Qualitative Heterogeneous Signal Drop on Chemical Shift (CS) MR Imaging: Correlative Quantitative Analysis between CS Signal Intensity Index and Contrast Washout Parameters Using T1-Weighted Sequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Imaging Protocol
2.3. Image Analysis
- The percentage of signal intensity reduction on CS sequence was estimated applying the formula of the adrenal signal intensity index (ASII) [15]:
- 2.
- The percentages of absolute and relative wash-out of AL at 5 and 10 min on DCE images using the following formulas already reported in a previous study [13]:
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Lesion Size Analysis
3.3. CS and DCE Quantitative Analysis
3.4. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Adam, S.Z.; Nikolaidis, P.; Horowitz, J.M.; Gabriel, H.; Hammond, N.A.; Patel, T.; Yaghmai, V.; Miller, F.H. Chemical Shift MR Imaging of the Adrenal Gland: Principles, Pitfalls, and Applications. RadioGraphics 2016, 36, 414–432. [Google Scholar] [CrossRef] [PubMed]
- Maurea, S.; Imbriaco, M.; D’Angelillo, M.; Mollica, C.; Camera, L.; Salvatore, M. Diagnostic accuracy of chemical-shift MR imaging to differentiate between adrenal adenomas and non adenoma adrenal lesions. Radiol. Med. 2006, 111, 674–686. [Google Scholar] [CrossRef][Green Version]
- Platzek, I.; Sieron, D.; Plodeck, V.; Borkowetz, A.; Laniado, M.; Hoffmann, R.-T. Chemical shift imaging for evaluation of adrenal masses: A systematic review and meta-analysis. Eur. Radiol. 2019, 29, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Davenport, M.S.; Pedrosa, I.; Shinagare, A.; Chandarana, H.; Curci, N.; Doshi, A.; Israel, G.; Remer, E.; Wang, J.; et al. Renal and adrenal masses containing fat at MRI: Proposed nomenclature by the society of abdominal radiology disease-focused panel on renal cell carcinoma. J. Magn. Reson. Imaging 2019, 49, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, H.; Pizzitola, V.; McComb, E.N.; Wiley, E.; Miller, F.H. Adrenal