Non-Hemorrhagic Adrenal Infarction during Pregnancy: The Diagnostic Imaging Keys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging Techniques
2.3. Imaging Analysis
2.4. Literature Analysis
3. Results
3.1. Clinical and Laboratory Findings
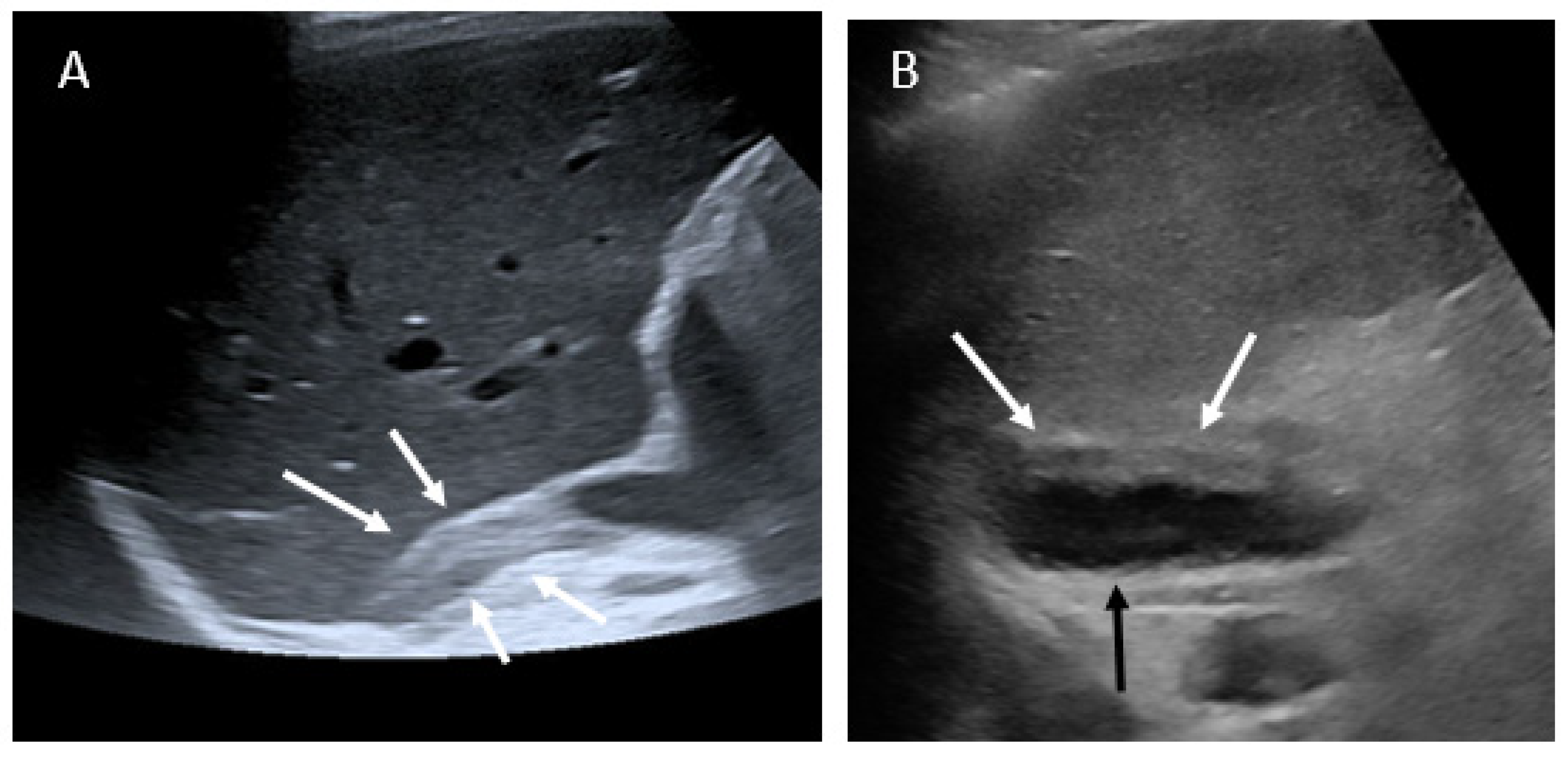
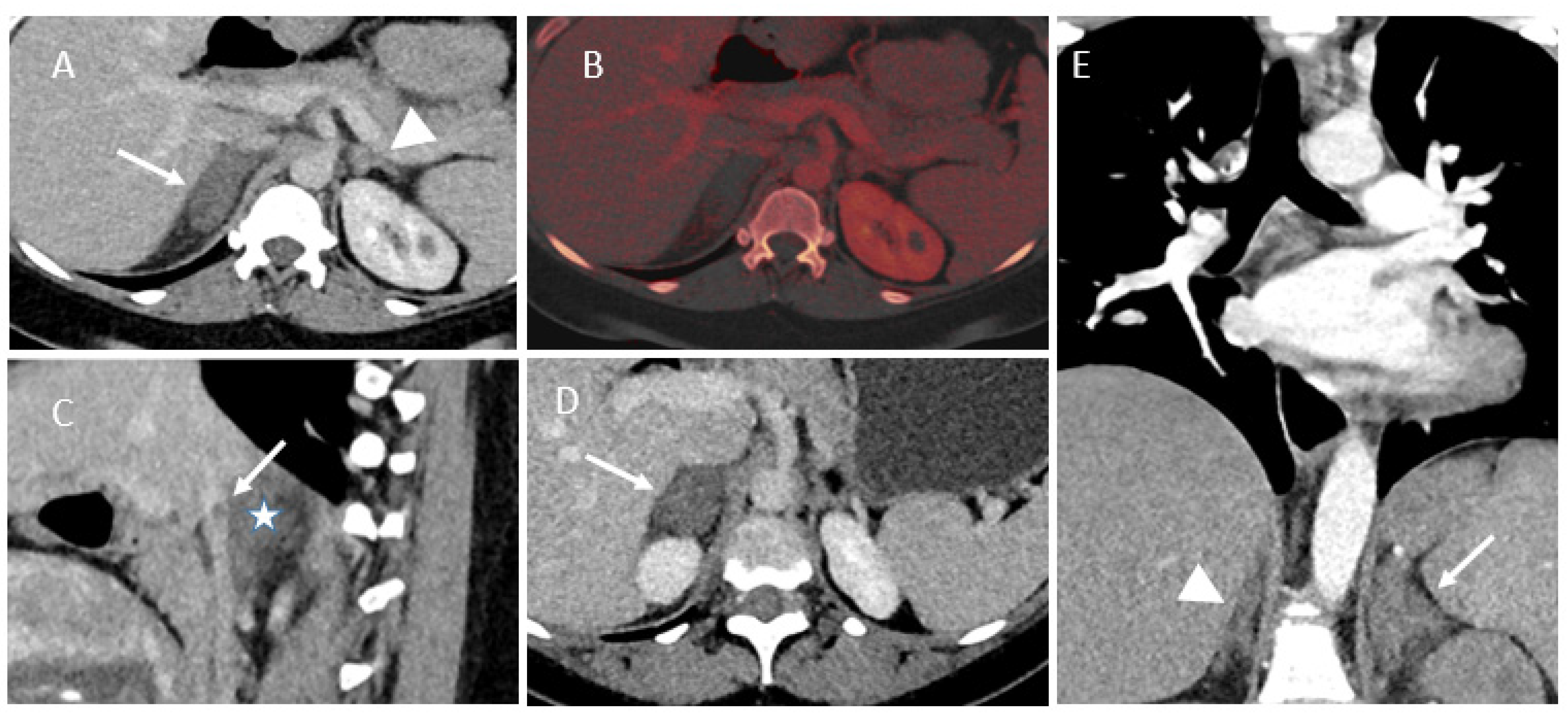
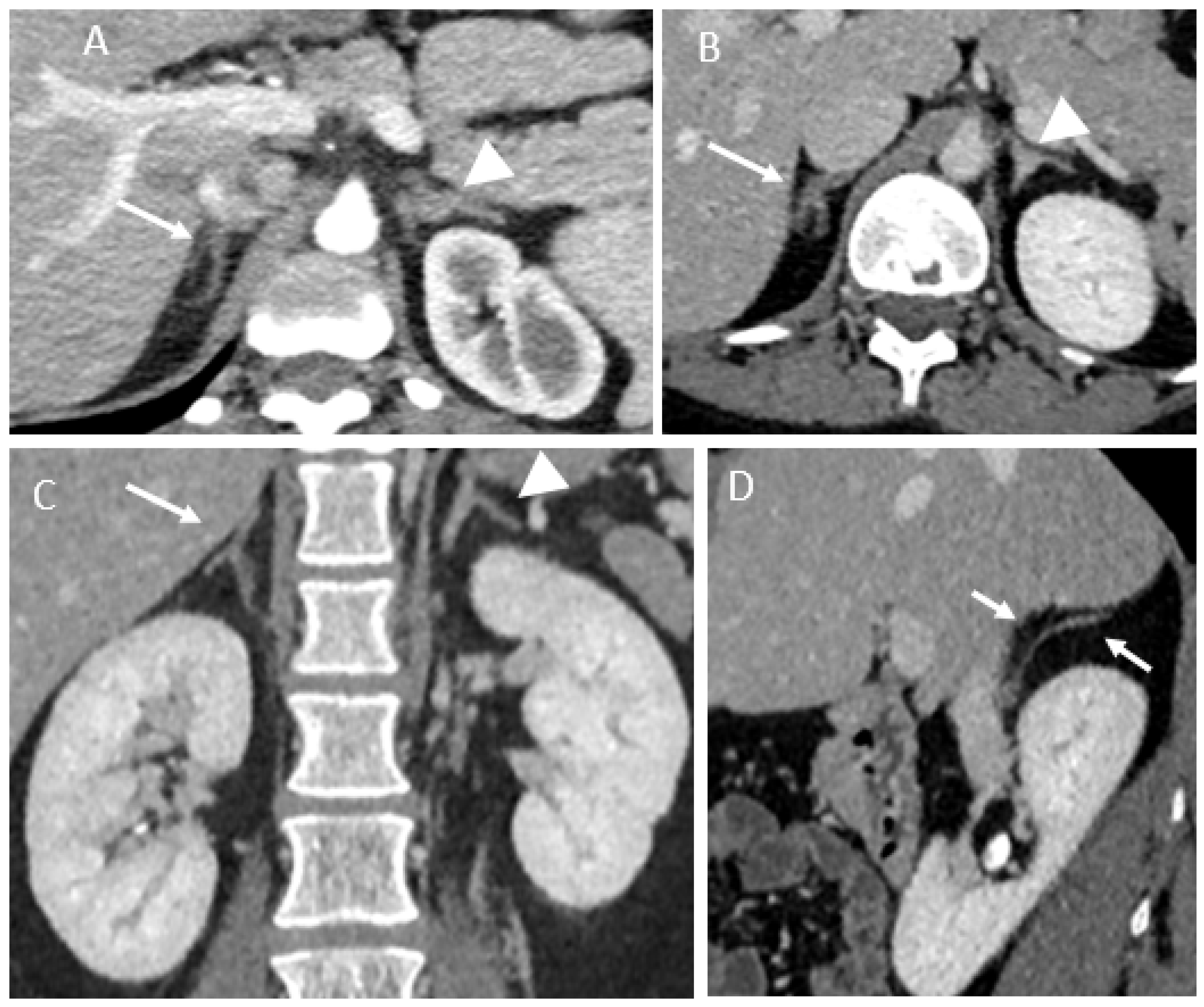
