Idiopathic Epiretinal Membrane: Microvasculature Analysis with Optical Coherence Tomography and Optical Coherence Tomography Angiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Examination Procedure
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERM | Epiretinal membrane |
| OCT | Optical coherence tomography |
| OCT-A | Optical coherence tomography angiography |
| BCVA | Best corrected visual acuity |
| FAZ | Foveal avascular zone |
| RNFL | Retinal nerve fiber layer |
| CFT | Central foveal thickness |
| ILM | Internal limiting membrane |
| DR | Diabetic retinopathy |
| PVD | Posterior vitreal detachment |
References
- Miguel, A.; Legris, A. Prognostic factors of epiretinal membranes: A systematic review. J. Français d’Ophtalmol. 2017, 40, 61–79. [Google Scholar] [CrossRef]
- Johnson, M.W. Important Role of Epiretinal Membrane in Tractional Vitreomaculopathies. Retina 2019, 39, 1031–1032. [Google Scholar] [CrossRef]
- Fraser-Bell, S.; Guzowski, M.; Rochtchina, E.; Wang, J.J.; Mitchell, P. Five-year cumulative incidence and progression of epiretinal membranes. Ophthalmology 2003, 110, 34–40. [Google Scholar] [CrossRef]
- Bu, S.-C.; Kuijer, R.; Li, X.-R.; Hooymans, J.M.M.; Los, L.I. Idiopathic Epiretinal Membrane. Retina 2014, 34, 2317–2335. [Google Scholar] [CrossRef]
