Diagnostic Accuracy of Coronary Artery Occlusion and Myocardial Perfusion Defect on Non-Gated Enhanced Chest CT in Predicting Acute Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Technique
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Incremental Value of CAO in the Diagnosis of AMI over Evaluation of MPD Alone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, S.M.; Chun, E.J.; Lee, H.Y.; Min, D.; White, C.S. Computed Tomography Diagnosis of Nonspecific Acute Chest Pain in the Emergency Department: From Typical Acute Coronary Syndrome to Various Unusual Mimics. J. Thorac. Imaging 2017, 32, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Pursnani, A.; Lee, A.; Mayrhofer, T.; Ahmed, W.; Uthamalingam, S.; Ferencik, M.; Ghoshhajra, B.B. Early resting myocardial computed tomography perfusion for the detection of acute coronary syndrome in patients with coronary artery disease. Circ. Cardiovasc. Imaging 2015, 8, e002404. [Google Scholar] [CrossRef] [PubMed]
- Branch, K.R.; Busey, J.; Mitsumori, L.M.; Strote, J.; Caldwell, J.H.; Busch, J.H.; Shuman, W.P. Diagnostic Performance of Resting CT Myocardial Perfusion in Patients with Possible Acute Coronary Syndrome. Am. J. Roentgenol. 2013, 200, W450–W457. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Anzai, T.; Yoshizawa, A.; Itabashi, Y.; Ohki, T. Role of non-electrocardiogram-gated contrast-enhanced computed tomography in the diagnosis of acute coronary syndrome. Hear. Vessel. 2015, 30, 1–8. [Google Scholar] [CrossRef]