lesions with heterogeneous suppression on chemical shift imaging: Clinical implications. J. Magn. Reson. Imaging 2004, 19, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Al Dandan, O.; Kielar, A.Z.; Flood, T.A.; McInnes, M.D.F.; Siegelman, E.S. Pitfalls of adrenal imaging with chemical shift MRI. Clin. Radiol. 2014, 69, 1186–1197. [Google Scholar] [CrossRef]
- Ecénarro-Montiel, A.; Baleato-González, S.; Santiago-Pérez, M.I.; Sánchez-González, J.; Montesinos, P.; García-Figueiras, R. Valoración mediante Dixon modificado de las lesiones suprarrenales incidentales en RM 3T. Radiologia 2018, 60, 485–492. [Google Scholar] [CrossRef]
- Tu, W.; Abreu-Gomez, J.; Udare, A.; Alrashed, A.; Schieda, N. Utility of T2-weighted MRI to Differentiate Adrenal Metastases from Lipid-Poor Adrenal Adenomas. Radiol. Imaging Cancer 2020, 2, e200011. [Google Scholar] [CrossRef]
- Tu, W.; Gerson, R.; Abreu-Gomez, J.; Udare, A.; Mcphedran, R.; Schieda, N. Comparison of MRI features in lipid-rich and lipid-poor adrenal adenomas using subjective and quantitative analysis. Abdom. Radiol. 2021, 46, 4864–4872. [Google Scholar] [CrossRef]
- Inan, N.; Arslan, A.; Akansel, G.; Anik, Y.; Balci, N.C.; Demirci, A. Dynamic contrast enhanced MRI in the differential diagnosis of adrenal adenomas and malignant adrenal masses. Eur. J. Radiol. 2008, 65, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Becker-Weidman, D.; Kalb, B.; Mittal, P.K.; Harri, P.A.; Arif-Tiwari, H.; Farris, A.B.; Chen, Z.; Sungjin, K.; Martin, D.R. Differentiation of lipid-poor adrenal adenomas from non-adenomas with magnetic resonance imaging: Utility of dynamic, contrast enhancement and single-shot T2-weighted sequences. Eur. J. Radiol. 2015, 84, 2045–2051. [Google Scholar] [CrossRef]