- Kumar, D.A.; Maitra, P.; Agarwal, A. Epiretinal membrane profile on spectral domain optical coherence tomography in patients with uveitis. Indian J. Ophthalmol. 2019, 67, 376. [Google Scholar] [CrossRef] [PubMed]
- Mokbul, M.I. Optical Coherence Tomography: Basic Concepts and Applications in Neuroscience Research. J. Med. Eng. 2017, 2017, 3409327. [Google Scholar] [CrossRef]
- Koustenis, A.K., Jr.; Harris, A.; Gross, J.; Januleviciene, I.; Shah, A.; Siesky, B. Optical coherence tomography angiography: An overview of the technology and an assessment of applications for clinical research. Br. J. Ophthalmol. 2016, 101, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Dansingani, K.; Inoue, M.; Engelbert, M.; Freund, K.B. Optical coherence tomographic angiography shows reduced deep capillary flow in paracentral acute middle maculopathy. Eye 2015, 29, 1620–1624. [Google Scholar] [CrossRef]
- Malihi, M.; Jia, Y.; Gao, S.S.; Flaxel, C.; Lauer, A.K.; Hwang, T.; Wilson, D.J.; Huang, D.; Bailey, S.T. Optical coherence tomographic angiography of choroidal neovascularization ill-defined with fluorescein angiography. Br. J. Ophthalmol. 