Enamel Remineralization Competence of a Novel Fluoride-Incorporated Bioactive Glass Toothpaste—A Surface Micro-Hardness, Profilometric, and Micro-Computed Tomographic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Enamel Blocks
2.2. AS Preparation
2.3. Demineralization Procedure
2.4. Grouping of Enamel Blocks
2.5. Simulated Tooth Brushing Protocol
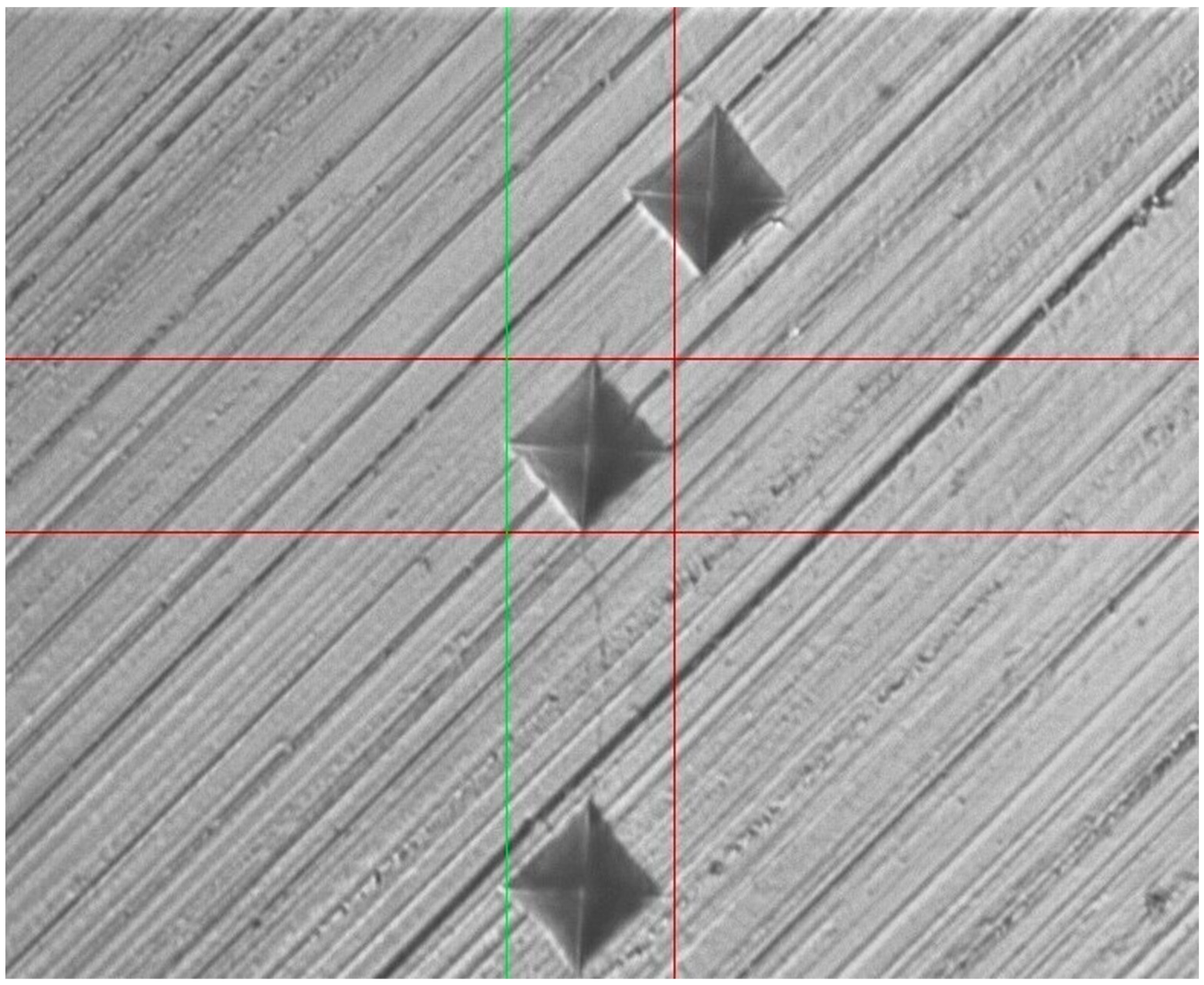
2.6. Surface Micro-Hardness Testing
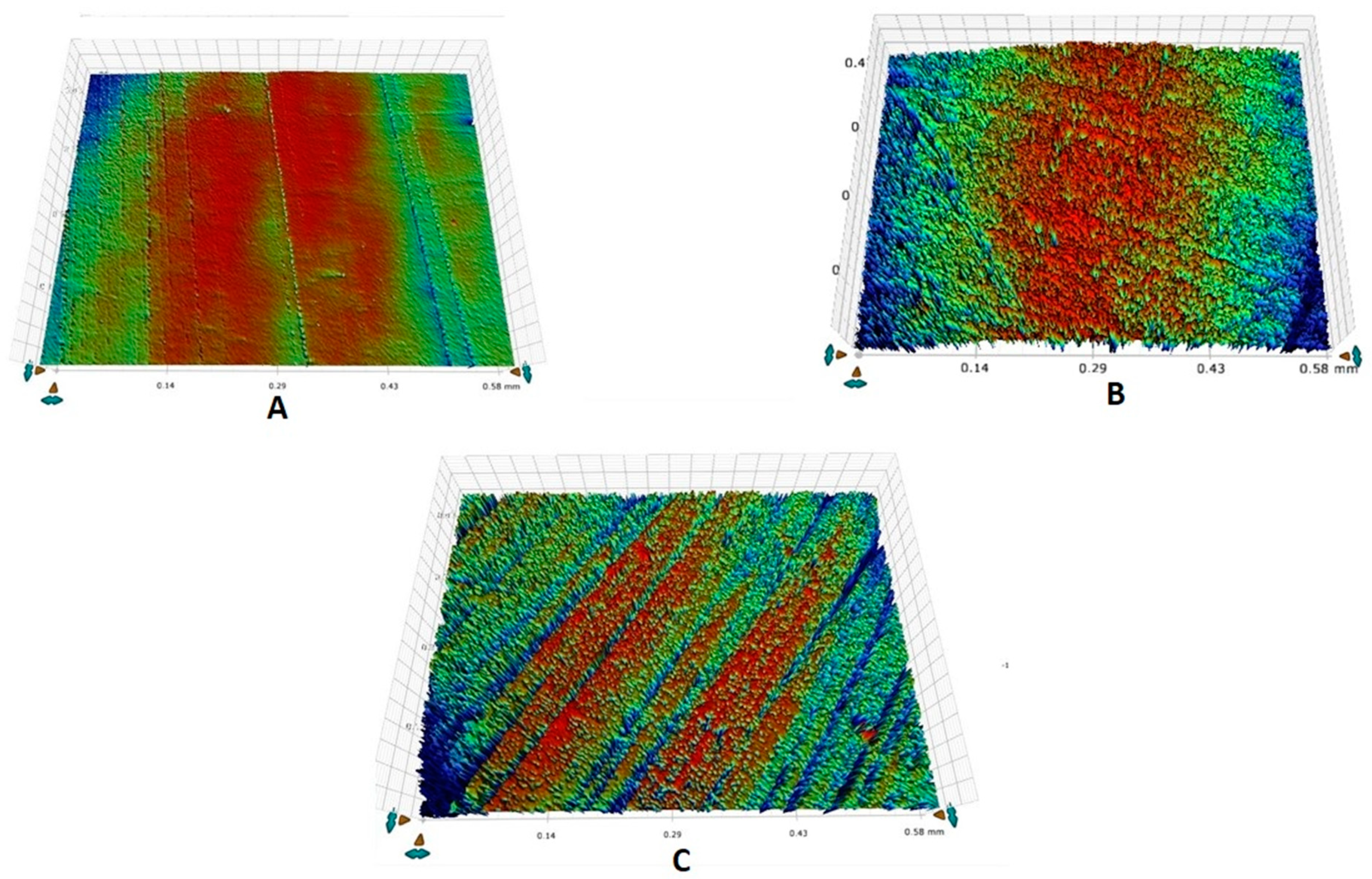
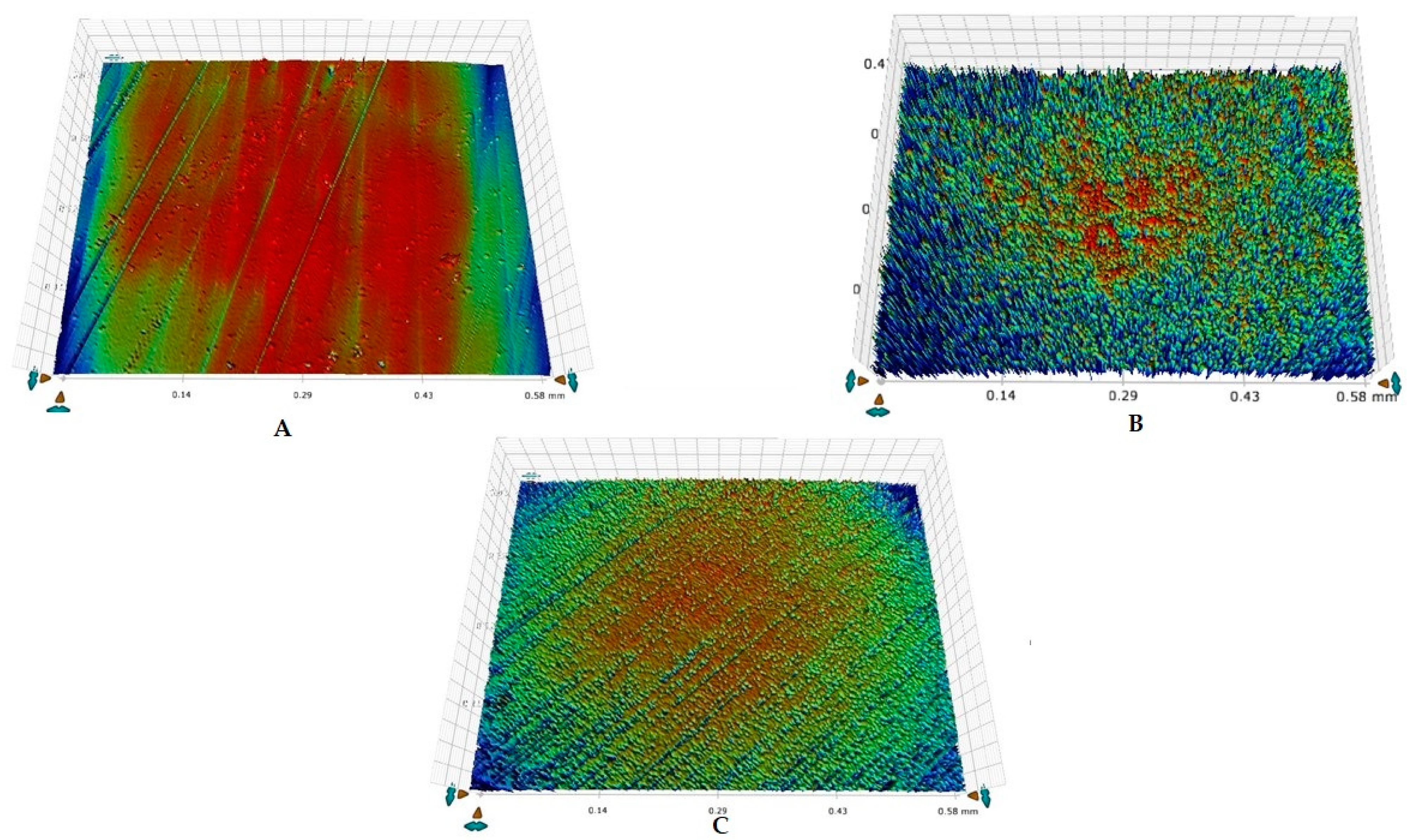
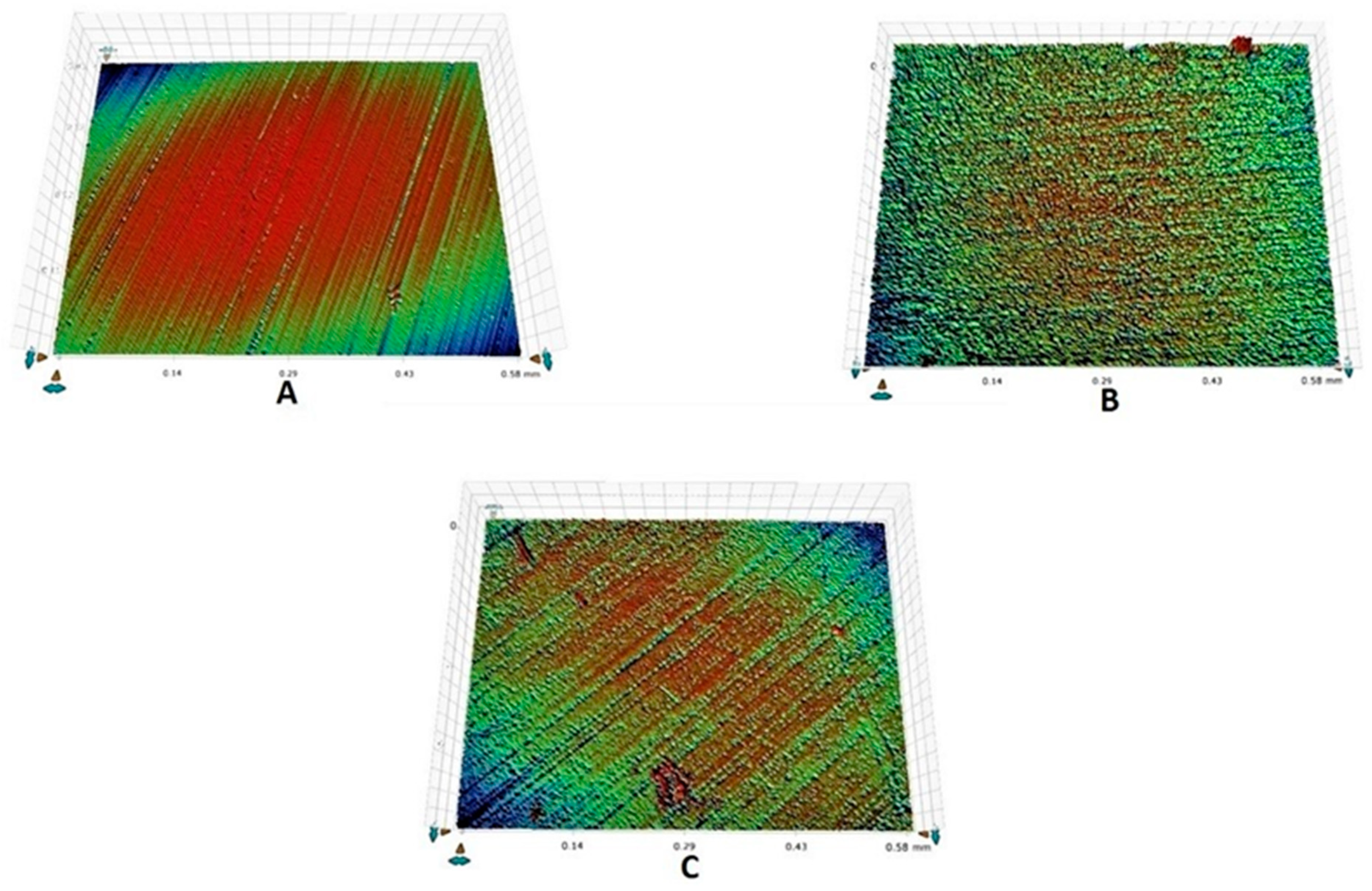
2.7. Surface Roughness Analysis
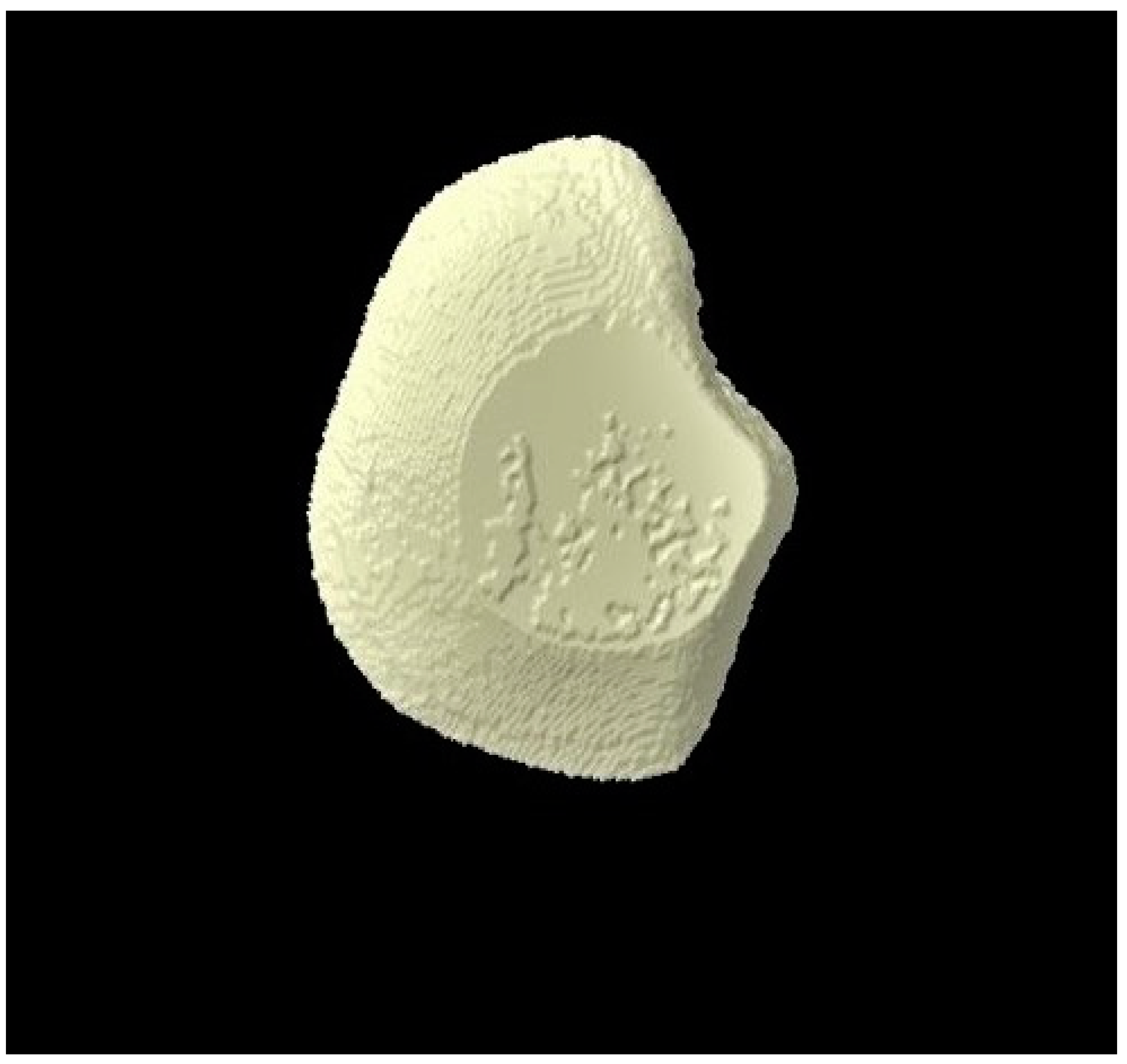
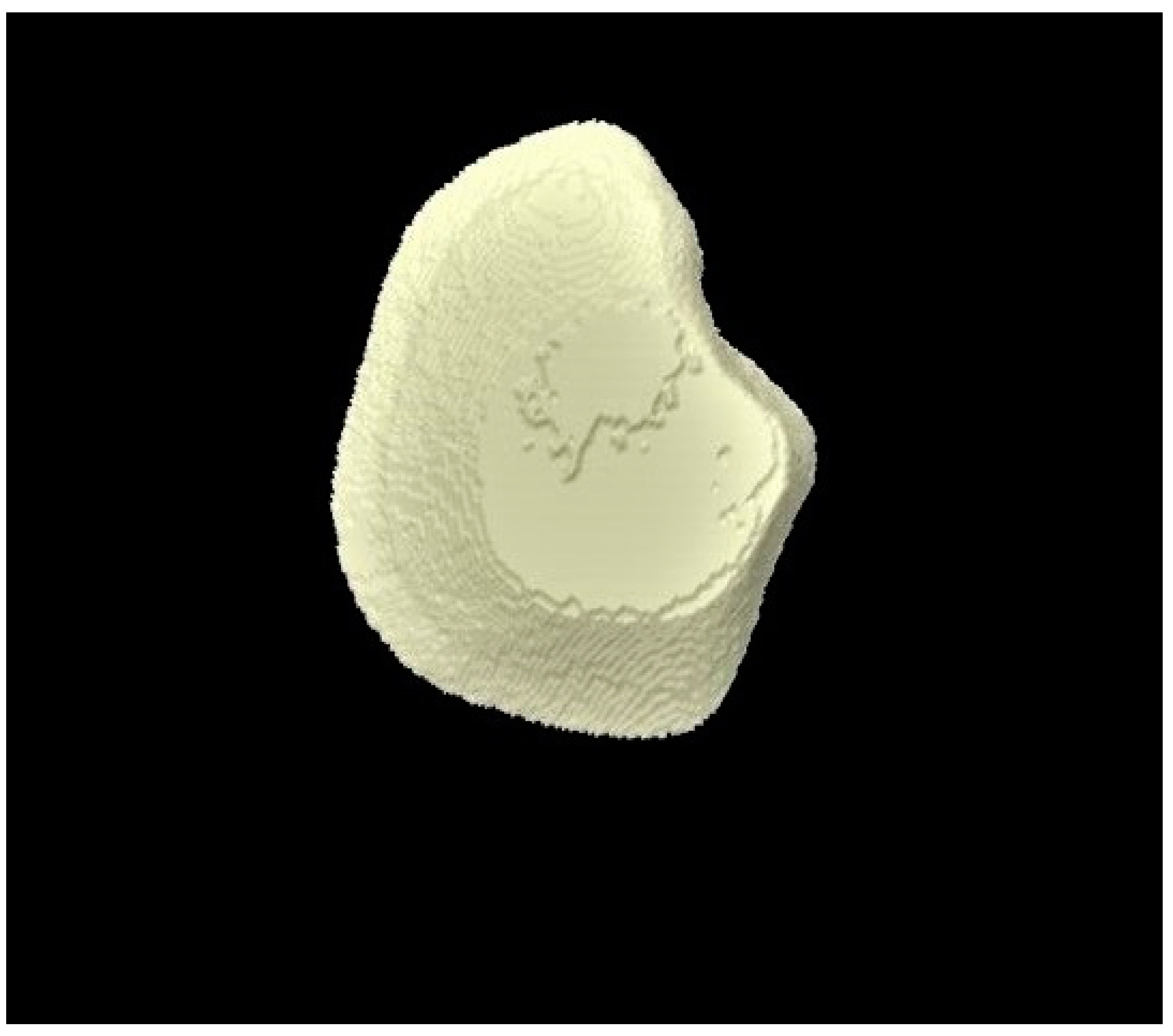
2.8. Micro-CT Investigation
2.9. Statistical Analysis
3. Results
3.1. Surface Micro-Hardness Outcomes
3.2. Surface Roughness Outcomes
3.3. Micro-CT Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Al-Qahtani, S.M.; Razak, P.A.; Khan, S.D. Knowledge and Practice of Preventive Measures for Oral Health Care among Male Intermediate Schoolchildren in Abha, Saudi Arabia. Int. J. Environ. Res. Public Health 2020, 17, 703. [Google Scholar] [CrossRef]
- Chen, R.; Santo, K.; Wong, G.; Sohn, W.; Spallek, H.; Chow, C.; Irving, M. Mobile Apps for Dental Caries Prevention: Systematic Search and Quality Evaluation. JMIR Mhealth Uhealth 2021, 9, e19958. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, D.; Varghese, A.; Prabath, S.; Sreeram, R.; Asha, J.; Ravi, A.B. Prevalence of Dental Caries in Adult South Indian Population in Association with Dietary Pattern: A Comparative Study. J. Pharm. Bioallied Sci. 2020, 12, S546–S549. [Google Scholar] [CrossRef]
- Maheswari, S.U.; Raja, J.; Kumar, A.; Seelan, R.G. Caries management by risk assessment: A review on current strategies for caries prevention and management. J. Pharm. Bioallied Sci. 2015, 7, S320–S324. [Google Scholar]