- Rodacki, K.; Ramalho, M.; Dale, B.M.; Battisti, S.; de Campos, R.O.P.; Giardino, A.; Semelka, R.C. Combined Chemical Shift Imaging With Early Dynamic Serial Gadolinium-Enhanced MRI in the Characterization of Adrenal Lesions. Am. J. Roentgenol. 2014, 203, 99–106. [Google Scholar] [CrossRef]
- Romeo, V.; Maurea, S.; Guarino, S.; Mainenti, P.P.; Liuzzi, R.; Petretta, M.; Cozzolino, I.; Klain, M.; Brunetti, A. The role of dynamic post-contrast T1-w MRI sequence to characterize lipid-rich and lipid-poor adrenal adenomas in comparison to non-adenoma lesions: Preliminary results. Abdom. Radiol. 2018, 43, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Alrashed, A.; Flood, T.A.; Samji, K.; Shabana, W.; McInnes, M.D.F. Comparison of Quantitative MRI and CT Washout Analysis for Differentiation of Adrenal Pheochromocytoma From Adrenal Adenoma. Am. J. Roentgenol. 2016, 206, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Ream, J.M.; Gaing, B.; Mussi, T.C.; Rosenkrantz, A.B. Characterization of Adrenal Lesions at Chemical-Shift MRI: A Direct Intraindividual Comparison of In- and Opposed-Phase Imaging at 1.5 T and 3 T. Am. J. Roentgenol. 2015, 204, 536–541. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241. [Google Scholar] [CrossRef]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Efron, B. Better Bootstrap Confidence Intervals. J. Am. Stat. Assoc. 1987, 82, 171. [Google Scholar] [CrossRef]
- Kumagae, Y.; Fukukura, Y.; Takumi, K.; Shindo, T.; Tateyama, A.; Kamiyama, T.; Kamimura, K.; Nakajo, M. Distinguishing adrenal adenomas from non-adenomas on dynamic enhanced CT: A comparison of 5 and 10 min delays after intravenous contrast medium injection. Clin. Radiol. 2013, 68, 696–703. [Google Scholar] [CrossRef]
- Angelelli, G.; Mancini, M.E.; Moschetta, M.; Pedote, P.; Pignataro, P.; Scardapane, A. MDCT in the Differentiation of Adrenal Masses: Comparison between Different Scan Delays for the Evaluation of Intralesional Washout. Sci. World J. 2013, 2013, 957680. [Google Scholar] [CrossRef]