2017, 101, 45–50. [Google Scholar] [CrossRef]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A Computational Tool for Quantitative Analysis of Vascular Networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luisi, J.; Liu, H.; Motamedi, M.; Zhang, W. OCT-Angiography for Non-Invasive Monitoring of Neuronal and Vascular Structure in Mouse Retina: Implication for Characterization of Retinal Neurovascular Coupling. EC Ophthalmol. 2017, 5, 89–98. [Google Scholar]
- Kumagai, K.; Uemura, A.; Furukawa, M.; Suetsugu, T.; Ogino, N. Decrease of the foveal avascular zone area after internal limiting membrane peeling: Single case study. Int. Med. Case Rep. J. 2017, 10, 81–85. [Google Scholar] [CrossRef]
- Kumagai, K.; Furukawa, M.; Suetsugu, T.; Ogino, N. Foveal Avascular Zone Area after Internal Limiting Membrane Peeling for Epiretinal Membrane and Macular Hole Compared with That of Fellow Eyes and Healthy Controls. Retina 2018, 38, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Shimada, H.; Shinojima, A.; Nakashizuka, H. Foveal avascular zone area analysis using optical coherence tomography angiography before and after idiopathic epiretinal membrane surgery. Retina 2019, 39, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Okawa, Y.; Maruko, I.; Kawai, M.; Hasegawa, T.; Arakawa, H.; Iida, T. Foveal structure and vasculature in eyes with idiopathic epiretinal membrane. PLoS ONE 2019, 14, e0214881. [Google Scholar] [CrossRef]
