Fat Fraction MRI for Longitudinal Assessment of Bone Marrow Heterogeneity in a Mouse Model of Myelofibrosis
Abstract
1. Introduction
2. Materials and Methods
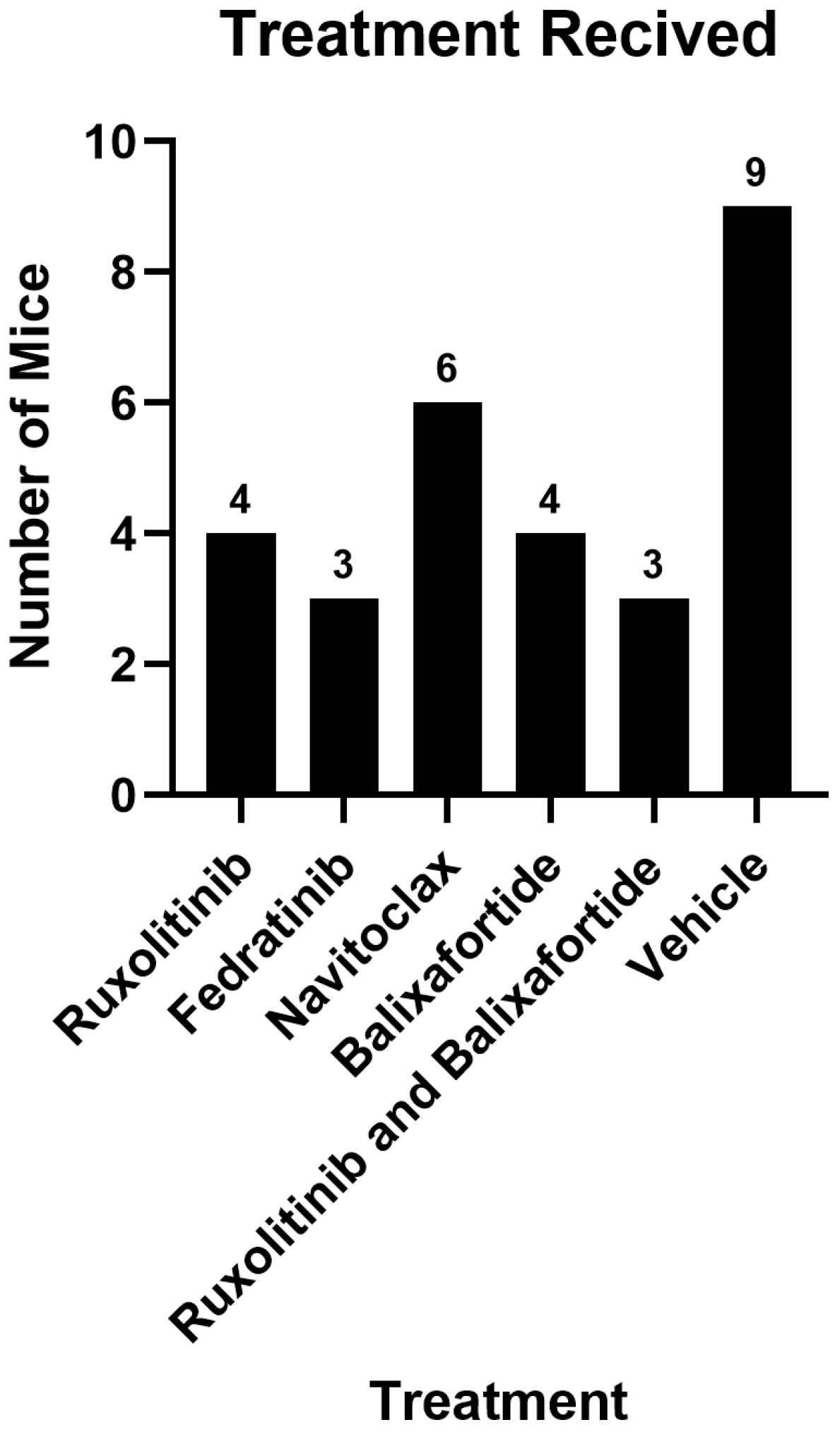
2.1. Study Populations
2.2. MRI Parameters
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
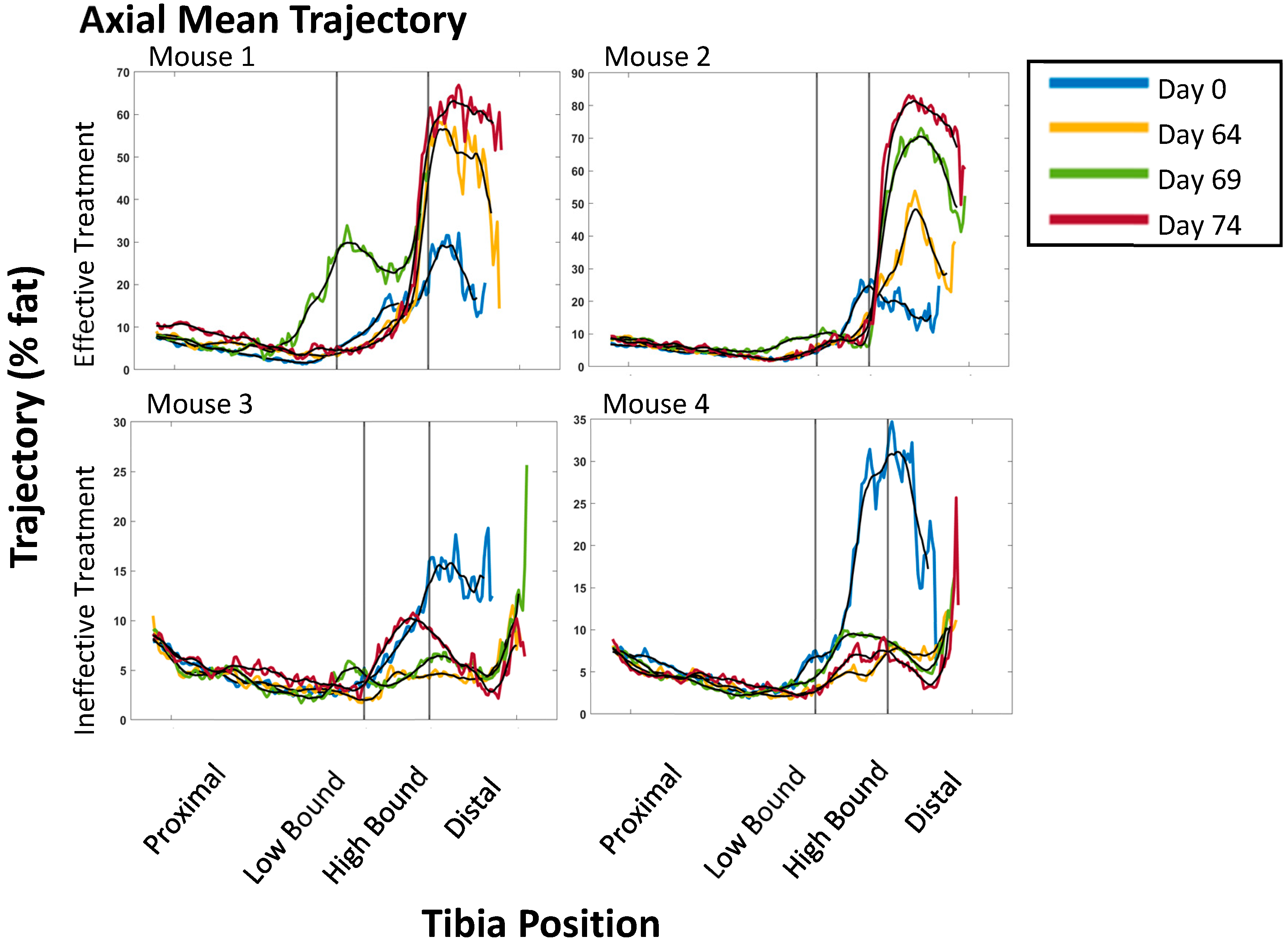
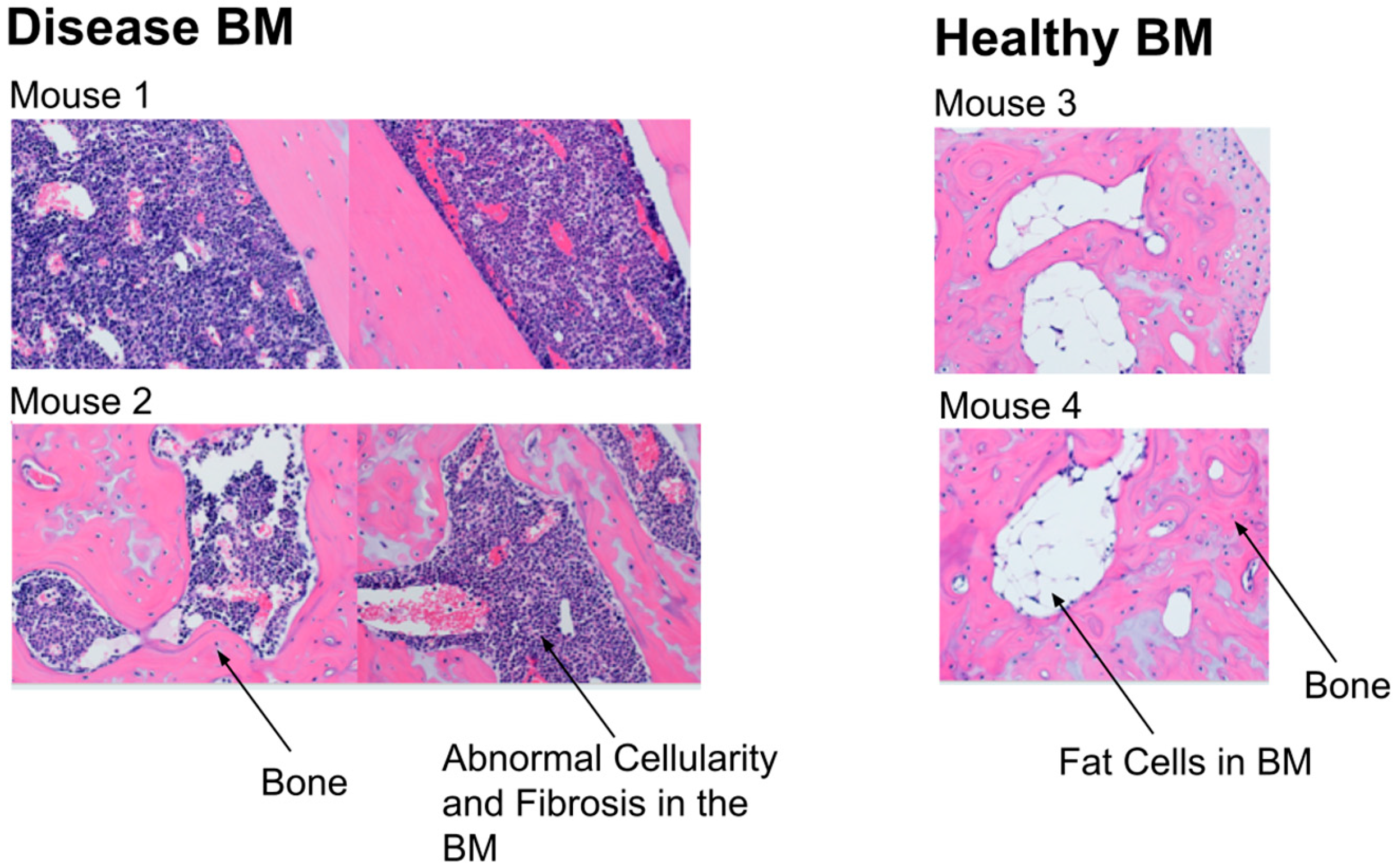
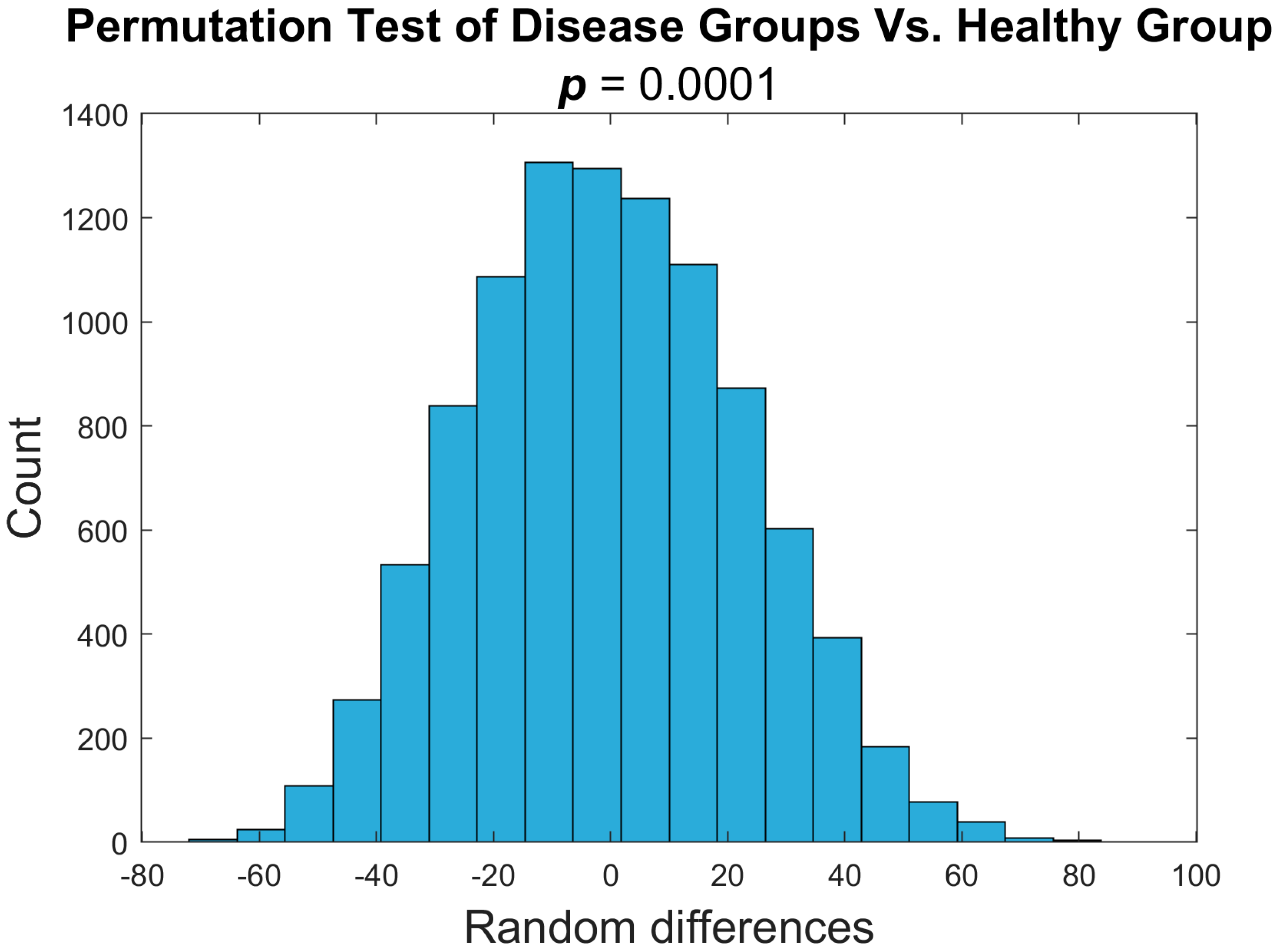
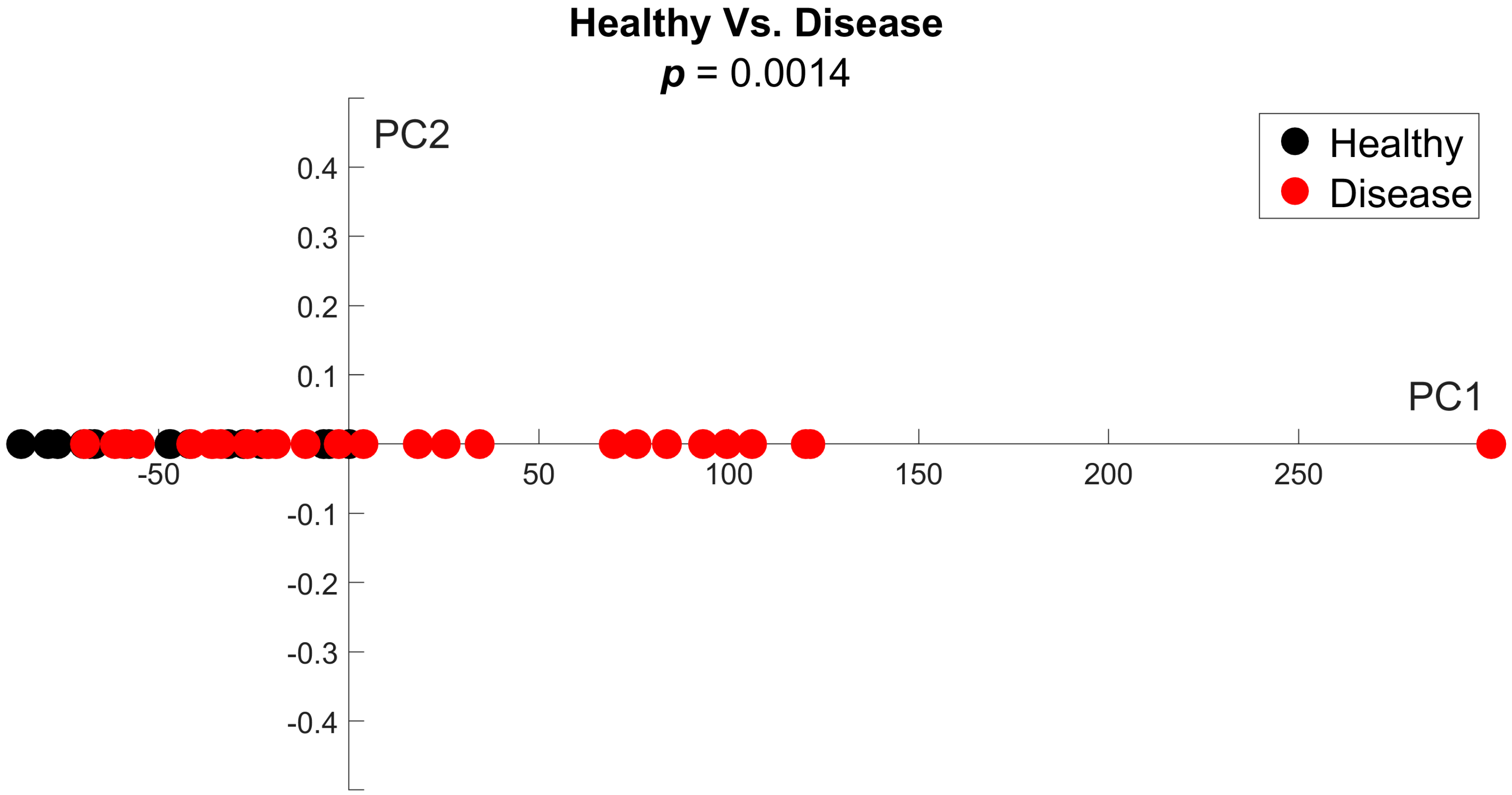
3.1. PDFF Image Analysis for Heterogeneity
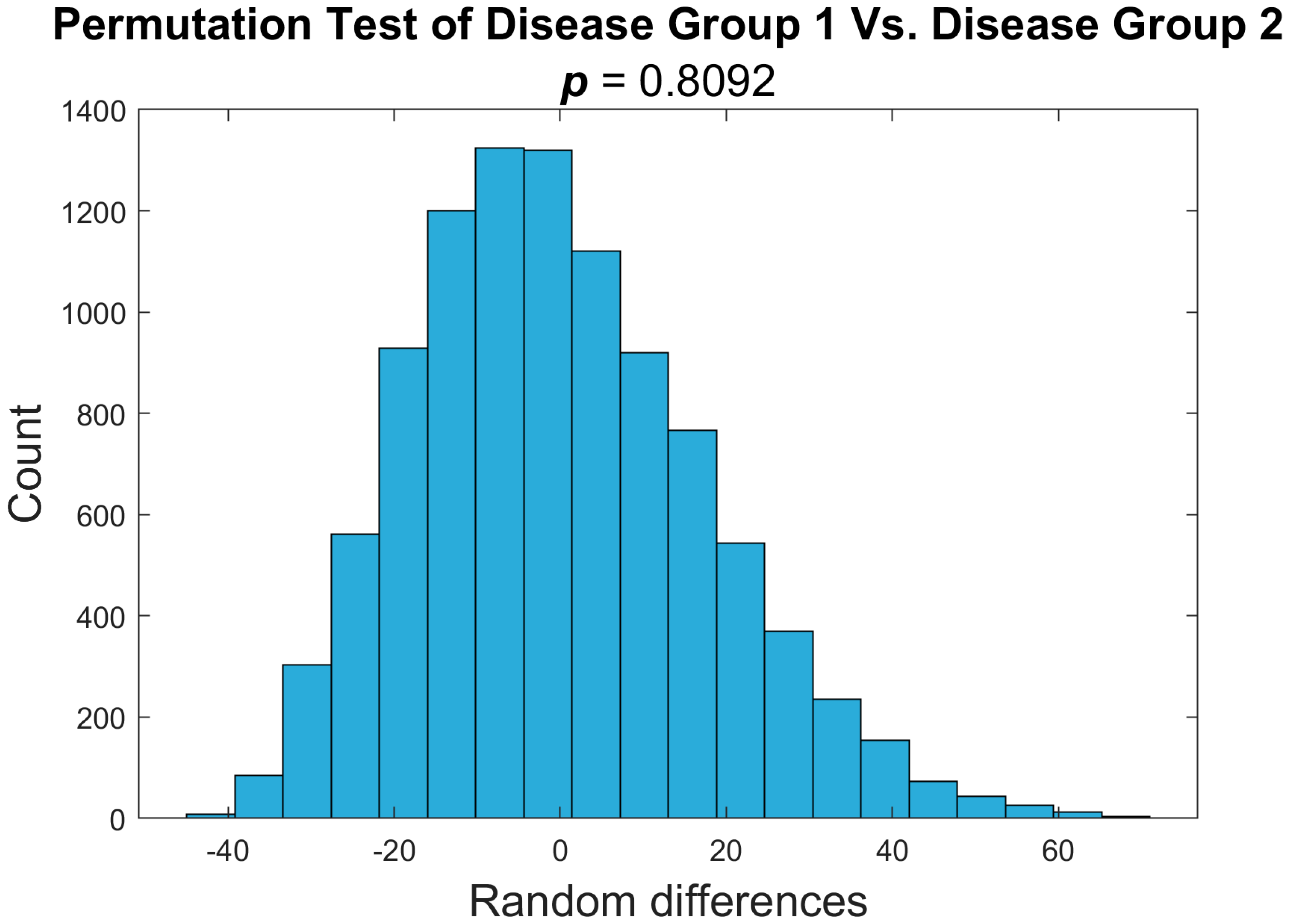
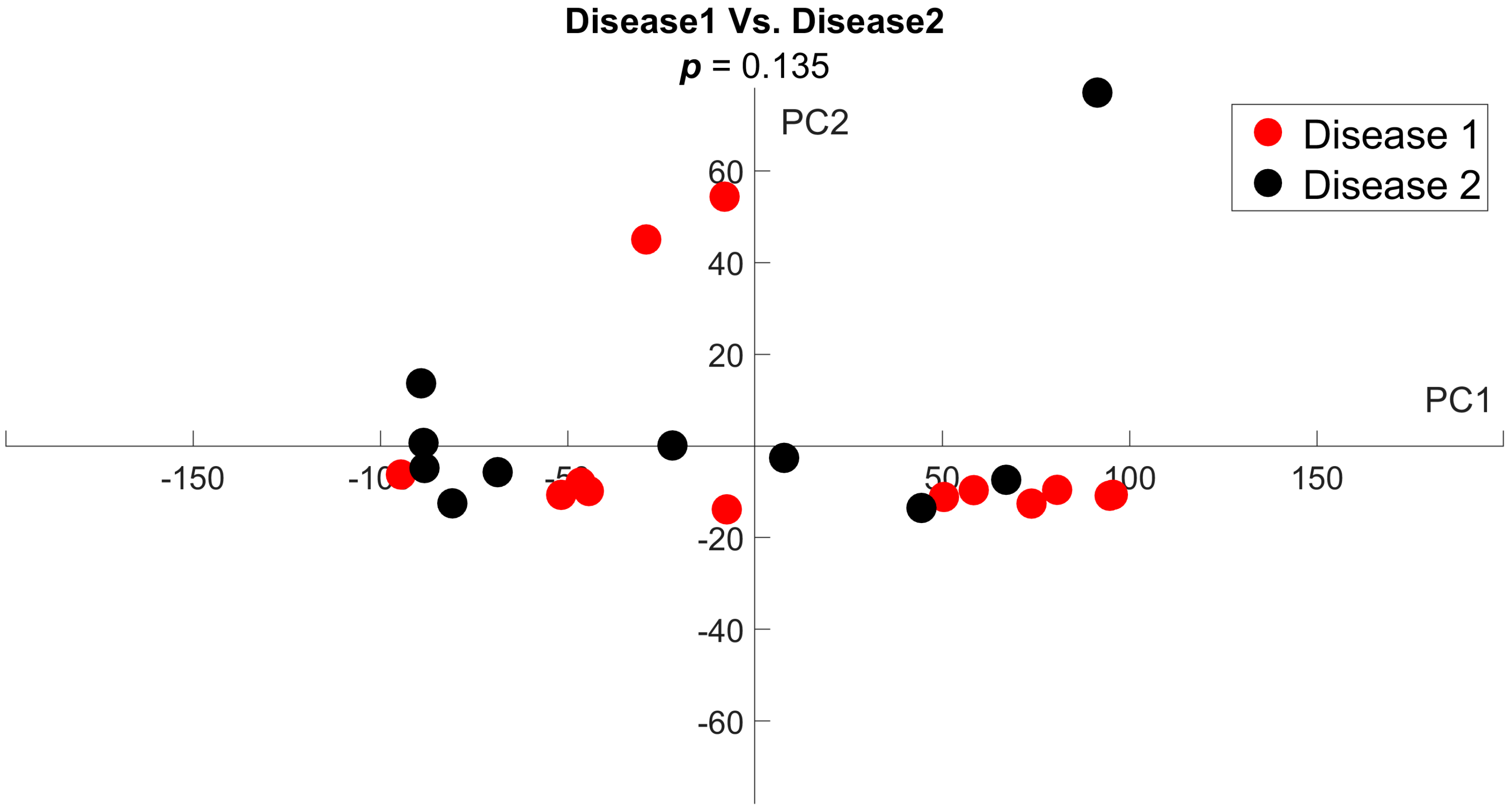