- Foti, G.; Malleo, G.; Faccioli, N.; Guerriero, A.; Furlani, L.; Carbognin, G. Characterization of adrenal lesions using MDCT wash-out parameters: Diagnostic accuracy of several combinations of intermediate and delayed phases. Radiol. Med. 2018, 123, 833–840. [Google Scholar] [CrossRef]
- Matos, A.P.; Semelka, R.C.; Herédia, V.; AlObaidiy, M.; Gomes, F.V.; Ramalho, M. Modified approach to the characterization of adrenal nodules using a standard abdominal magnetic resonance imaging protocol. Radiol. Bras. 2017, 50, 19–25. [Google Scholar] [CrossRef][Green Version]
- Slapa, R.Z.; Jakubowski, W.; Januszewicz, A.; Kasperlik-Zaluska, A.A.; Dabrowska, E.; Fijuth, J.; Feltynowski, T.; Tarnawski, R.; Królicki, L. Discriminatory power of MRI for differentiation of adrenal non-adenomas vs adenomas evaluated by means of ROC analysis: Can biopsy be obviated? Eur. Radiol. 2000, 10, 95–104. [Google Scholar] [CrossRef]
- Stanzione, A.; Cuocolo, R.; Verde, F.; Galatola, R.; Romeo, V.; Mainenti, P.P.; Aprea, G.; Guadagno, E.; Del Basso De Caro, M.; Maurea, S. Handcrafted MRI radiomics and machine learning: Classification of indeterminate solid adrenal lesions. Magn. Reson. Imaging 2021, 79, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Maurea, S.; Imbriaco, M.; Mollica, C.; Pace, L.; Salvatore, M. Quantitative imaging characterization of hypersecreting or nonhypersecreting adrenal adenomas. Nucl. Med. Commun. 2011, 32, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Maurea, S.; Cuocolo, R.; Petretta, M.; Mainenti, P.P.; Verde, F.; Coppola, M.; Dell’Aversana, S.; Brunetti, A. Characterization of Adrenal Lesions on Unenhanced MRI Using Texture Analysis: A Machine-Learning Approach. J. Magn. Reson. Imaging 2018, 48, 198–204. [Google Scholar] [CrossRef]
- Ho, L.M.; Samei, E.; Mazurowski, M.A.; Zheng, Y.; Allen, B.C.; Nelson, R.C.; Marin, D. Can Texture Analysis Be Used to Distinguish Benign From Malignant Adrenal Nodules on Unenhanced CT, Contrast-Enhanced CT, or In-Phase and Opposed-Phase MRI? Am. J. Roentgenol. 2019, 212, 554–561. [Google Scholar] [CrossRef]