- Samara, W.A.; Say, E.A.T.; Khoo, C.T.L.; Higgins, T.P.; Magrath, G.; Ferenczy, S.; Shields, C.L. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina 2015, 35, 2188–2195. [Google Scholar] [CrossRef]
- Muftuoglu, I.K.; Amador, M.; Meshi, A.; Nudleman, E.; Lin, T.; Freeman, W.R. Foveal Avascular Zone Distortion in Epiretinal Membrane by Optical Coherence Tomography Angiography. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Woo, J.M.; Min, J.K. Superficial foveal avascular zone area changes before and after idiopathic epiretinal membrane surgery. Int. J. Ophthalmol. 2018, 11, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Shiihara, H.; Terasaki, H.; Sonoda, S.; Kakiuchi, N.; Yamaji, H.; Yamaoka, S.; Uno, T.; Watanabe, M.; Sakamoto, T. Association of foveal avascular zone with the metamorphopsia in epiretinal membrane. Sci. Rep. 2020, 10, 17092. [Google Scholar] [CrossRef]
- Hirata, A.; Nakada, H.; Mine, K.; Masumoto, M.; Sato, T.; Hayashi, K. Relationship between the morphology of the foveal avascular zone and the degree of aniseikonia before and after vitrectomy in patients with unilateral epiretinal membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 507–515. [Google Scholar] [CrossRef]
