; et al. Hypoxaemia related to COVID-19: Vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020, 20, 1365–1366. [Google Scholar] [CrossRef]
- Fischer, A.M.; Yacoub, B.; Savage, R.H.; Martinez, J.D.; Wichmann, J.L.; Sahbaee, P.; Grbic, S.; Varga-Szemes, A.; Schoepf, U.J. Machine Learning/Deep Neuronal Network: Routine Application in Chest Computed Tomography and Workflow Considerations. J. Thorac. Imaging 2020, 35 (Suppl. 1), S21–S27. [Google Scholar] [CrossRef]
| Parameter | Female | Male | Total |
|---|---|---|---|
| Patient Population | |||
| Absolute (n) | 54 | 51 | 105 |
| Reference | 18 | 17 | 35 |
| Pneumonitis | 18 | 17 | 35 |
| COVID-19 | 18 | 17 | 35 |
| Mean age (y) | 62 ± 13 | 63 ± 14 | 62 ± 13 |
| Mean BMI | 26 ± 1 | 27 ± 2 | 27 ± 2 |
| CO-RADS Score and Level of Suspicion | Reference | Pneumonitis | COVID-19 | Total (n) | ||
|---|---|---|---|---|---|---|
| Level of Suspicion | ||||||
| 1 | Very low | Normal or noninfectious | 175 | 22 | 13 | 210 |
| 2 | Low | Infectious abnormalities other than COVID-19 | 82 | 11 | 93 | |
| 3 | Indeterminate | Unclear whether COVID-19 is present | 59 | 33 | 92 | |
| 4 | High | Infectious abnormalities suspicious for COVID-19 | 12 | 49 | 61 | |
| 5 | Very high | Infectious abnormalities typical for COVID-19 | 69 | 69 | ||
| Estimate (B) | SE | Wald χ2 | p | Odds Ratio Exp (B) | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| Differentiation from COVID-19 | Pneumonitis | Reader | 0.24 | 0.15 | 2.57 | 0.109 | 1.3 | 0.95–1.70 |
| CO-RADS score | −1.60 | 0.19 | 71.40 | <0.001 | 0.20 | 0.14–0.29 | ||
| Iodine Uptake | 0.60 | 0.25 | 5.52 | 0.019 | 1.82 | 1.10–2.99 | ||
| Volume | 0.47 | 0.25 | 3.47 | 0.062 | 1.60 | 0.98–2.62 | ||
| Iodine Concentration | 0.41 | 0.25 | 2.75 | 0.097 | 1.51 | 0.93–2.46 | ||
| Reference | Reader | 0.29 | 0.15 | 3.69 | 0.06 | 1.3 | 0.99–1.79 | |
| CO-RADS score | −0.11 | 0.02 | 25.10 | <0.000 | 0.9 | 0.86–0.94 | ||
| Iodine Uptake | 1.40 | 0.31 | 20.42 | <0.000 | 4.07 | 1.03–3.42 | ||
| Volume | 0.63 | 0.30 | 4.29 | 0.038 | 1.88 | 1.03–3.42 | ||
| Iodine Concentration | 0.86 | 0.28 | 9.31 | 0.002 | 2.37 | 1.36–4.13 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brendlin, A.S.; Mader, M.; Faby, S.; Schmidt, B.; Othman, A.E.; Gassenmaier, S.; Nikolaou, K.; Afat, S. AI Lung Segmentation and Perfusion Analysis of Dual-Energy CT Can Help to Distinguish COVID-19 Infiltrates from Visually Similar Immunotherapy-Related Pneumonitis Findings and Can Optimize Radiological Workflows. Tomography 2022, 8, 22-32. https://doi.org/10.3390/tomography8010003
Brendlin AS, Mader M, Faby S, Schmidt B, Othman AE, Gassenmaier S, Nikolaou K, Afat S. AI Lung Segmentation and Perfusion Analysis of Dual-Energy CT Can Help to Distinguish COVID-19 Infiltrates from Visually Similar Immunotherapy-Related Pneumonitis Findings and Can Optimize Radiological Workflows. Tomography. 2022; 8(1):22-32. https://doi.org/10.3390/tomography8010003
Chicago/Turabian StyleBrendlin, Andreas S., Markus Mader, Sebastian Faby, Bernhard Schmidt, Ahmed E. Othman, Sebastian Gassenmaier, Konstantin Nikolaou, and Saif Afat. 2022. "AI Lung Segmentation and Perfusion Analysis of Dual-Energy CT Can Help to Distinguish COVID-19 Infiltrates from Visually Similar Immunotherapy-Related Pneumonitis Findings and Can Optimize Radiological Workflows" Tomography 8, no. 1: 22-32. https://doi.org/10.3390/tomography8010003
APA StyleBrendlin, A. S., Mader, M., Faby, S., Schmidt, B., Othman, A. E., Gassenmaier, S., Nikolaou, K., & Afat, S. (2022). AI Lung Segmentation and Perfusion Analysis of Dual-Energy CT Can Help to Distinguish COVID-19 Infiltrates from Visually Similar Immunotherapy-Related Pneumonitis Findings and Can Optimize Radiological Workflows. Tomography, 8(1), 22-32. https://doi.org/10.3390/tomography8010003