- Umanodan, T.; Fukukura, Y.; Kumagae, Y.; Shindo, T.; Nakajo, M.; Takumi, K.; Nakajo, M.; Hakamada, H.; Umanodan, A.; Yoshiura, T. ADC histogram analysis for adrenal tumor histogram analysis of apparent diffusion coefficient in differentiating adrenal adenoma from pheochromocytoma. J. Magn. Reson. Imaging 2017, 45, 1195–1203. [Google Scholar] [CrossRef] [PubMed]

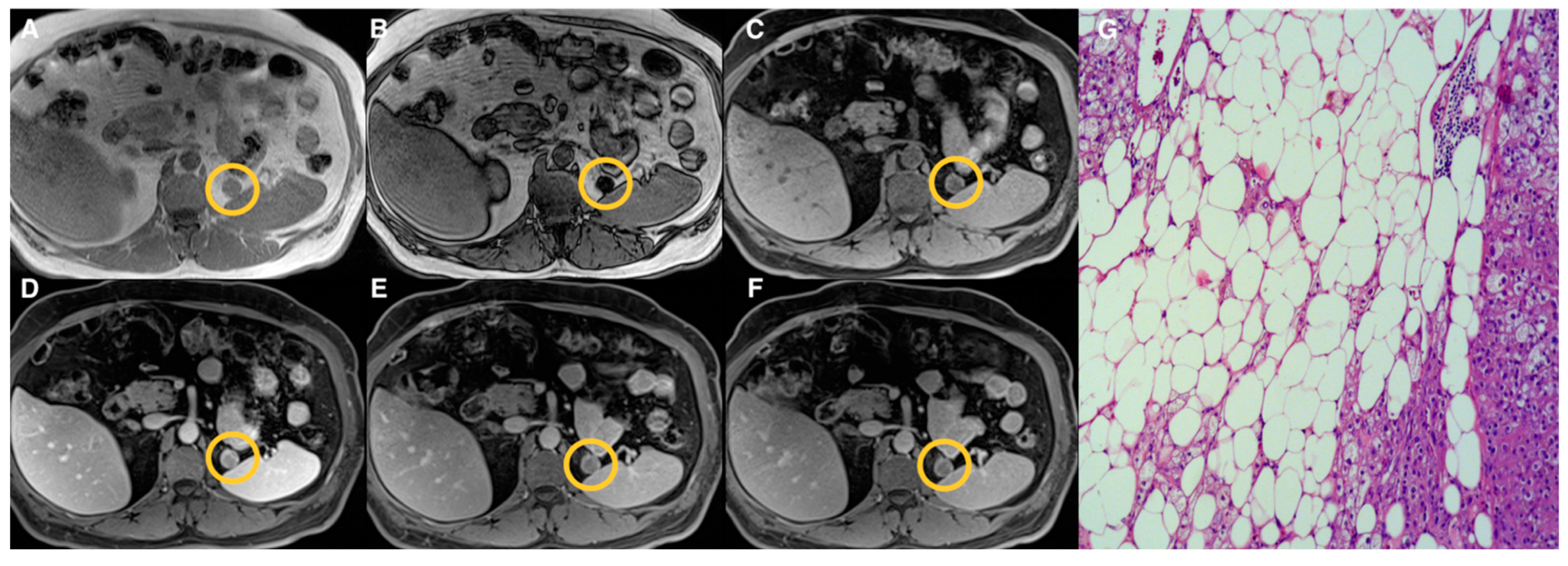
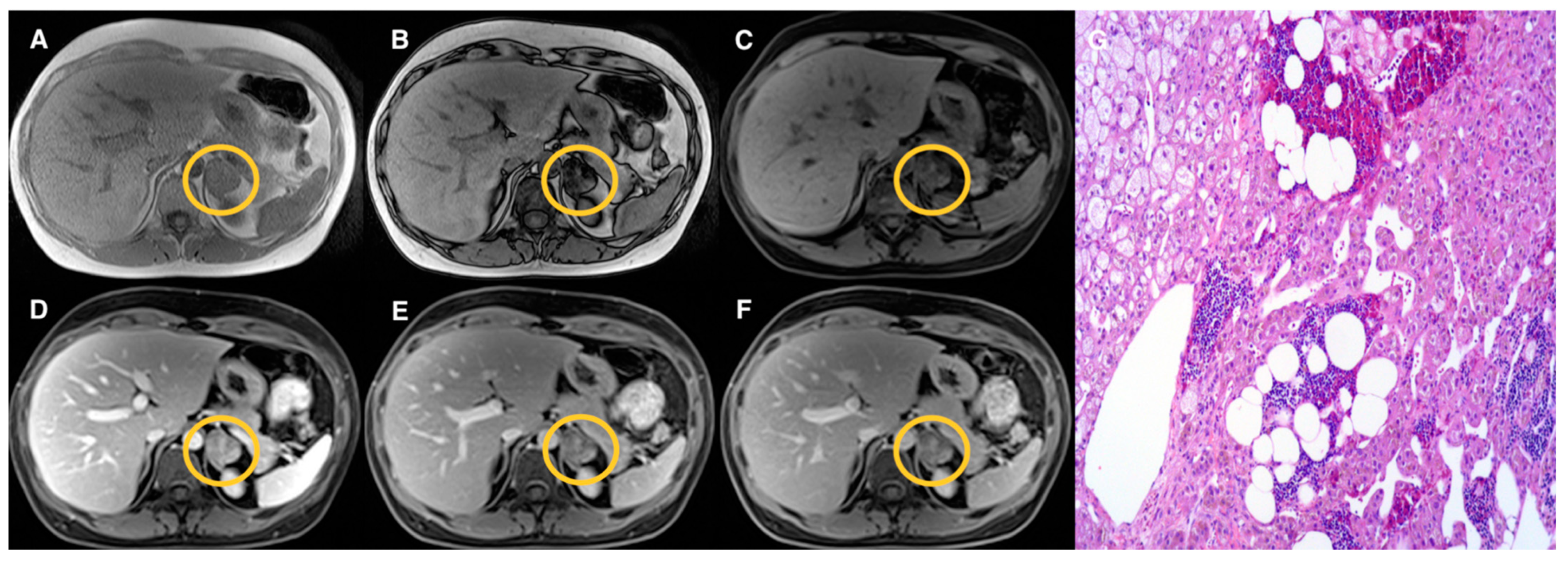
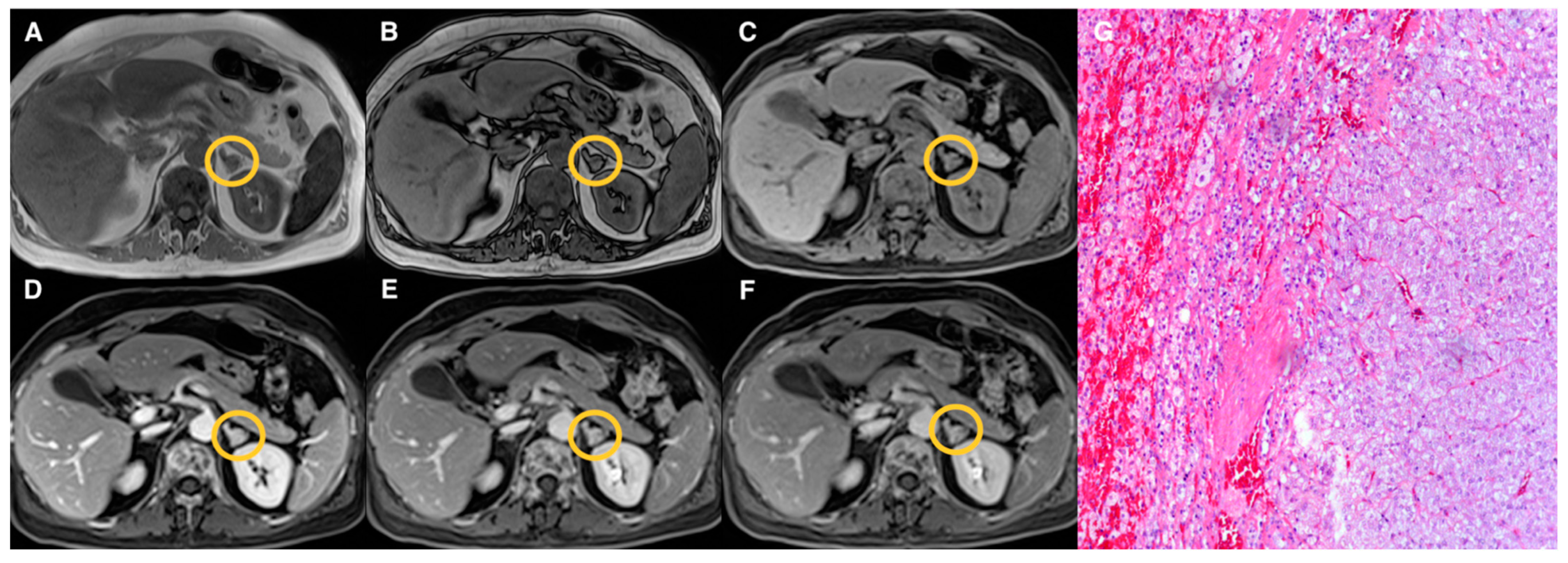

| Groups | Qualitative CS Signal Drop | Lesion Type | Total |
|---|---|---|---|
| 1 | Homogeneous | Adenomas (n = 19) | 19 |
| 2 | Heterogeneous | Adenomas (n = 17) Pheochromocytomas (n = 3) Myelolipomas (n = 3) | 23 |
| 3 | Absent | Adenomas (n = 10) Pheochromocytomas (n = 9) Primary malignant tumors (n = 5) Metastasis (n = 5) Oncocytoma (n = 1) | 30 |
| MRI Parameter | Group 1 Median (Range) | Group 2 Median (Range) | Group 3 Median (Range) |
|---|---|---|---|
| ASII | 71 # (46–87) | 53 # (22–76) | 3 # (−41–75) |
| AWO5min | 23 (−49–51) | 20 (−133–52) | 1 * (−259–45) |
| AWO10min | 54 (6–68) | 46 (−160–66) | 15 * (−372–70) |
| RWO5min | 17 (−30–36) | 12 (−37–35) | −1 * (−84–31) |
| RWO10min | 33 (4–48) | 29 (−13–43) | 5 * (−100–42) |
| MRI Parameter | Group 1 and Group 2 | Group 1 and Group 3 | Group 2 and Group 3 | |||
|---|---|---|---|---|---|---|
| Cut-off (95% CI) | AUC (95% CI) | Cut-off (95% CI) | AUC (95% CI) | Cut-off (95% CI) | AUC (95% CI) | |
| ASII | 60 (55–62) | 0.85 # (0.71–0.94) | 37 (32–37) | 0.96 * (0.87–1.00) | 20 (7–32) | 0.93 # (0.82–0.98) |
| AWO5min | 14 (−36–51) | 0.55 (0.38–0.70) | 9 (8–25) | 0.75 (0.60–0.86) | 9 (8–45) | 0.70 (0.56–0.82) |
| AWO10min | 38 (31–61) | 0.61 (0.44–0.75) | 36 (35–53) | 0.87 (0.74–0.95) | 36 (35–53) | 0.73 (0.59–0.84) |
| RWO5min | 12 (−23–23) | 0.54 (0.38–0.70) | 6 (3–21) | 0.78 (0.64–0.89) | 6 (2–24) | 0.73 (0.59–0.85) |