- Raskin, S.E.; Tranby, E.P.; Ludwig, S.; Okunev, I.; Frantsve-Hawley, J.; Boynes, S. Survival of silver diamine fluoride among patients treated in community dental clinics: A naturalistic study. BMC Oral Health 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Horst, J.A.; Tanzer, J.M.; Milgrom, P.M. Fluorides and Other Preventive Strategies for Tooth Decay. Dent. Clin. North Am. 2018, 62, 207–234. [Google Scholar] [CrossRef]
- Aoun, A.; Darwiche, F.; Al Hayek, S.; Doumit, J. The Fluoride Debate: The Pros and Cons of Fluoridation. Prev. Nutr. Food Sci. 2018, 23, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.; Jeroncic, A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [PubMed]
- Slayton, R.L.; Urquhart, O.; Araujo, M.W.B.; Fontana, M.; Guzman-Armstrong, S.; Nascimento, M.M.; Novy, B.B.; Tinanoff, N.; Weyant, R.J.; Wolff, M.S.; et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J. Am. Dent. Assoc. 2018, 149, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, J.; Hannig, M.; Winkel, P.; Basche, S.; Leis, B.; Putz, N.; Kensche, A.; Hannig, C. Influence of pure fluorides and stannous ions on the initial bacterial colonization in situ. Sci. Rep. 2019, 9, 18499. [Google Scholar] [CrossRef]
- Mocquot, C.; Attik, N.; Pradelle-Plasse, N.; Grosgogeat, B.; Colon, P. Bioactivity assessment of bioactive glasses for dental applications: A critical review. Dent. Mater. 2020, 36, 1116–1143. [Google Scholar] [CrossRef]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Verne, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef]