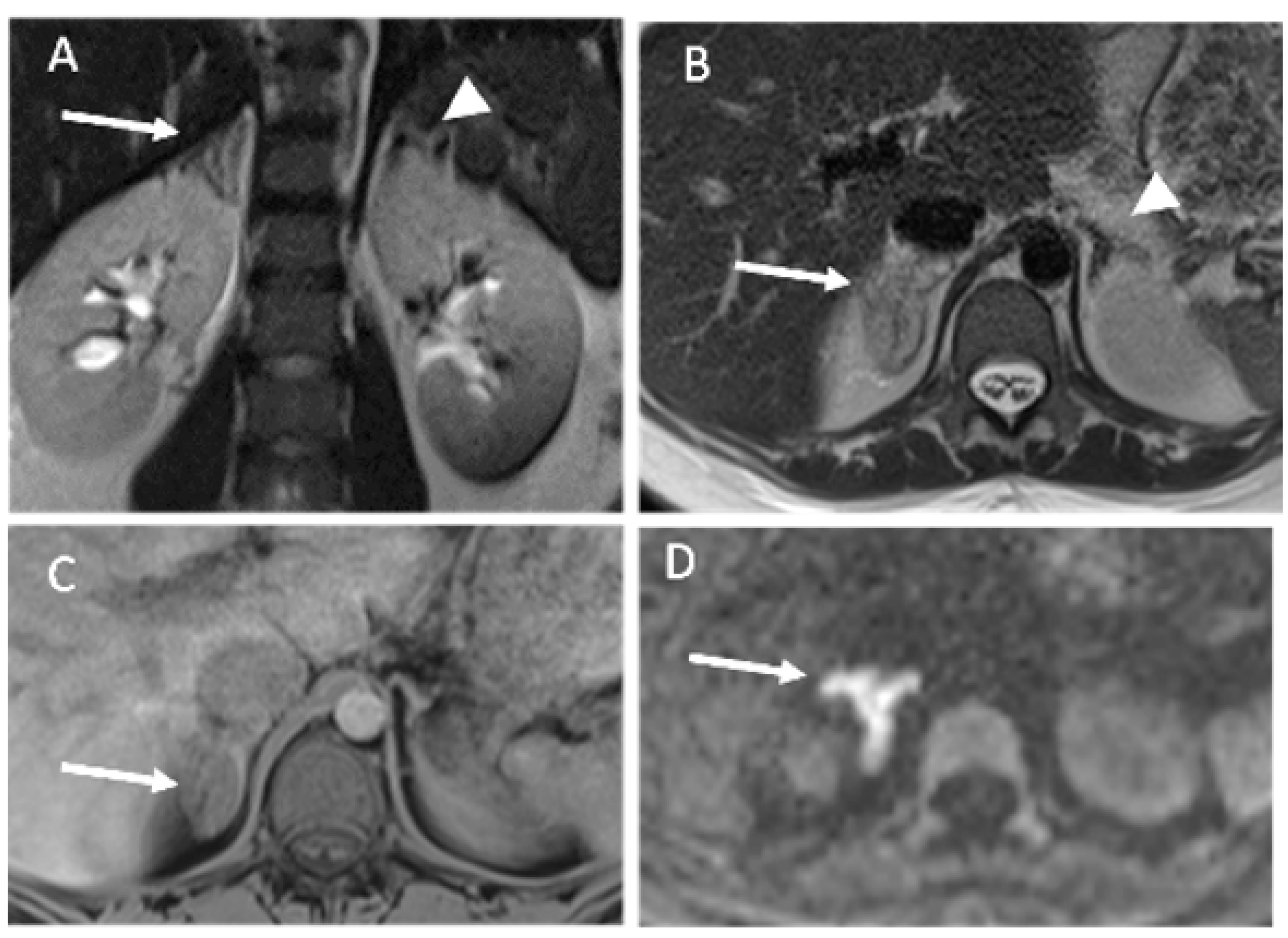
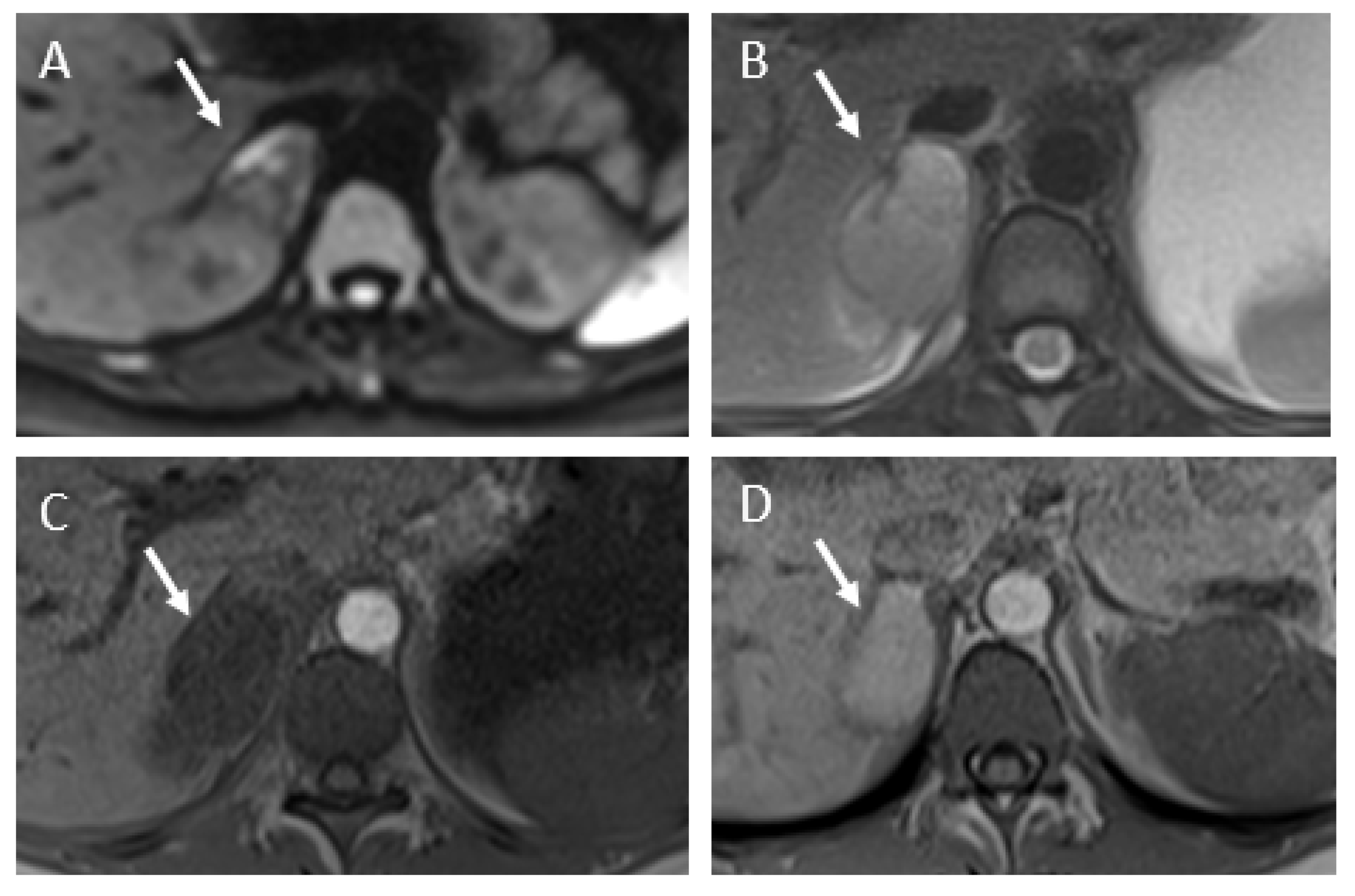
3.2. Imaging Findings
3.3. Literature Review
4. Discussion
5. Conclusions
- Imaging plays a central role in the management of abdominal pain during pregnancy. The clinical presentation of NHAI is non-specific, but imaging findings are typical and highly similar from patient-to-patient.
- US examination performed for abdominal/flank pain should screen the adrenal gland region.
- Our results highlight the usefulness of unenhanced MRIs, which should include diffusion-weighted imaging.