- Munk, M.R.; Giannakaki-Zimmermann, H.; Berger, L.; Huf, W.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. OCT-angiography: A qualitative and quantitative comparison of 4 OCT-A devices. PLoS ONE 2017, 12, e0177059. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.K.; Nesper, P.L.; Gill, M.K.; Fawzi, A.A. Semiautomated quantitative approach to characterize treatment response in neovascular age-related macular degeneration. Retina 2017, 37, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Sugano, Y.; Sekiryu, T.; Furuta, M.; Tomita, R.; Shintake, H.; Maehara, H.; Ojima, A. Morphometrical evaluation of the choriocapillaris imaged by swept-source optical coherence tomography angiography. Clin. Ophthalmol. 2018, 12, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Zeydanli, E.O.; Gurelik, G. Optical coherence tomography angiography markers associated with treatment response in neovascular age-related macular degeneration. Eur. J. Ophthalmol. 2021, 31, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
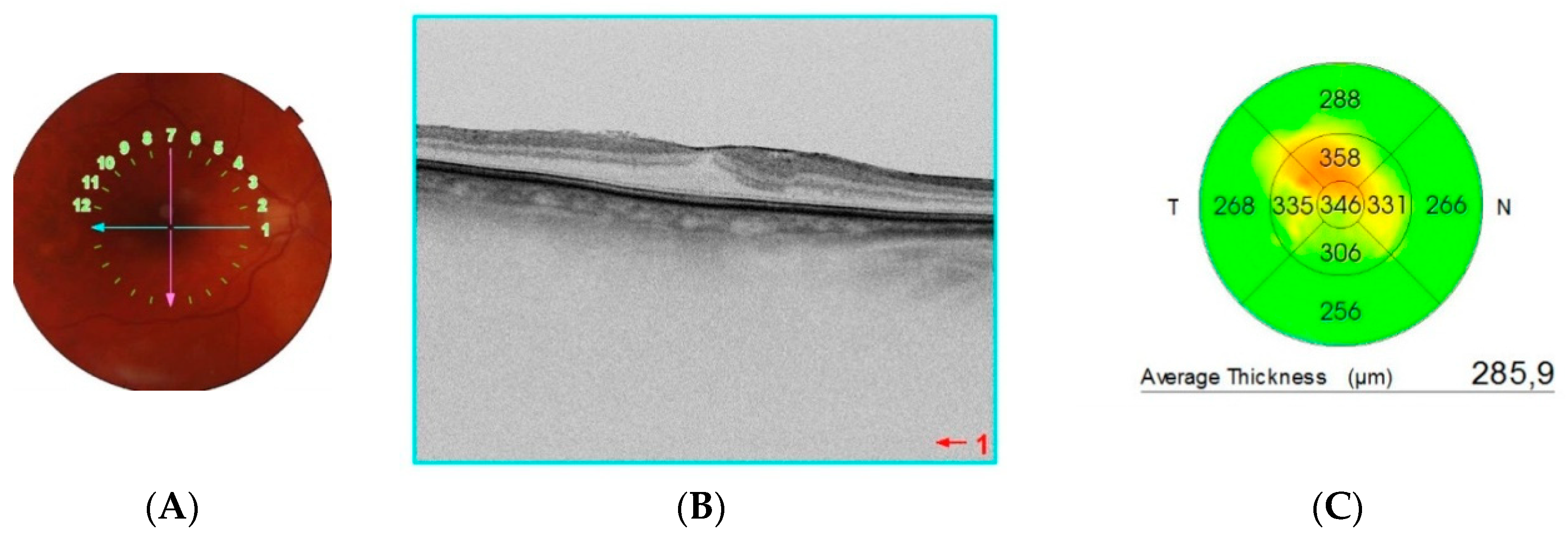
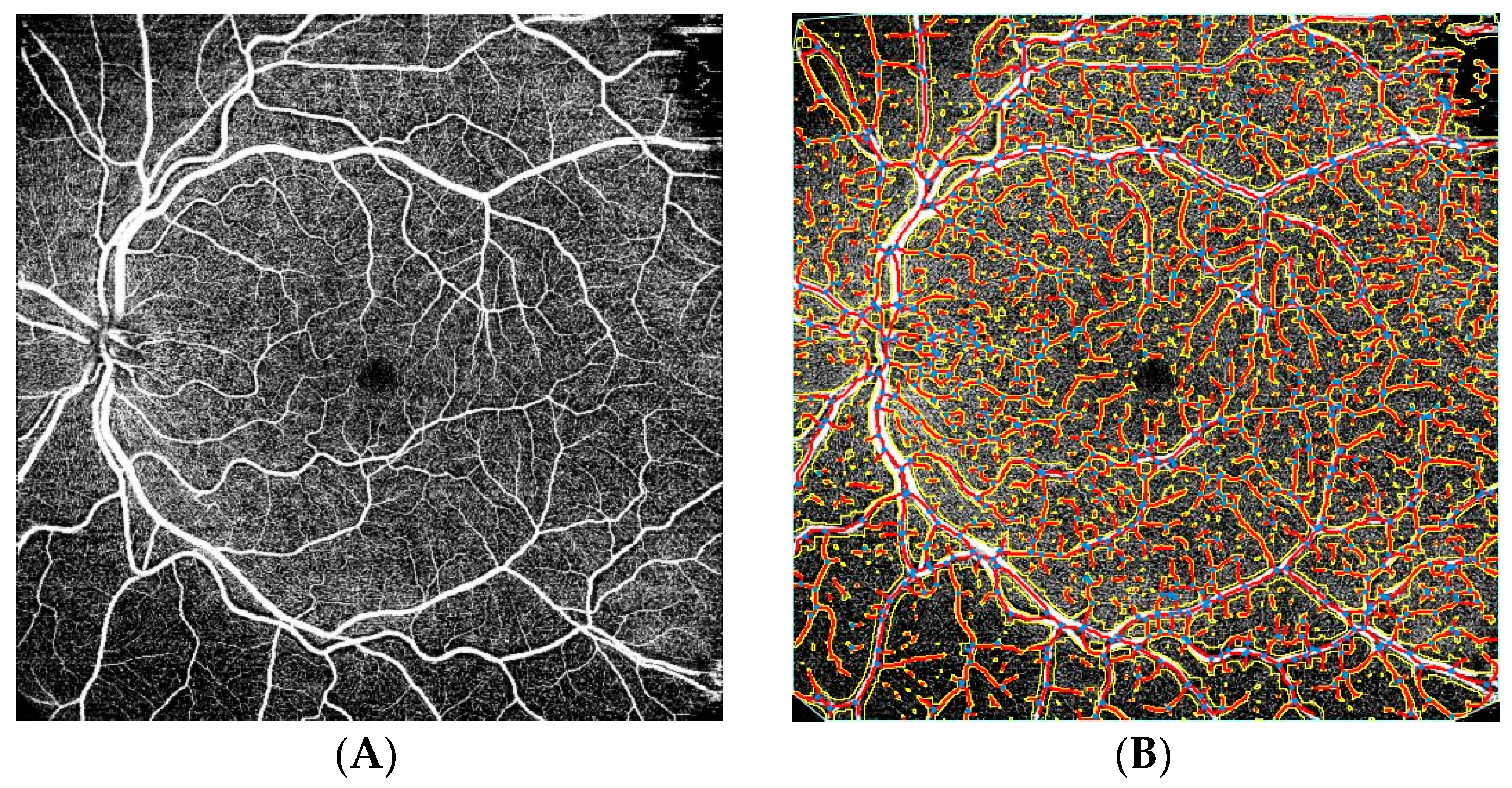