- Earl, J.S.; Leary, R.K.; Muller, K.H.; Langford, R.M.; Greenspan, D.C. Physical and chemical characterization of dentin surface following treatment with NovaMin technology. J. Clin. Dent. 2011, 22, 62–67. [Google Scholar] [PubMed]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Mechanisms of Bioactive Glass on Caries Management: A Review. Materials 2019, 12, 4183. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Farooq, I.; Iqbal, K. A review of the effect of various ions on the properties and the clinical applications of novel bioactive glasses in medicine and dentistry. Saudi. Dent. J. 2014, 26, 1–5. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Da Cruz, L.P.D.; Hill, R.G.; Chen, X.; Gillam, D.G. Dentine Tubule Occlusion by Novel Bioactive Glass-Based Toothpastes. Int. J. Dent. 2018, 2018, 5701638. [Google Scholar] [CrossRef]
- Patel, V.R.; Shettar, L.; Thakur, S.; Gillam, D.; Kamala, D.N. A randomised clinical trial on the efficacy of 5% fluorocalcium phosphosilicate-containing novel bioactive glass toothpaste. J. Oral Rehabil. 2019, 46, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, V.; Neamtu, B.; Picu, O.; Stefanache, M.A.M.; Cioranu, V.S.I. The comparitive evaluation of salivary biomarkers (calcium, phosphate, salivary pH) in the cario-resistance versus cario-activity. Rev. Chim. (Bucharest) 2016, 68, 821–824. [Google Scholar]
- Bijle, M.N.; Ekambaram, M.; Lo, E.C.; Yiu, C.K.Y. The enamel remineralization potential of fluoride varnishes containing arginine. J. Dent. 2020, 99, 103411. [Google Scholar] [CrossRef]
- Fusayama, T.; Katayori, T.; Nomoto, S. Corrosion of Gold and Amalgam Placed in Contact with Each Other. J. Dent. Res. 1963, 42, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Rehder Neto, F.C.; Maeda, F.A.; Turssi, C.P.; Serra, M.C. Potential agents to control enamel caries-like lesions. J. Dent. 2009, 37, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Molaasadolah, F.; Eskandarion, S.; Ehsani, A.; Sanginan, M. In Vitro Evaluation of Enamel Microhardness after Application of Two Types of Fluoride Varnish. J. Clin. Diagn. Res. 2017, 11, ZC64–ZC66. [Google Scholar] [CrossRef]