- Yoo, S.M.; Rho, J.Y.; Lee, H.Y.; Song, I.S.; Moon, J.Y.; White, C.S. Current Concepts in Cardiac CT Angiography for Patients with Acute Chest Pain. Korean Circ. J. 2010, 40, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Yoo, S.M.; White, C.S. Coronary CT angiography in emergency department patients with acute chest pain: Triple rule-out protocol versus dedicated coronary CT angiography. Int. J. Cardiovasc. Imaging 2008, 25, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Yoo, S.M.; Rho, J.Y.; Lee, H.Y.; White, C.S. MDCT evaluation of atherosclerotic coronary artery disease: What should radiologists know? Int. J. Cardiovasc. Imaging 2014, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T. Plaque Characterization by Coronary Computed Tomography Angiography and Association with Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2016, 67, 458–459. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. Circulation 2012, 126, 2020–2035. [Google Scholar] [CrossRef] [PubMed]
- Berko, N.S.; Clark, E.T.; Levsky, J.M. Acute left circumflex coronary artery occlusion detected on nongated CT. Clin. Imaging 2015, 39, 897–900. [Google Scholar] [CrossRef]
- Chun, E.J.; Han, J.H.; Yoo, S.M.; Lee, H.Y.; Song, I.S.; White, C.S. Differences in the CT findings between vulnerable plaque and culprit lesions in acute coronary syndrome. J. Cardiovasc. Comput. Tomogr. 2018, 12, 115–117. [Google Scholar] [CrossRef]