- A prompt diagnosis of NHAI induced the implementation of anticoagulation and screening for acute adrenal insufficiency.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Satement
Abbreviations
| NHAI | non-hemorrhagic adrenal infarction |
| MRI | magnetic resonance imaging |
| IQR | interquartile range |
| CRP | C-reactive protein |
| FS | fat-suppressed |
| CT | computed tomography |
| GA | gestational age |
| ACTH | adrenocorticotropic hormone |
| US | ultrasound |
References
- Baheti, A.D.; Nicola, R.; Bennett, G.L.; Bordia, R.; Moshiri, M.; Katz, D.S.; Bhargava, P. Magnetic Resonance Imaging of Abdominal and Pelvic Pain in the Pregnant Patient. Magn. Reson. Imaging Clin. North Am. 2016, 24, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Glomski, S.A.; Guenette, J.; Landman, W.; Tatli, S. Acute Nonhemorrhagic Adrenal Infarction in Pregnancy: 10-Year MRI Incidence and Patient Outcomes at a Single Institution. Am. J. Roentgenol. 2018, 210, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Chasseloup, F.; Bourcigaux, N.; Christin-Maitre, S. Unilateral non-haemorrhagic adrenal infarction as a cause of abdominal pain during pregnancy. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Molière, S.; Gaudineau, A.; Koch, A.; Leroi, T.; Roedlich, M.-N.; Veillon, F. Usefulness of diffusion-weighted imaging for diagnosis of adrenal ischemia during pregnancy: A preliminary report. Emerg. Radiol. 2017, 24, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.K.; Maydanovych, S.; Draper, H.; Antoniades, G.; Allen, J. Adrenal infarction in the immediate postnatal period†. J. Obstet. Gynaecol. 2018, 39, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Riddell, A.M.; Khalili, K. Sequential Adrenal Infarction without MRI-Detectable Hemorrhage in Primary Antiphospholipid-Antibody Syndrome. Am. J. Roentgenol. 2004, 183, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Guenette, J.; Tatli, S. Nonhemorrhagic Adrenal Infarction with Magnetic Resonance Imaging Features during Pregnancy. Obstet. Gynecol. 2015, 126, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.; Dahlbäck, B.; Marŝál, K. Thrombotic risk during pregnancy: A population study. Obstet. Gynecol. 1999, 94, 95–99. [Google Scholar] [CrossRef]
- Schmitt, C.; Debord, M.-P.; Grange, C.; Ben Cheikh, A.; Krauth, J.-P.; Dupuis, O.; Golfier, F.; Raudrant, D. Adrenal vein thrombosis during pregnancy. J. Gynecol. Obstet Biol. Reprod. 2010, 39, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Green, P.-A.D.; Ngai, I.M.; Lee, T.T.; Garry, D.J. Unilateral adrenal infarction in pregnancy. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, B. Venous infarction of the adrenal glands. J. Pathol. 1976, 119, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Blanco, L.T.; Socarras, M.R.; Montero, R.F.; Diez, E.L.; Calvo, A.O.; Gregorio, S.A.Y.; Cansino, J.R.; Galan, J.A.; Rivas, J.G. Renal colic during pregnancy: Diagnostic and therapeutic aspects. Literature review. Central Eur. J. Urol. 2016, 70, 93–100. [Google Scholar] [CrossRef]
- Bafaraj, S.M. Value of Magnetic Resonance Urography Versus Computerized Tomography Urography (CTU) in Evaluation of Obstructive Uropathy: An Observational Study. Med Imaging Rev. 2017, 14, 129–134. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, A.J.; Reimer, P.; Dekkers, I.A.; Bongartz, G.; Bellin, M.-F.; M Bertolotto, M.; Clement, O.; Heina-Peer, G.; Stacul, F.; Webb, A.W.; et al. Post-contrast acute kidney injury-Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2018, 28, 2845–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient | Age | Gravidity and Parity | Gest. Age | Clinical Presentation/Initial Suspected Diagnosis | Laboratory Findings | Imaging Findings | Side | Treatment | Imaging Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33y | G4P3 | 37w | Right-sided abdominal pain/appendicitis | Leukocytosis: 19x10*9/L, CRP: 49 mg/L, D-dimer: 1070 ng/mL | US: swelling adrenal gland MRI: typical findings ‡, without any diffusion imaging CT: typical findings † | Right | After giving birth, Oral anticoagulation and antiplatelet during 11 months | CT at 3 and 7 months: Atrophic adrenal with partially restored glandular enhancement |