- Srivastava, S.K.P.; Choudhary, E. Assessment of Enamel Remineralising Capability of Three Different Remineralising Agents Using Vicker’s Surface Microhardness Test. Int. J. Pharm. Bio. Sci. 2019, 10, 151–157. [Google Scholar] [CrossRef]
- Mohapatra, S.K.P.; Arumugham, I.M.; Sakthi, D.S.; Prabakar, J. Assessment of Microhardness of Enamel Carious Like Lesions After Treatment with Nova Min, Bio Min and Remin Pro Containing Toothpastes: An in Vitro Study. Ind. J. Public Health Res. Dev. 2018, 10, 375. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, I.; Al-Thobity, A.M.; Al-Khalifa, K.S.; Alhooshani, K.; Sauro, S. An in-vitro evaluation of fluoride content and enamel remineralization potential of two toothpastes containing different bioactive glasses. Biomed. Mater. Eng. 2020, 30, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.A.; Staiger, M.; Kouji, O.; Modric, J.; Pierchalla, T.; Rybka, M.; Hill, R.G.; Arnold, W.H. Randomized investigation of the bioavailability of fluoride in saliva after administration of sodium fluoride, amine fluoride and fluoride containing bioactive glass dentifrices. BMC Oral Health 2019, 19, 119. [Google Scholar] [CrossRef]
- Lynch, E.; Brauer, D.S.; Karpukhina, N.; Gillam, D.G.; Hill, R.G. Multi-component bioactive glasses of varying fluoride content for treating dentin hypersensitivity. Dent. Mater. 2012, 28, 168–178. [Google Scholar] [CrossRef]
- Mielczarek, A.; Michalik, T. The effect of nano-hydroxyapatite toothpaste on enamel surface remineralization. An in vitro study. Am. J. Dent. 2014, 27, 287–290. [Google Scholar]