- Sommer, W.H.; Schenzle, J.C.; Becker, C.R.; Nikolaou, K.; Graser, A.; Michalski, G.; Neumaier, K.; Reiser, M.F.; Johnson, T.R.C. Saving Dose in Triple-Rule-Out Computed Tomography Examination Using a High-Pitch Dual Spiral Technique. Investig. Radiol. 2010, 45, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Higuchi, T.; Shimokoshi, T.; Kiguchi, T.; Horii, Y.; Yoshimura, N.; Aoyama, H. Acute coronary syndrome: Evaluation of detection capability using non-electrocardiogram-gated parenchymal phase CT imaging. Jpn. J. Radiol. 2016, 34, 331–338. [Google Scholar] [CrossRef] [PubMed]
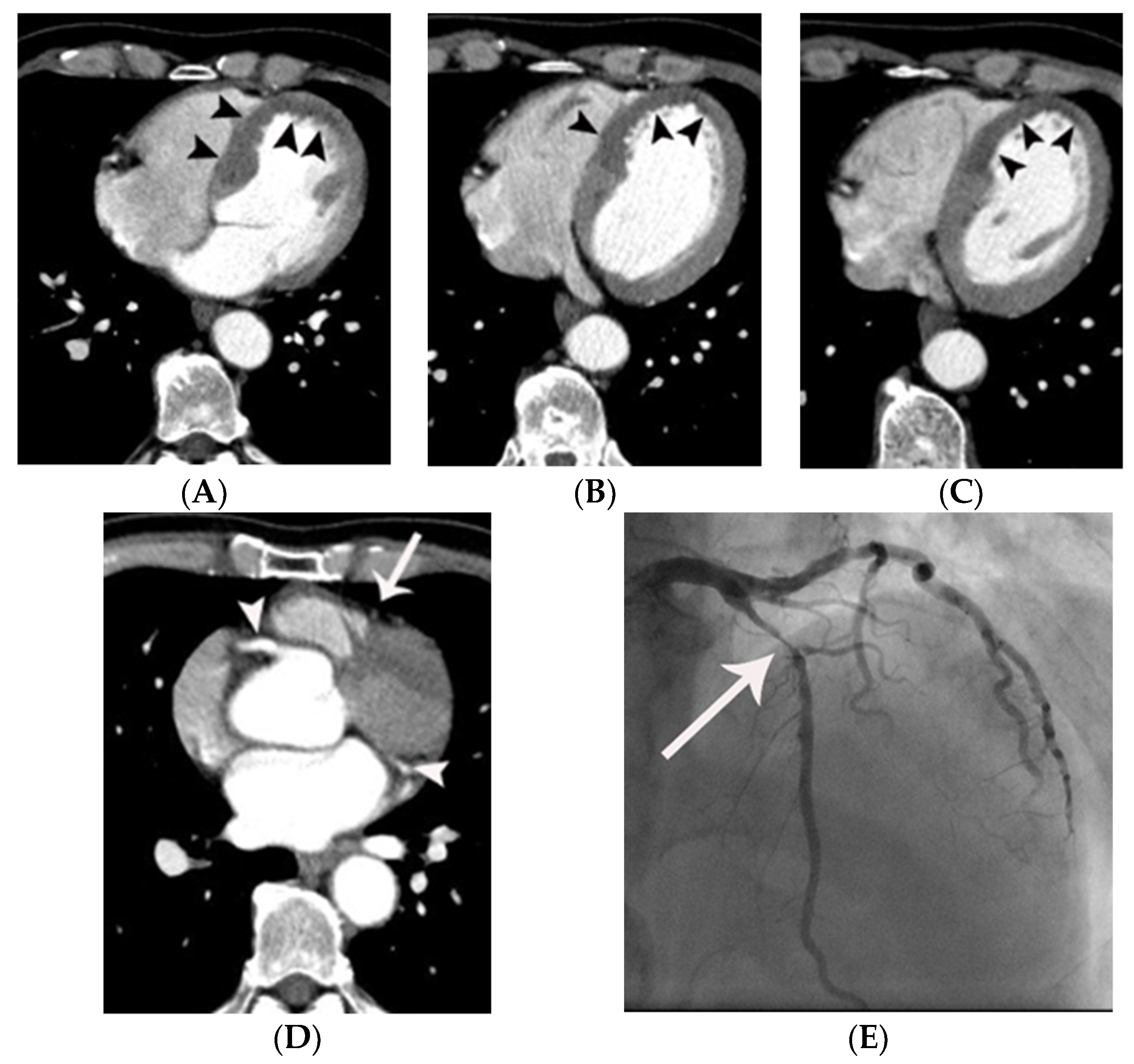
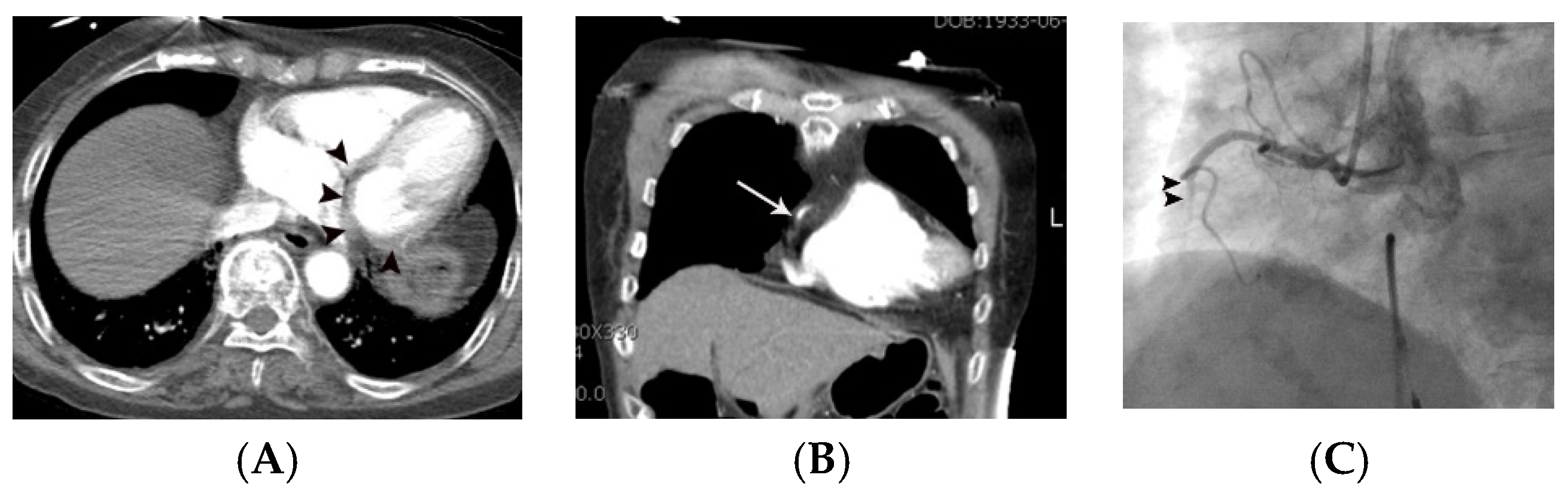
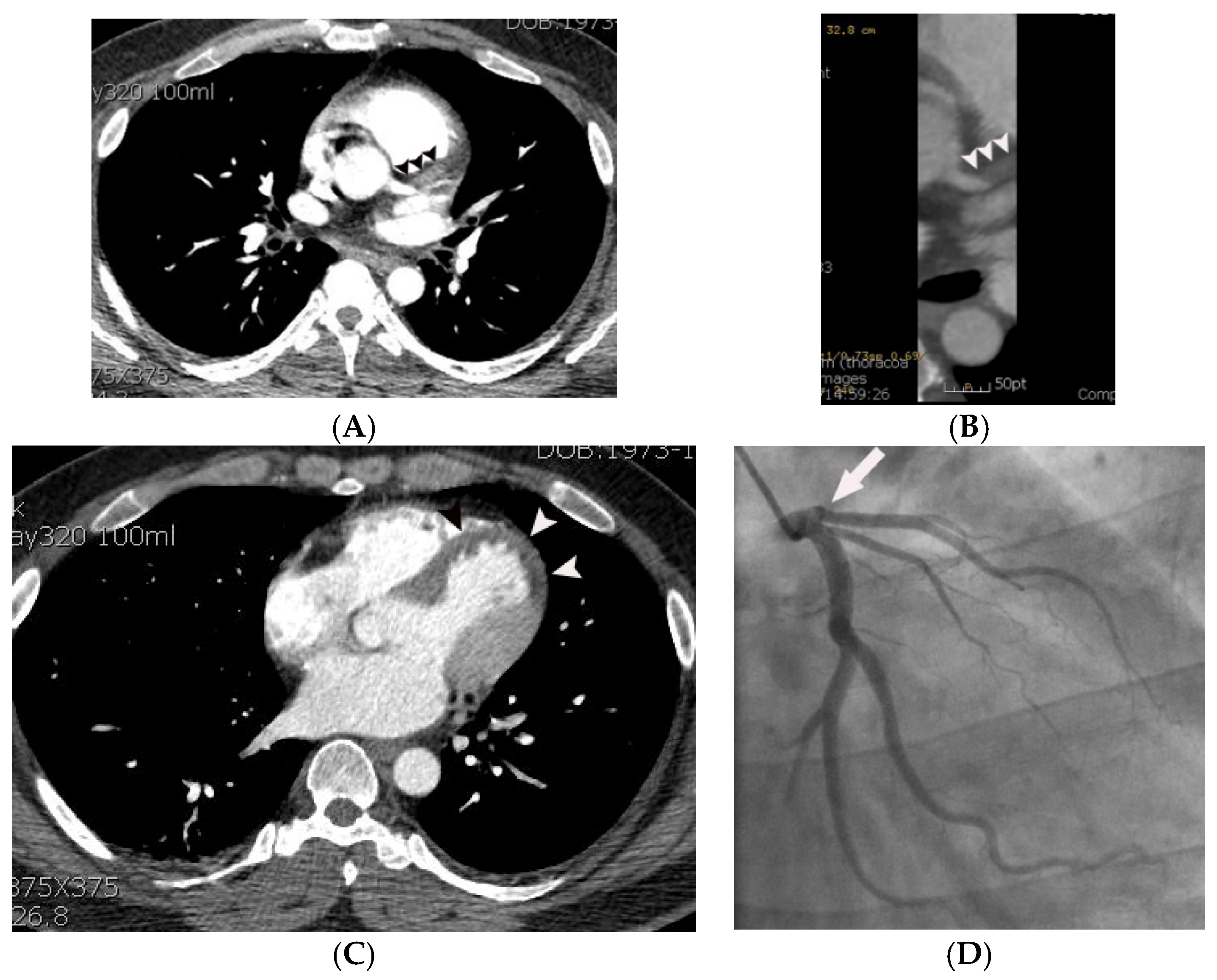

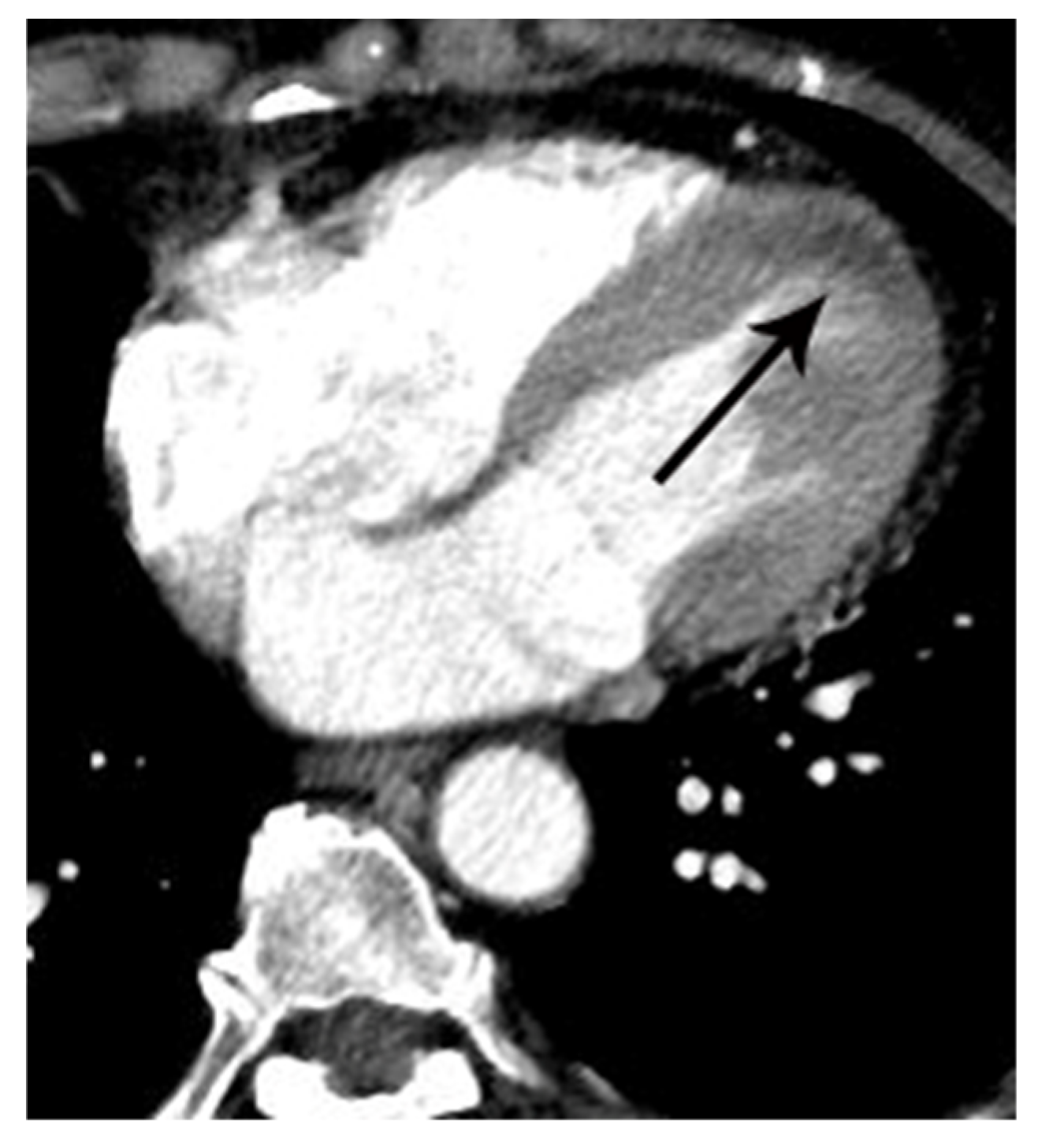
| Variables | Means ± SD or Prevalence | ||
|---|---|---|---|
| Group 1 | Group 2 | p Value | |
| Age (years) | 62 ± 14.1 | 63 ± 25.2 | >0.05 |
| Sex (male/female) | 25/8 | 49/17 | >0.05 |
| Body mass index (kg/m2) | 23.9 ± 3.7 | 23.7 ± 3.0 | >0.05 |
| Hypertension | 22/33 (66.7) | 39/66 (59.1) | >0.05 |
| Systolic blood pressure (mmHg) | 133.2 ± 17.9 | 132.2 ± 16.8 | >0.05 |
| Diastolic blood pressure (mmHg) | 77.9 ± 80.1 | 78.9 ± 78.1 | >0.05 |
| Smoking | 20/33 (60.6) | 36/66 (54.5) | >0.05 |
| Diabetes mellitus | 14/33 (42.4) | 23/66 (34.8) | >0.05 |