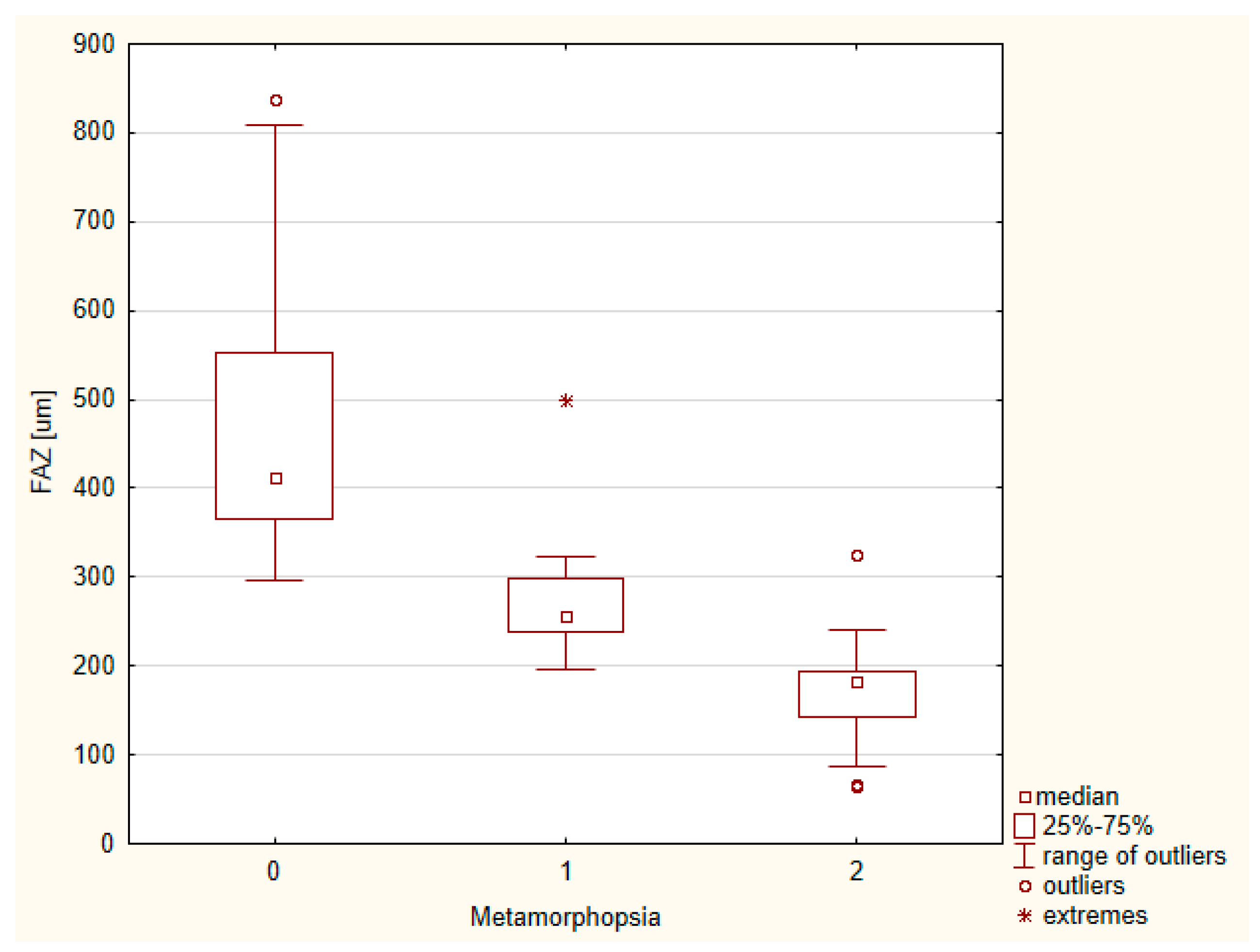
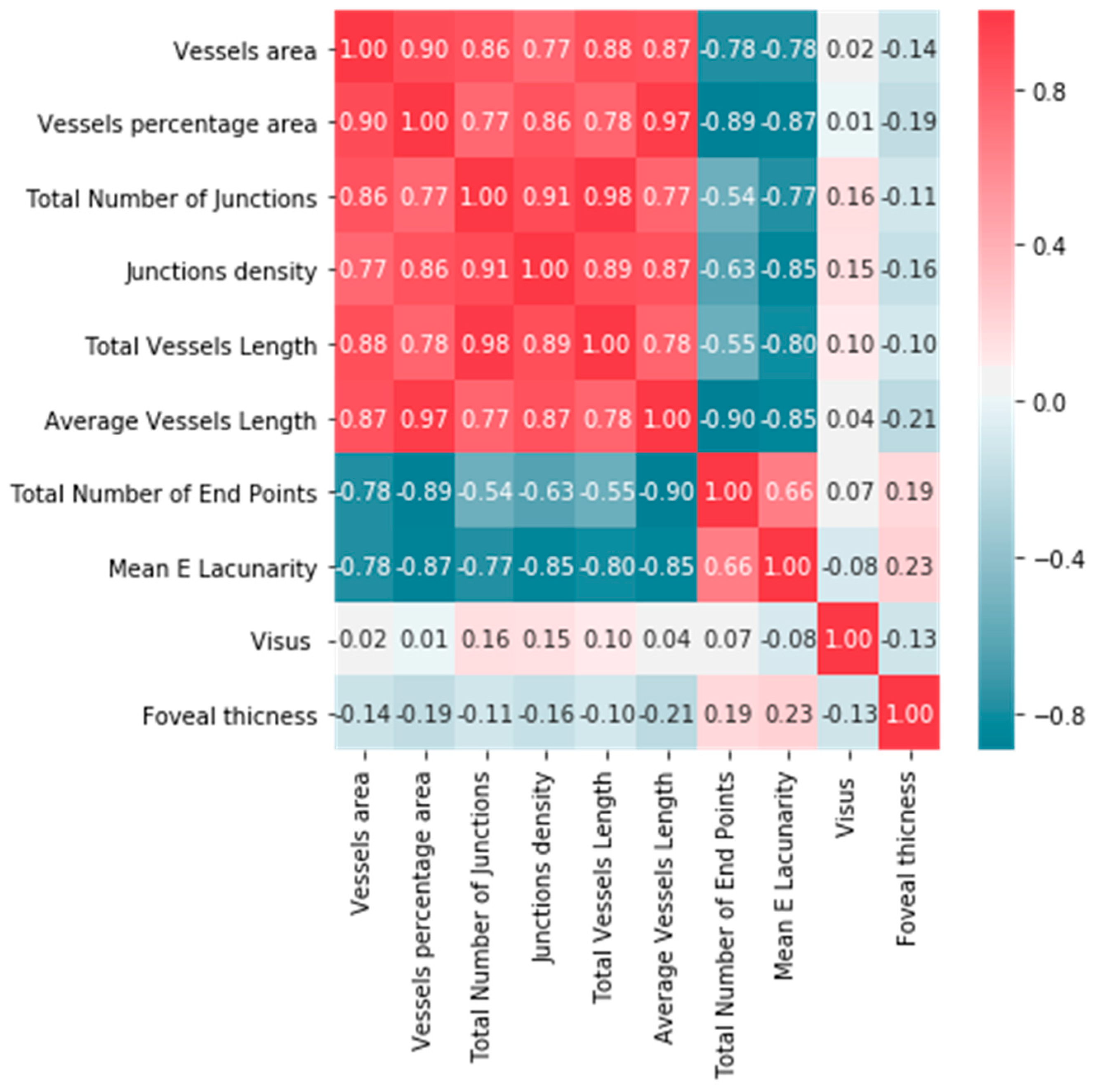

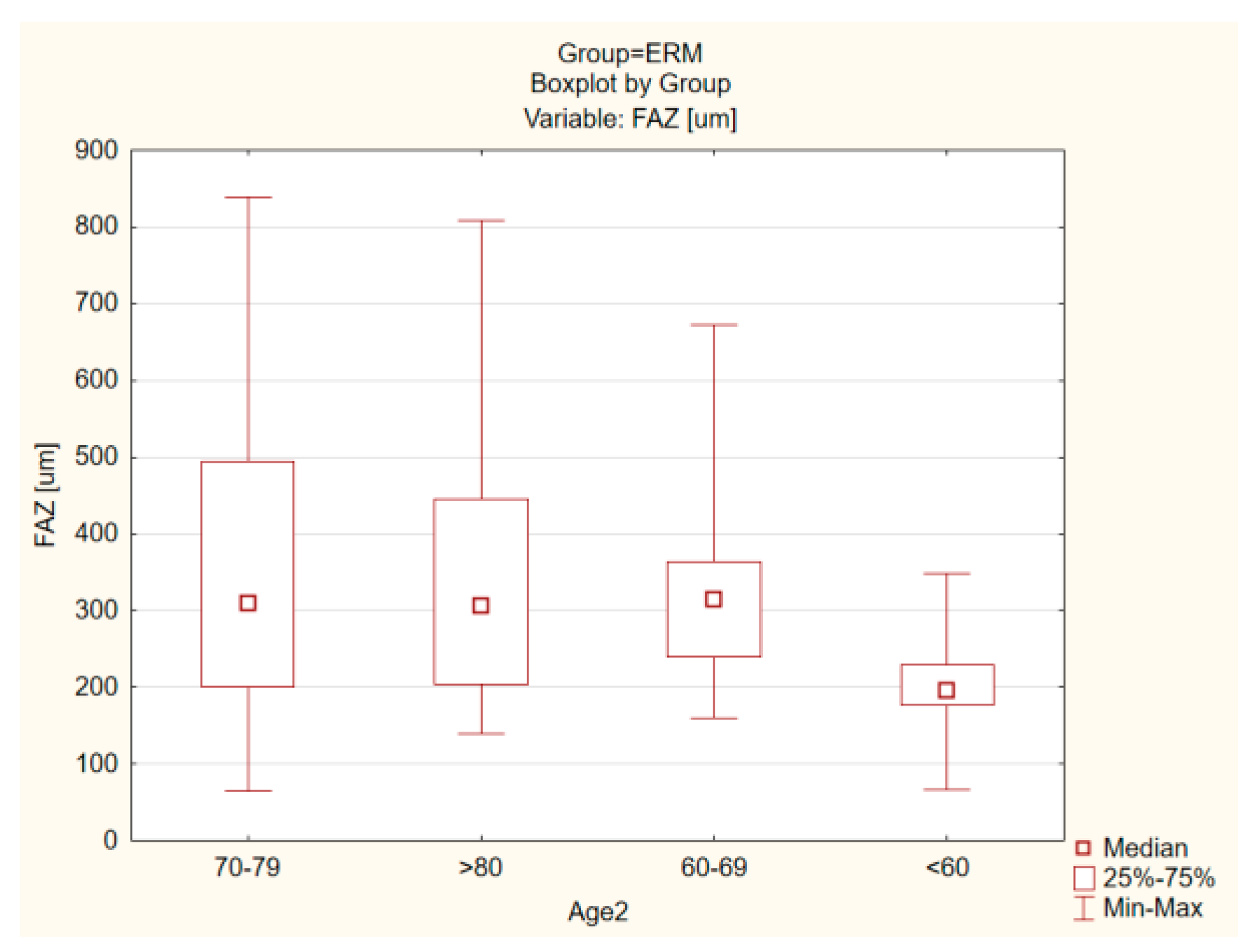
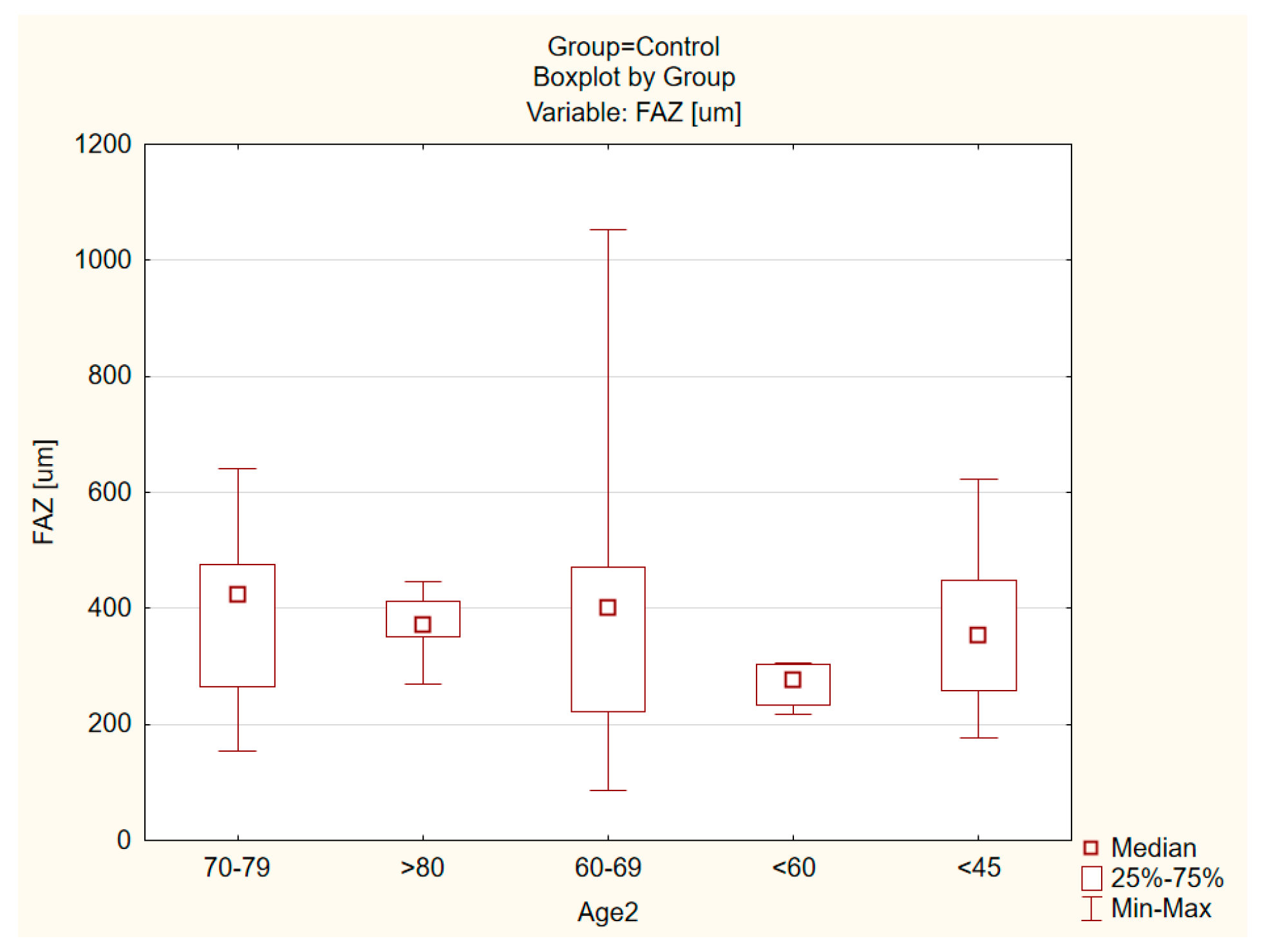
| ERM Group | Control Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean ± Standard Deviation | IC 95 | Median | Minimum | Maximum | p * | Mean ± Standard Deviation | IC95 | Median | Minimum | Maximum | p * |
| Foveal avascular zone (FAZ) (um) | 329. 57 ± 171.14 | 373.78 | 302.73 | 64.39 | 838.62 | 0.001 | 378.67 ± 171.23 | 426.8387 | 364.1510 | 86.5740 | 1053.428 | <0.001 |
| Foveal thickness (um) | 265.64 ± 72.96 | 282.21 | 260.00 | 104.00 | 432.00 | 0.35 | 212.08 ± 38.25 | 221.88 | 200.00 | 159.00 | 325.00 | 0.0002 |
| Averange macular thickness (um) | 282.99 ± 33.62 | 290.62 | 281.30 | 127.90 | 397.60 | 0.631 | 268.94 ± 13.77 | 272.47 | 269.90 | 226.90 | 307.20 | 0.12 |
| Average retinal nerve fiber layer (RNFL) (um) | 96.91 ± 17.29 | 100.95 | 99.00 | 42.00 | 128.00 | 0.0011 | 96.09 ± 17.07 | 100.47 | 98.00 | 43.00 | 126.00 | 0.0005 |
| Superior quadrant RNFL thickness (um) | 114.50 ± 28.87 | 121.24 | 118.00 | 40.00 | 177.00 | 0.0003 | 111.09 ± 22.67 | 116.90 | 113.00 | 46.00 | 148.00 | 0.007 |