- Mullan, F.; Austin, R.S.; Parkinson, C.R.; Hasan, A.; Bartlett, D.W. Measurement of surface roughness changes of unpolished and polished enamel following erosion. PLoS ONE 2017, 12, e0182406. [Google Scholar] [CrossRef]
- Goel, A.; Singh, A.; Gupta, T.; Gambhir, R.S. Evaluation of surface roughness of enamel after various bonding and clean-up procedures on enamel bonded with three different bonding agents: An in-vitro study. J. Clin. Exp. Dent 2017, 9, e608–e616. [Google Scholar] [CrossRef][Green Version]
- Sugsompian, K.; Tansalarak, R.; Piyapattamin, T. Comparison of the Enamel Surface Roughness from Different Polishing Methods: Scanning Electron Microscopy and Atomic Force Microscopy Investigation. Eur. J. Dent. 2020, 14, 299–305. [Google Scholar]
- Bakry, A.S.; Abbassy, M.A.; Alharkan, H.F.; Basuhail, S.; Al-Ghamdi, K.; Hill, R. A Novel Fluoride Containing Bioactive Glass Paste is Capable of Re-Mineralizing Early Caries Lesions. Materials 2018, 11, 1636. [Google Scholar] [CrossRef] [PubMed]
- Farooq, I.M.A.; AlShwaimi, E.; Almas, K. Efficacy of a novel fluoride containing bioactive glass based dentifrice in remineralizing artificially induced demineralization in human enamel. Fluoride 2019, 52, 447–455. [Google Scholar]
- Brauer, D.S.; Karpukhina, N.; O’Donnell, M.D.; Law, R.V.; Hill, R.G. Fluoride-containing bioactive glasses: Effect of glass design and structure on degradation, pH and apatite formation in simulated body fluid. Acta Biomater. 2010, 6, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Tie, S.F.H.R.; Gillam, D.G. The Influence of Glass Content on the Hydraulic Conductance and Tubule Occlusion of Novel Bioactive GlassToothpastes. J. Dent. Maxillofac. Res. 2020, 3, 1–7. [Google Scholar]
- Akbulut, N.; Cetin, S.; Bilecenoglu, B.; Altan, A.; Akbulut, S.; Ocak, M.; Orhan, K. The micro-CT evaluation of enamel-cement thickness, abrasion, and mineral density in teeth in the postmortem interval (PMI): New parameters for the determination of PMI. Int. J. Legal Med. 2020, 134, 645–653. [Google Scholar] [CrossRef]