3.2. Disease Groups Show Comparable Imaging Findings
3.3. Variance as an Indicator of Disease Status
4. Discussion
4.1. Current Applications of Quantitative PDFF MRI
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDFF | Proton Density Fat Fraction |
| MF | Myelofibrosis |
| BM | Bone Marrow |
| HSPC | Hematopoietic Stem and Progenitor Cells |
| MPN | Myeloproliferative Neoplasm |
| ROIs | Region of Interest |
| PCA | Principal Component Analysis |
| ADC | Apparent Diffusion Coefficient |
| MTR | Magnetization Transfer Ratio |
| NASH | Nonalcoholic Steatohepatitis |
References
- Tefferi, A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 801–821. [Google Scholar] [CrossRef]
- Beekman, K.M.; Regenboog, M.; Nederveen, A.J.; Bravenboer, N.; den Heijer, M.; Bisschop, P.H.; Hollak, C.E.; Akkerman, E.M.; Maas, M. Gender- and Age-Associated Differences in Bone Marrow Adipose Tissue and Bone Marrow Fat Unsaturation Throughout the Skeleton, Quantified Using Chemical Shift Encoding-Based Water-Fat MRI. Front. Endocrinol 2022, 13, 815835. [Google Scholar] [CrossRef]
- Yan, J.; Hammami, M.B.; Wei, J.X.; Shah, N.; Goldfinger, M.; Mantzaris, I.; Kornblum, N.; Gritsman, K.; Sica, A.; Cooper, D.; et al. Socio-demographic determinants of myelofibrosis outcomes in an underserved center and the SEER national database. Ann. Hematol. 2024, 103, 3543–3551. [Google Scholar] [CrossRef]
- Song, M.-K.; Park, B.-B.; Uhm, J.-E. Understanding Splenomegaly in Myelofibrosis: Association with Molecular Pathogenesis. Int. J. Mol. Sci. 2018, 19, 898. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.A. How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood 2009, 113, 5394–5400. [Google Scholar] [CrossRef] [PubMed]