| Inferior quadrant RNFL Thickness (um) | 116.35 ± 28.5489 | 123.01 | 121.00 | 23.00 | 155.00 | <0.001 | 122.75 ± 29.05 | 130.19 | 125.00 | 34.00 | 193.00 | 0.006 |
| ERM Group | Control Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean ± Standard Deviation | IC 95 | Median | Minimum | Maximum | p * | Mean ± Standard Deviation | IC95 | Median | Minimum | Maximum | p * | p ** |
| Vessels area | 102,783.3 ± 16,912.49 | 107,312.5 | 103,929.0 | 54,550.0 | 134,812.0 | 0.36 | 84,591.0 ± 26,185.56 | 91,881.1 | 89,033.0 | 29,643.0 | 127,731.0 | 0.09 | 0.0017 |
| Vessels percentage area | 40.1 ± 6.50 | 41.9 | 40.2 | 26.6 | 57.8 | 0.75 | 37.8 ± 8.58 | 40.2 | 38.8 | 20.8 | 59.9 | 0.86 | 0.0444 |
| Total number of junctions | 1079.0 ± 249.13 | 1145.8 | 1074.5 | 557.0 | 1722.0 | 0.91 | 847.0 ± 315.09 | 934.7 | 829.5 | 288.0 | 1533.0 | 0.13 | <0.0001 |
| Junctions den sity | 0.004 ± 0.001 | 0.004 | 0.004 | 0.002 | 0.007 | 0.76 | 0.004 ± 0.001 | 0.004 | 0.004 | 0.001 | 0.007 | 0.57 | 0.577 |
| Total vessels length | 21,547.6 ± 2889.62 | 22,321.4 | 21,715.0 | 10,635.7 | 27,502.0 | 0.014 | 17,974.3 ± 5166.66 | 19,412.7 | 19,328.4 | 7303.5 | 25,800.3 | 0.003 | 0.0002 |