- Selig, K.R.; Lopez-Torres, S.; Hartstone-Rose, A.; Nash, L.T.; Burrows, A.M.; Silcox, M.T. A Novel Method for Assessing Enamel Thickness Distribution in the Anterior Dentition as a Signal for Gouging and Other Extractive Foraging Behaviors in Gummivorous Mammals. Folia Primatol. 2020, 91, 365–384. [Google Scholar] [CrossRef]
- Fredholm, Y.C.; Karpukhina, N.; Brauer, D.S.; Jones, J.R.; Law, R.V.; Hill, R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R Soc. Interface 2012, 9, 880–889. [Google Scholar] [CrossRef]
- Shah, F.A. Fluoride-containing bioactive glasses: Glass design, structure, bioactivity, cellular interactions, and recent developments. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.A.; Sandulescu, T.; Bochnig, C.; Gaengler, P.; Zimmer, S.; Arnold, W.H. Kinetics of fluoride bioavailability in supernatant saliva and salivary sediment. Arch. Oral Biol. 2012, 57, 870–876. [Google Scholar] [CrossRef]
- Cafferata, E.A.; Alvarez, C.; Diaz, K.T.; Maureira, M.; Monasterio, G.; González, F.E.; Covarrubias, C.; Vernal, R. Multifunctional nanocarriers for the treatment of periodontitis: Immunomodulatory, antimicrobial, and regenerative strategies. Oral Dis. 2019, 25, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, K.; Saraiya, V.; Laage, T.A.; Harris, M.; Blieden, M.; Karimbux, N. An evaluation of bioactive glass in the treatment of periodontal defects: A meta-analysis of randomized controlled clinical trials. J. Periodontol. 2012, 83, 453–464. [Google Scholar] [CrossRef]
- Janaphan, K.; Hill, R.G.; Gillam, D. Air-Polishing in Subgingival Root Debridement during Supportive Periodontal Care: A Review. J. Orthod Craniofac Res. 2020, 2, 113. [Google Scholar]