| High density lipoprotein (mg/dL) | 41.0 ± 9.8 | 41.7 ± 8.5 | >0.05 |
| Low density lipoprotein (mg/dL) | 105.6 ± 33.6 | 108.6 ± 41.4 | >0.05 |
| C-reactive protein (mg/dL) | 2.2 ± 5.1 | 2.1 ± 5.1 | >0.05 |
| Group 1 | Group 2 | p Value | |
|---|---|---|---|
| No defect (CT score of 0) | 4/33 (12.1) | 42/66 (63.6) | 0.0001 |
| Probable artifact or equivocal MPD (CT score of 1) | 15/33 (45.5) | 16/66 (24.2) | 0.04 |
| Probable MPD (CT score of 2) | 14/33 (42.4) | 8/66 (12.1) | 0.002 |
| Left main artery | 1 |
| Proximal left anterior descending artery | 9 |
| Middle left anterior descending artery | 14 |
| First diagonal branch | 1 |
| Proximal right coronary artery | 1 |
| Middle right coronary artery | 3 |
| Distal right coronary artery | 1 |
| Posterolateral branch | 1 |
| Proximal left circumflex artery | 1 |
| Distal left circumflex artery | 1 |
| Positive Wall Motion Abnormality | No Wall Motion Abnormality | |
|---|---|---|
| No defect (CT score of 0) | 0/33 (0) | 4/33 (12.1) |
| Probable artifact or equivocal MPD (CT score of 1) | 12/33 (36.4) | 3/33 (9.1) |
| Probable MPD (CT score of 2) | 14/33 (42.4) | 0/33 (0) |
| Coronary artery occlusion | 6/6 (100) | 0/33 (0) |
| Variables | Sensitivity | Specificity | Negative Predictive Value | Positive Predictive Value |
|---|---|---|---|---|
| MPD ≥ 1 | 29/33 (87.9) | 42/66 (63.6) | 42/46 (91.3) | 29/53 (54.7) |