| Total number of end points | 1088.1 ± 215.22 | 1145.7 | 1100.0 | 248.0 | 1472.0 | 0.0022 | 959.0 ± 355.29 | 1057.9 | 1039.5 | 209.0 | 1623.0 | 0.004 | 0.074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulfik-Dembska, K.; Teper, S.; Dembski, M.; Nowińska, A.; Wylęgała, E. Idiopathic Epiretinal Membrane: Microvasculature Analysis with Optical Coherence Tomography and Optical Coherence Tomography Angiography. Tomography 2022, 8, 189-199. https://doi.org/10.3390/tomography8010016
Ulfik-Dembska K, Teper S, Dembski M, Nowińska A, Wylęgała E. Idiopathic Epiretinal Membrane: Microvasculature Analysis with Optical Coherence Tomography and Optical Coherence Tomography Angiography. Tomography. 2022; 8(1):189-199. https://doi.org/10.3390/tomography8010016
Chicago/Turabian StyleUlfik-Dembska, Klaudia, Sławomir Teper, Michał Dembski, Anna Nowińska, and Edward Wylęgała. 2022. "Idiopathic Epiretinal Membrane: Microvasculature Analysis with Optical Coherence Tomography and Optical Coherence Tomography Angiography" Tomography 8, no. 1: 189-199. https://doi.org/10.3390/tomography8010016
APA StyleUlfik-Dembska, K., Teper, S., Dembski, M., Nowińska, A., & Wylęgała, E. (2022). Idiopathic Epiretinal Membrane: Microvasculature Analysis with Optical Coherence Tomography and Optical Coherence Tomography Angiography. Tomography, 8(1), 189-199. https://doi.org/10.3390/tomography8010016