- Al-Eesa, N.A.; Fernandes, S.D.; Hill, R.G.; Wong, F.S.L.; Jargalsaikhan, U.; Shahid, S. Remineralising fluorine containing bioactive glass composites. Dent. Mater. 2021, 37, 672–681. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin-based composite performance: Are there some things we can’t predict? Dent. Mater. 2013, 29, 51–58. [Google Scholar] [CrossRef]
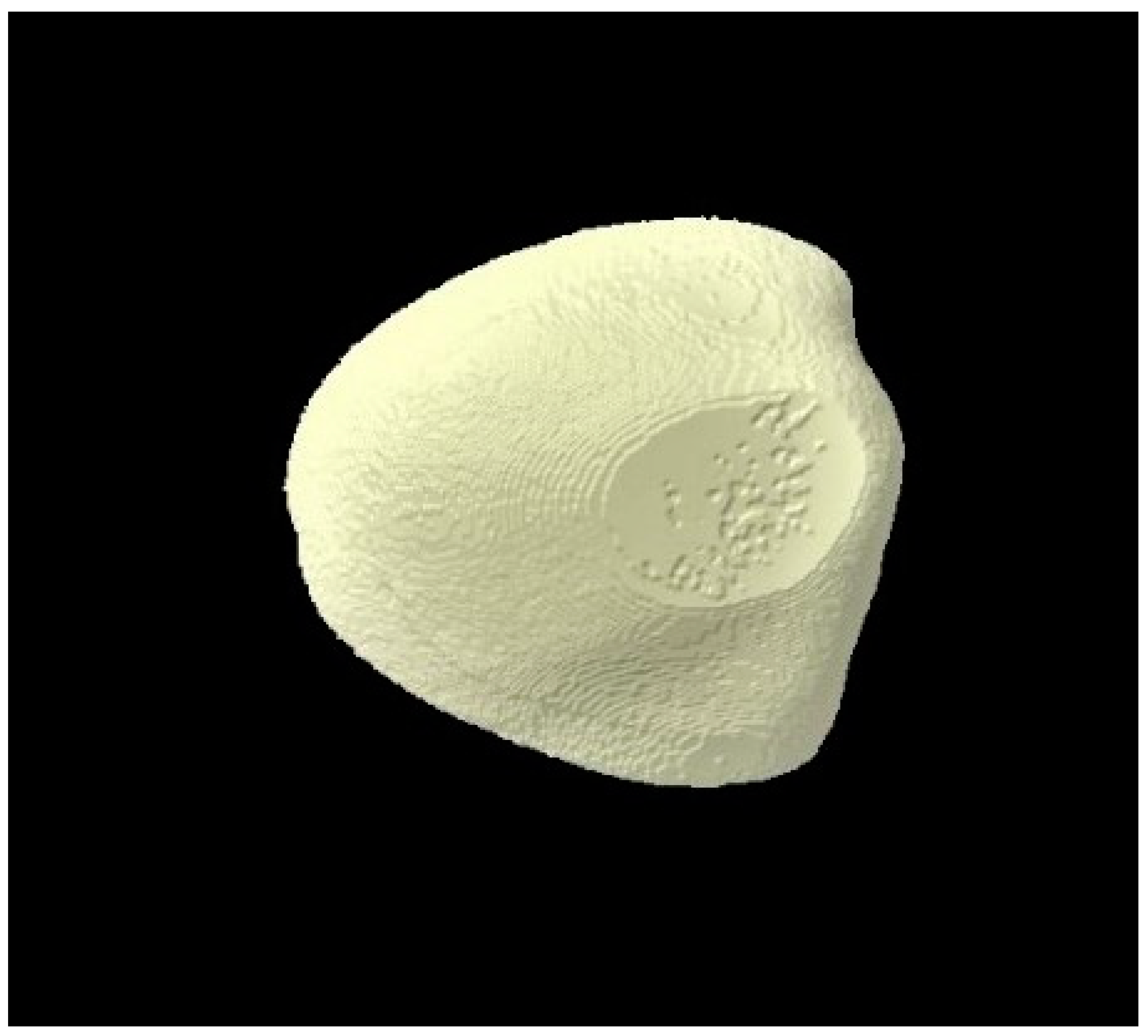
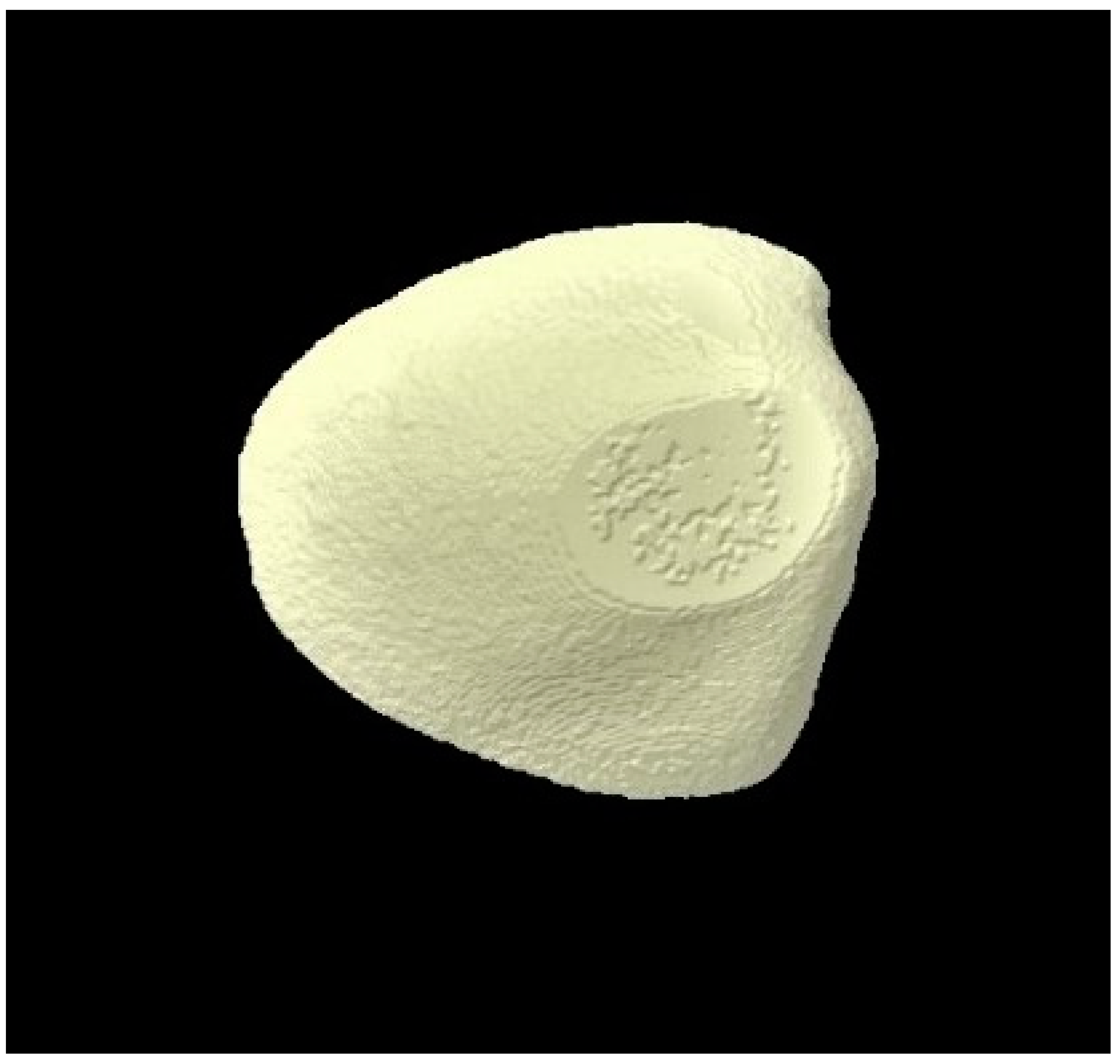
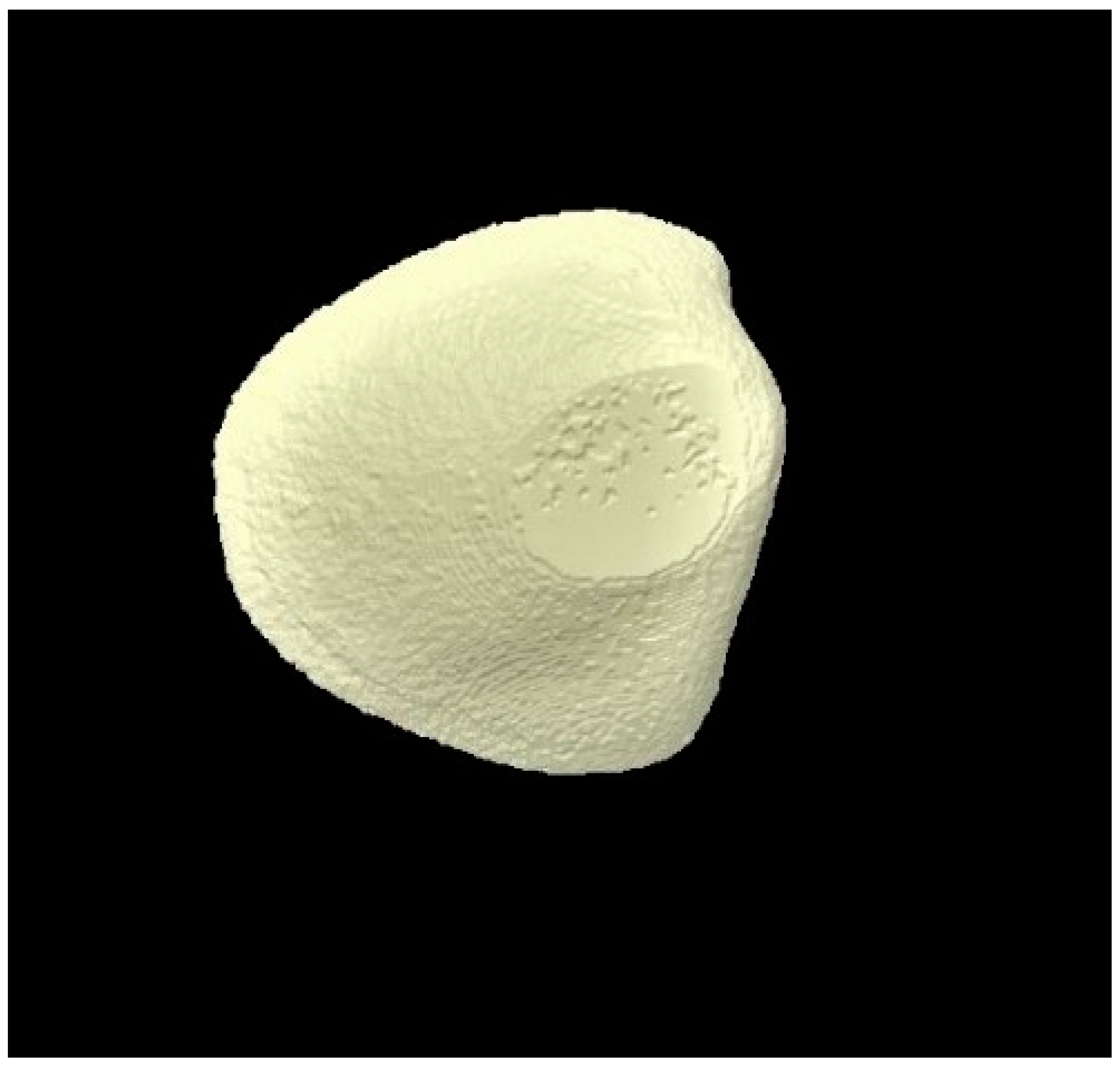
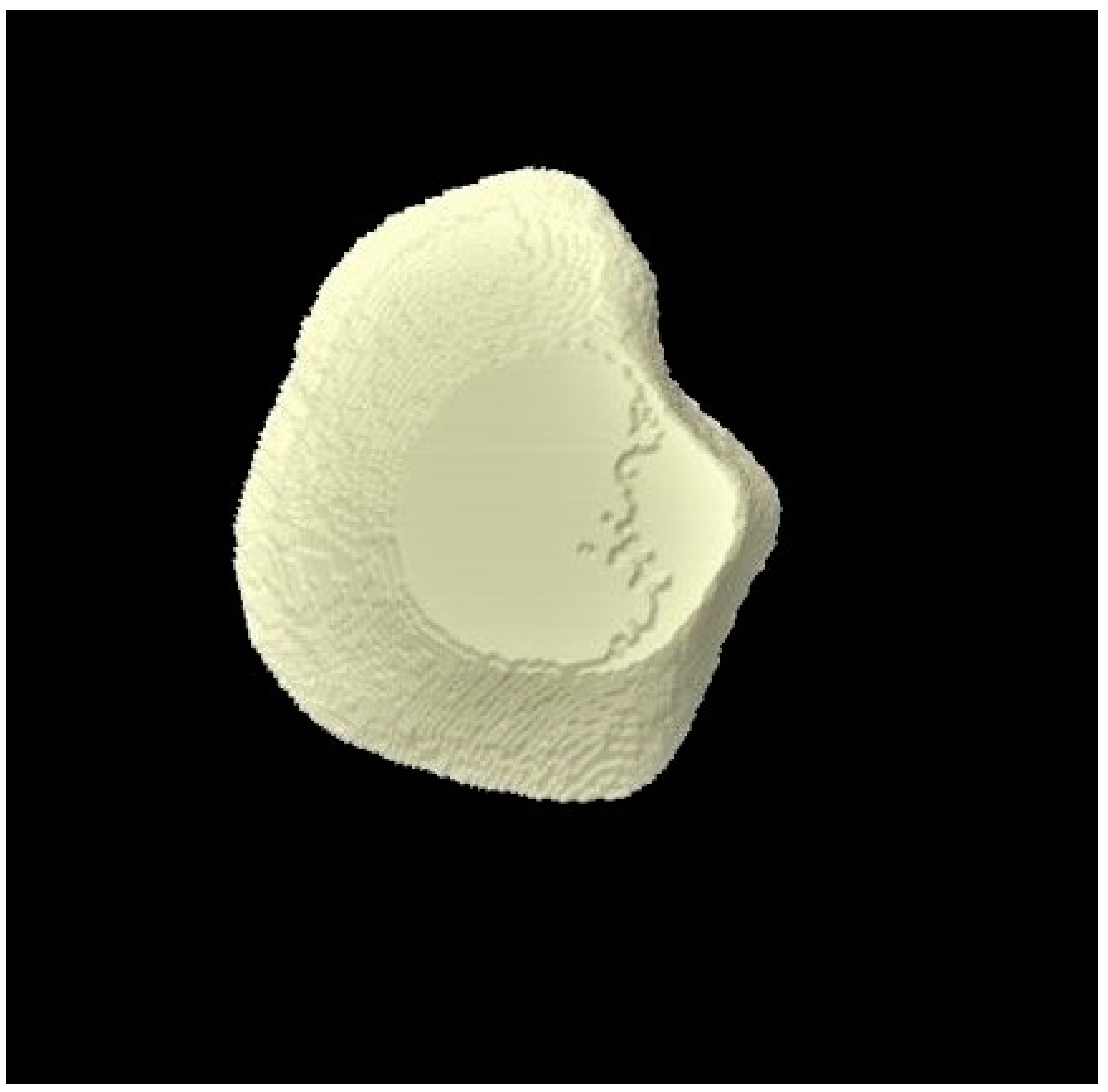
| Surface Micro-Hardness Analysis Time Points | Group 1 (Distilled Water) | Group 2 (Fluoride Toothpaste) | Group 3 (BG Toothpaste) | p-Values * |
|---|---|---|---|---|
| Baseline | 572.11 ± 28.38 + | 480.75 ± 66.04 + | 502.46 ± 74.5 | 0.017 |
| Post-demineralization | 123.87 ± 54.60 a | 211.28 ± 150.09 a | 167.71 ± 170.8 a | 0.471 |
| Post-remineralization | 173.13 ± 73.94 a | 272.13 ± 137.01 a | 285.52 ± 161.18 a | 0.27 |
| p-values ** | 0.0001 | 0.003 | 0.003 | |
| Difference (post-remin–post-demin) | 49.26 | 61.13 | 117.81 |
| Surface Roughness Analysis Time Points | Group 1 (Distilled Water) | Group 2 (Fluoride Toothpaste) | Group 3 (BG Toothpaste) | p-Values * |
|---|---|---|---|---|
| Baseline | 249.2 ± 127.0 | 276.2 ± 190.0 | 221.4 ± 106.3 | 0.925 |
| Post-demineralization | 1148.3 ± 816.8 a | 1114.1 ± 506.1 a | 992.0 ± 479.2 a | 0.912 |
| Post-remineralization | 1105.8 ± 825.9 a | 1058.5 ± 492.5 a | 768.8 ± 300.8 a | 0.547 |
| p-values ** | 0.02 | 0.001 | 0.0001 | |
| Difference (post-remin–post-demin) | −42.2 | −55.6 | −223.2 |
| Micro-CT Analysis Time Points | Group 1 (Distilled Water) | Group 2 (Fluoride Toothpaste) | Group 3 (BG Toothpaste) | p-Values * |
|---|---|---|---|---|