| MPD ≥ 2 | 14/33 (42.4) | 58/66 (87.9) | 58/77 (75.3) | 14/22 (63.6) |
| CAO alone | 6/33 (18.2) | 66/66 (100) | 66/93 (71) | 6/6 (100) |
| MPD ≥ 2 and CAO | 4/33 (12.1) | 66/66 (100) | 66/95 (69.5) | 4/4 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, M.J.; Lee, D.; Yoo, S.M.; White, C.S. Diagnostic Accuracy of Coronary Artery Occlusion and Myocardial Perfusion Defect on Non-Gated Enhanced Chest CT in Predicting Acute Myocardial Infarction. Tomography 2021, 7, 504-512. https://doi.org/10.3390/tomography7040043
Son MJ, Lee D, Yoo SM, White CS. Diagnostic Accuracy of Coronary Artery Occlusion and Myocardial Perfusion Defect on Non-Gated Enhanced Chest CT in Predicting Acute Myocardial Infarction. Tomography. 2021; 7(4):504-512. https://doi.org/10.3390/tomography7040043
Chicago/Turabian StyleSon, Min Ji, Dongjun Lee, Seung Min Yoo, and Charles S. White. 2021. "Diagnostic Accuracy of Coronary Artery Occlusion and Myocardial Perfusion Defect on Non-Gated Enhanced Chest CT in Predicting Acute Myocardial Infarction" Tomography 7, no. 4: 504-512. https://doi.org/10.3390/tomography7040043
APA StyleSon, M. J., Lee, D., Yoo, S. M., & White, C. S. (2021). Diagnostic Accuracy of Coronary Artery Occlusion and Myocardial Perfusion Defect on Non-Gated Enhanced Chest CT in Predicting Acute Myocardial Infarction. Tomography, 7(4), 504-512. https://doi.org/10.3390/tomography7040043