| RWO10min | 3 (−4–29) | 0.63 (0.47–0.78) | 23 (18–34) | 0.88 (0.75–0.95) | 27 (11–34) | 0.76 (0.62–0.87) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanzione, A.; Verde, F.; Galatola, R.; Romeo, V.; Liuzzi, R.; Mainenti, P.P.; Aprea, G.; Klain, M.; Guadagno, E.; Del Basso De Caro, M.; et al. Qualitative Heterogeneous Signal Drop on Chemical Shift (CS) MR Imaging: Correlative Quantitative Analysis between CS Signal Intensity Index and Contrast Washout Parameters Using T1-Weighted Sequences. Tomography 2021, 7, 961-971. https://doi.org/10.3390/tomography7040079
Stanzione A, Verde F, Galatola R, Romeo V, Liuzzi R, Mainenti PP, Aprea G, Klain M, Guadagno E, Del Basso De Caro M, et al. Qualitative Heterogeneous Signal Drop on Chemical Shift (CS) MR Imaging: Correlative Quantitative Analysis between CS Signal Intensity Index and Contrast Washout Parameters Using T1-Weighted Sequences. Tomography. 2021; 7(4):961-971. https://doi.org/10.3390/tomography7040079
Chicago/Turabian StyleStanzione, Arnaldo, Francesco Verde, Roberta Galatola, Valeria Romeo, Raffaele Liuzzi, Pier Paolo Mainenti, Giovanni Aprea, Michele Klain, Elia Guadagno, Marialaura Del Basso De Caro, and et al. 2021. "Qualitative Heterogeneous Signal Drop on Chemical Shift (CS) MR Imaging: Correlative Quantitative Analysis between CS Signal Intensity Index and Contrast Washout Parameters Using T1-Weighted Sequences" Tomography 7, no. 4: 961-971. https://doi.org/10.3390/tomography7040079
APA StyleStanzione, A., Verde, F., Galatola, R., Romeo, V., Liuzzi, R., Mainenti, P. P., Aprea, G., Klain, M., Guadagno, E., Del Basso De Caro, M., & Maurea, S. (2021). Qualitative Heterogeneous Signal Drop on Chemical Shift (CS) MR Imaging: Correlative Quantitative Analysis between CS Signal Intensity Index and Contrast Washout Parameters Using T1-Weighted Sequences. Tomography, 7(4), 961-971. https://doi.org/10.3390/tomography7040079