| Baseline | 15.58 ± 6.38 | 14.72 ± 2.35 | 17.24 ± 10.39 | 0.968 |
| Post-demineralization | 15.02 ± 6.31 | 14.54 ± 2.25 | 16.82 ± 10.33 | 0.983 |
| Post-remineralization | 15.03 ± 6.39 | 14.55 ± 2.26 | 17.01 ± 10.40 | 0.949 |
| p-values ** | 0.779 | 0.835 | 0.852 | |
| Difference (post-remin–post-demin) | 0.01 | 0.01 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, I.; Ali, S.; Farooqi, F.A.; AlHumaid, J.; Binhasan, M.; Shabib, S.; Vohra, F.; Abduljabbar, T. Enamel Remineralization Competence of a Novel Fluoride-Incorporated Bioactive Glass Toothpaste—A Surface Micro-Hardness, Profilometric, and Micro-Computed Tomographic Analysis. Tomography 2021, 7, 752-766. https://doi.org/10.3390/tomography7040063
Farooq I, Ali S, Farooqi FA, AlHumaid J, Binhasan M, Shabib S, Vohra F, Abduljabbar T. Enamel Remineralization Competence of a Novel Fluoride-Incorporated Bioactive Glass Toothpaste—A Surface Micro-Hardness, Profilometric, and Micro-Computed Tomographic Analysis. Tomography. 2021; 7(4):752-766. https://doi.org/10.3390/tomography7040063
Chicago/Turabian StyleFarooq, Imran, Saqib Ali, Faraz Ahmed Farooqi, Jehan AlHumaid, Mashael Binhasan, Sara Shabib, Fahim Vohra, and Tariq Abduljabbar. 2021. "Enamel Remineralization Competence of a Novel Fluoride-Incorporated Bioactive Glass Toothpaste—A Surface Micro-Hardness, Profilometric, and Micro-Computed Tomographic Analysis" Tomography 7, no. 4: 752-766. https://doi.org/10.3390/tomography7040063
APA StyleFarooq, I., Ali, S., Farooqi, F. A., AlHumaid, J., Binhasan, M., Shabib, S., Vohra, F., & Abduljabbar, T. (2021). Enamel Remineralization Competence of a Novel Fluoride-Incorporated Bioactive Glass Toothpaste—A Surface Micro-Hardness, Profilometric, and Micro-Computed Tomographic Analysis. Tomography, 7(4), 752-766. https://doi.org/10.3390/tomography7040063