| 2 | 38y | G3P1 | 26w | history of kidney stone/Right back flank pain/renal colic | Leukocytosis: 20x10*9/L, CRP: 17 mg/L | US: no abnormality CT: typical findings with vein thrombus | Right | Heparin and then oral anticoagulation during 6 months | MRI at 1 month and CT-enhanced at 3 months: Atrophic adrenal with partially restored glandular enhancement |
| 3 | 19y | G1P0/twin pregnancy | 32w | Previous left acute obstructive pyelonephritis during the same pregnancy/right back flank pain/renal colic/ | Leukocytosis: 18x10*9/L, CRP: 82 mg/L | US: Pyelocaliceal dilatation and kidney stones CT: typical findings with vein thrombus MRI: typical findings | Right | Heparin injection follow by oral anticoagulation for 3 months | CT at 3 months: isolated residual atrophy of the lateral arm of the gland |
| 4 | 34y | G1P0 | 31w | Right upper quadrant pain/hepatic colic or pulmonary embolism | Leukocytosis: 15x10*9/L, CRP: 25 mg/L, D-dimer: 1500 ng/L | US: No abnormality CT: typical findings. MRI: typical findings with fluid collection | Right | Heparin injection during the pregnancy | MRI at one week: no change. MRI at one month: Appearance of T1-weighted hyperintensity |
| 5 | 31y | G3P0 | 36w | Left upper back and low back chest pain/ pulmonary embolism | Leukocytosis: 12.4x10*9/L, CRP: 187 mg/L, D-dimer: 820 ng/L | US: no initial abnormality/swelling adrenal gland and fluid collection on review CT: typical findings. MRI: typical findings with fluid collection | Left | Heparin injection during the pregnancy | MRI at 4 months: swollen left adrenal gland. Collection decreased with partially restored glandular enhancement |
| 6 | 22y | G1P0 | 30w | Left pain then one day later right flank pain/ appendicitis | Leukocytosis: 10.3x10*9/L, CRP: 52 mg/L | US: no abnormality CT: bilateral typical findings with right vein thrombus. MRI: bilateral typical findings | Right & Left | Heparin injection during the pregnancy | No follow-up (recent case) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chagué, P.; Marchi, A.; Fechner, A.; Hindawi, G.; Tranchart, H.; Carrara, J.; Vivanti, A.J.; Rocher, L. Non-Hemorrhagic Adrenal Infarction during Pregnancy: The Diagnostic Imaging Keys. Tomography 2021, 7, 533-544. https://doi.org/10.3390/tomography7040046
Chagué P, Marchi A, Fechner A, Hindawi G, Tranchart H, Carrara J, Vivanti AJ, Rocher L. Non-Hemorrhagic Adrenal Infarction during Pregnancy: The Diagnostic Imaging Keys. Tomography. 2021; 7(4):533-544. https://doi.org/10.3390/tomography7040046
Chicago/Turabian StyleChagué, Pierre, Antoine Marchi, Alix Fechner, Ghina Hindawi, Hadrien Tranchart, Julie Carrara, Alexandre J. Vivanti, and Laurence Rocher. 2021. "Non-Hemorrhagic Adrenal Infarction during Pregnancy: The Diagnostic Imaging Keys" Tomography 7, no. 4: 533-544. https://doi.org/10.3390/tomography7040046
APA StyleChagué, P., Marchi, A., Fechner, A., Hindawi, G., Tranchart, H., Carrara, J., Vivanti, A. J., & Rocher, L. (2021). Non-Hemorrhagic Adrenal Infarction during Pregnancy: The Diagnostic Imaging Keys. Tomography, 7(4), 533-544. https://doi.org/10.3390/tomography7040046







