Systemic Artery to Pulmonary Artery Shunt Mimicking Acute Pulmonary Embolism, Unmasked by a Multimodality Imaging Approach
Abstract
:1. Introduction
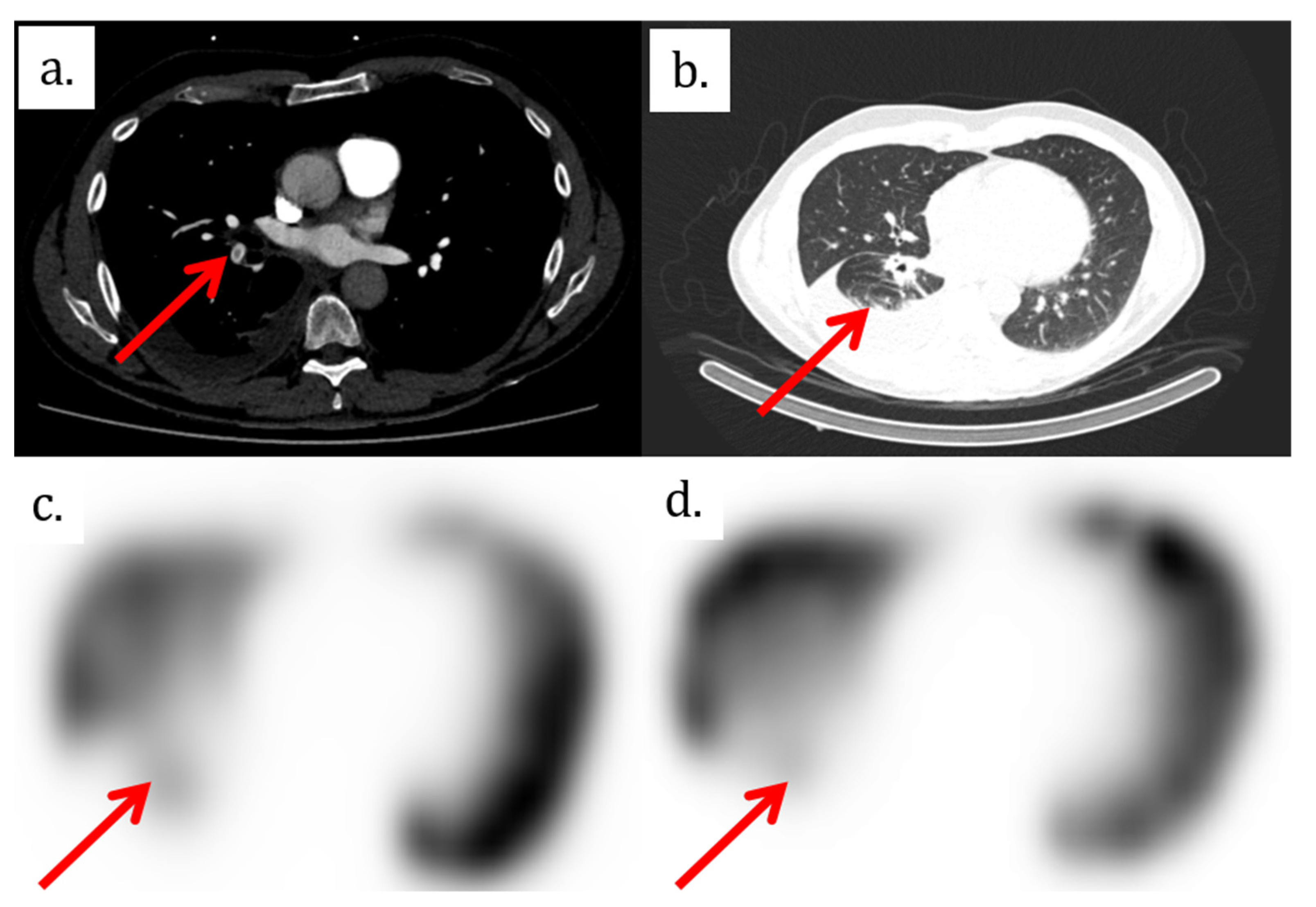
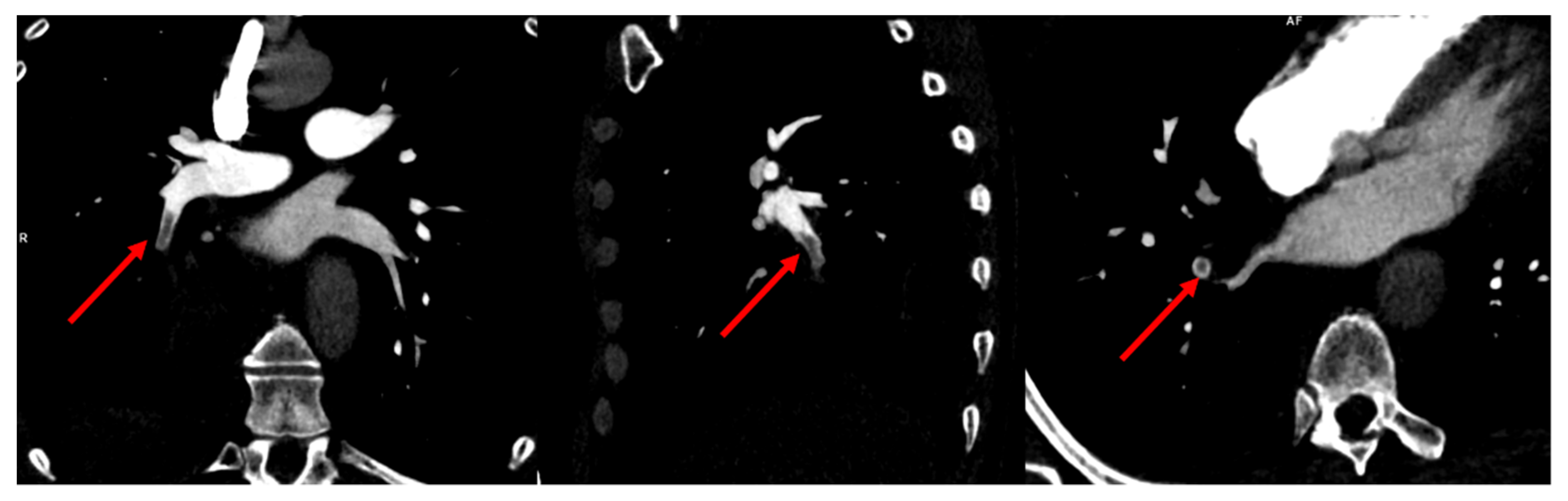
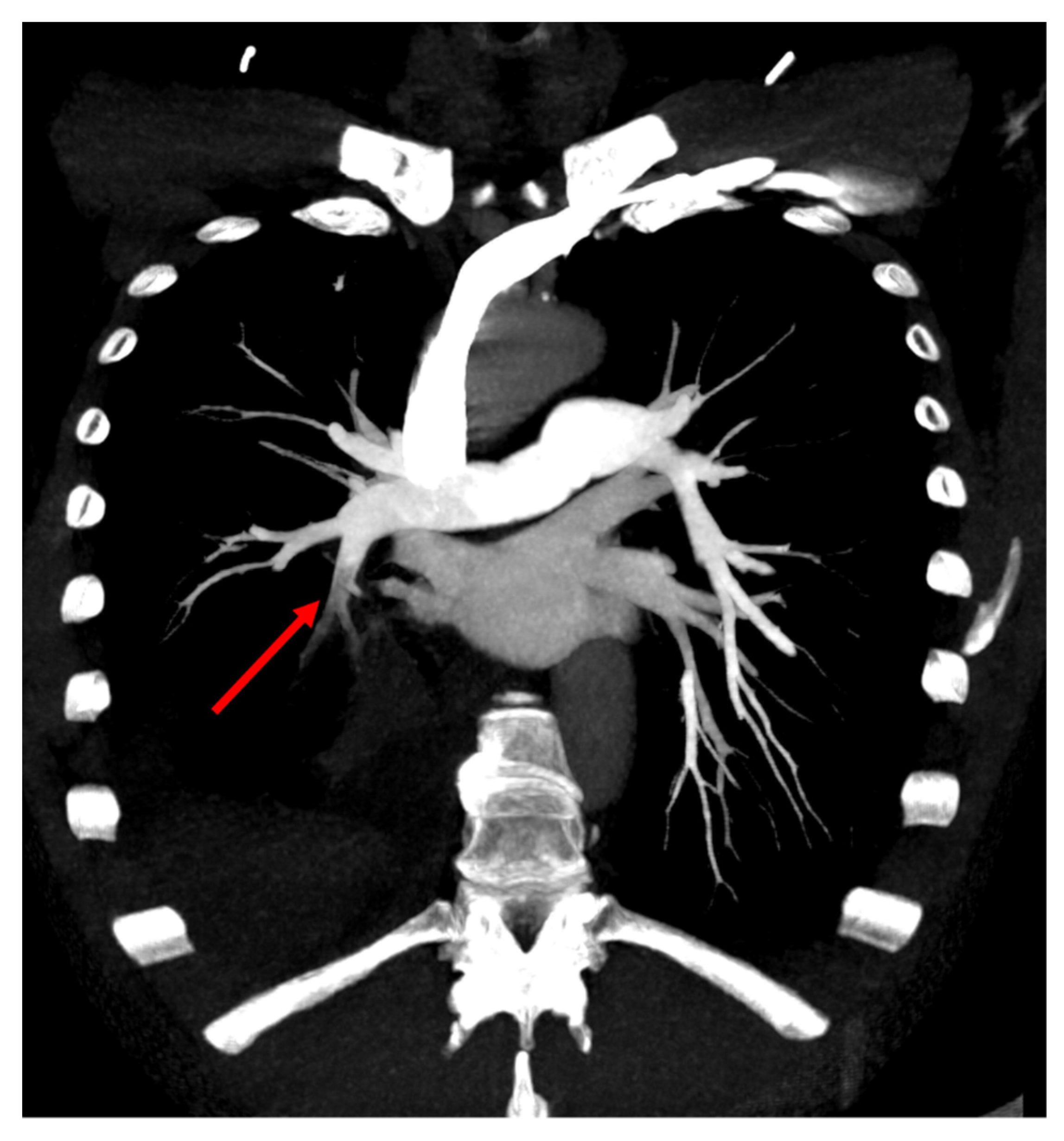
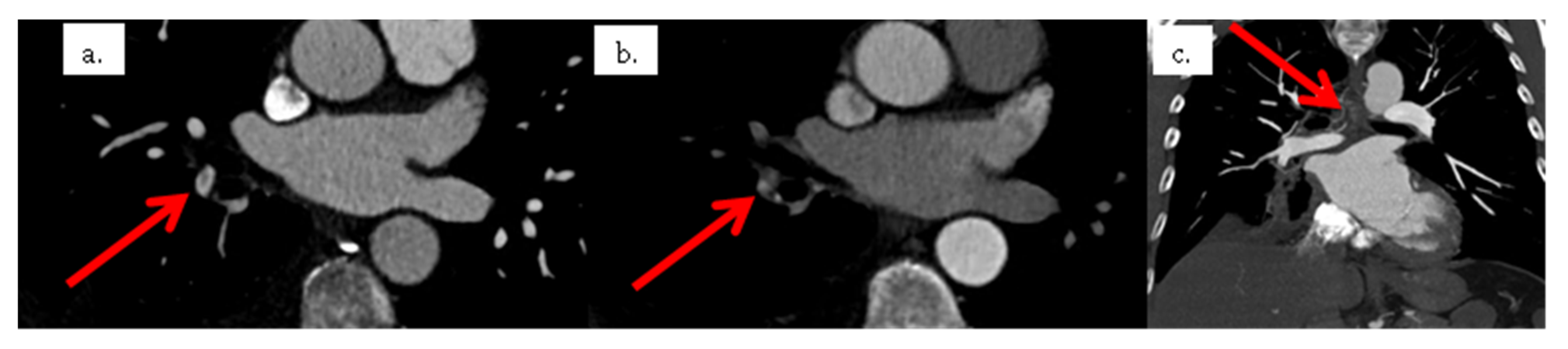
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dissaux, B.; Le Floch, P.Y.; Robin, P.; Bourhis, D.; Couturaud, F.; Salaun, P.Y.; Nonent, M.; Le Roux, P.Y. Pulmonary perfusion by iodine subtraction maps CT angiography in acute pulmonary embolism: Comparison with pulmonary perfusion SPECT (PASEP trial). Eur. Radiol. 2020, 30, 4857–4864. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.Y.; Robin, P.; Tromeur, C.; Davis, A.; Robert-Ebadi, H.; Carrier, M.; Le Gal, G.; Salaun, P.Y. Ventilation/perfusion SPECT for the diagnosis of pulmonary embolism: A systematic review. J. Thromb. Haemost. 2020, 18, 2910–2920. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.Y.; Robin, P.; Delluc, A.; Abgral, R.; Le Duc-Pennec, A.; Nowak, E.; Couturaud, F.; Le Gal, G.; Salaun, P.Y. V/Q SPECT interpretation for pulmonary embolism diagnosis: Which criteria to use? J. Nucl. Med. 2013, 54, 1077–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, A.; Bosco, J.; Lim, T.C.; Gaikwad, V.; Chung, R. Systemic-pulmonary artery shunt: A rare cause of false-positive filling defect in the pulmonary arteries. J. Med. Imaging Radiat. Oncol. 2017, 61, 82–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari-Gilani, K.; Gilkeson, R.C.; Hsiao, E.M.; Rajiah, P. Unusual Pulmonary Arterial Filling Defect caused by Systemic to Pulmonary Shunt in the Setting of Chronic Lung Disease Demonstrated by Dynamic 4D CTA. J. Radiol. Case Rep. 2015, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Do, K.H.; Goo, J.M.; Im, J.G.; Kim, K.W.; Chung, J.W.; Park, J.H. Systemic arterial supply to the lungs in adults: Spiral CT findings. Radiographics 2001, 21, 387–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacout, A.; El Hajjam, M.; Khalil, A.; Lacombe, P.; Marcy, P.Y. Retrograde systemic to pulmonary shunt simulating a pulmonary embolism. Diagn. Interv. Imaging 2013, 94, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, P.D.; Fowler, S.E.; Goodman, L.R.; Gottschalk, A.; Hales, C.A.; Hull, R.D.; Leeper, K.V., Jr.; Popovich, J., Jr.; Quinn, D.A.; Sos, T.A.; et al. Multidetector computed tomography for acute pulmonary embolism. N. Engl. J. Med. 2006, 354, 2317–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivali, N.; Yi, E.S.; Ryu, J.H. Pulmonary artery sarcoma mimicking pulmonary embolism: A case series. QJM 2017, 110, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.Y.; Robin, P.; Salaun, P.Y. New developments and future challenges of nuclear medicine and molecular imaging for pulmonary embolism. Thromb. Res. 2018, 163, 236–241. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dissaux, B.; Le Floch, P.-Y.; Le Pennec, R.; Tromeur, C.; Le Roux, P.-Y. Systemic Artery to Pulmonary Artery Shunt Mimicking Acute Pulmonary Embolism, Unmasked by a Multimodality Imaging Approach. Tomography 2022, 8, 175-179. https://doi.org/10.3390/tomography8010014
Dissaux B, Le Floch P-Y, Le Pennec R, Tromeur C, Le Roux P-Y. Systemic Artery to Pulmonary Artery Shunt Mimicking Acute Pulmonary Embolism, Unmasked by a Multimodality Imaging Approach. Tomography. 2022; 8(1):175-179. https://doi.org/10.3390/tomography8010014
Chicago/Turabian StyleDissaux, Brieg, Pierre-Yves Le Floch, Romain Le Pennec, Cécile Tromeur, and Pierre-Yves Le Roux. 2022. "Systemic Artery to Pulmonary Artery Shunt Mimicking Acute Pulmonary Embolism, Unmasked by a Multimodality Imaging Approach" Tomography 8, no. 1: 175-179. https://doi.org/10.3390/tomography8010014
APA StyleDissaux, B., Le Floch, P.-Y., Le Pennec, R., Tromeur, C., & Le Roux, P.-Y. (2022). Systemic Artery to Pulmonary Artery Shunt Mimicking Acute Pulmonary Embolism, Unmasked by a Multimodality Imaging Approach. Tomography, 8(1), 175-179. https://doi.org/10.3390/tomography8010014