- Masarova, L.; Bose, P.; Pemmaraju, N.; Daver, N.G.; Sasaki, K.; Chifotides, H.T.; Zhou, L.; Kantarjian, H.M.; Estrov, Z.; Verstovsek, S. Improved survival of patients with myelofibrosis in the last decade: Single-center experience. Cancer 2022, 128, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef]
- Pikman, Y.; Lee, B.H.; Mercher, T.; McDowell, E.; Ebert, B.L.; Gozo, M.; Cuker, A.; Wernig, G.; Moore, S.; Galinsky, I.; et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006, 3, e270. [Google Scholar] [CrossRef]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013, 69, 2379–2390. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Livingston, R.A.; Hu, W.; Mascarenhas, J. Ten years of treatment with ruxolitinib for myelofibrosis: A review of safety. J. Hematol. Oncol. 2023, 16, 82. [Google Scholar] [CrossRef]
- Duek, A.; Leviatan, I.; Dolberg, O.J.; Ellis, M.H. Fedratinib for the treatment of myelofibrosis: A critical appraisal of clinical trial and “real-world” data. Blood Cancer J. 2025, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2021, 96, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.T.; Verstovsek, S.; Gupta, V.; Platzbecker, U.; Devos, T.; Kiladjian, J.-J.; McLornan, D.P.; Perkins, A.; Fox, M.L.; McMullin, M.F.; et al. Changes in bone marrow fibrosis during momelotinib or ruxolitinib therapy do not correlate with efficacy outcomes in patients with myelofibrosis. EJHaem 2024, 5, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Somervaille, T.C.; Palandri, F.; Harrison, C.; Komrokji, R.S.; Perkins, A.; Diaz, R.M.A.; Lavie, D.; Tomita, A.; Feng, Y.; et al. Addition of navitoclax to ruxolitinib for patients with myelofibrosis with progression or suboptimal response. Blood Neoplasia 2024, 2, 100056. [Google Scholar] [CrossRef]
- Anuar, N.N.M.; Hisam, N.S.N.; Liew, S.L.; Ugusman, A. Clinical Review: Navitoclax as a Pro-Apoptotic and Anti-Fibrotic Agent. Front. Pharmacol. 2020, 11, 564108. [Google Scholar] [CrossRef]
- Xiang, J.; Hurchla, M.A.; Fontana, F.; Su, X.; Amend, S.R.; Esser, A.K.; Douglas, G.J.; Mudalagiriyappa, C.; Luker, K.E.; Pluard, T.; et al. CXCR4 Protein Epitope Mimetic Antagonist POL5551 Disrupts Metastasis and Enhances Chemotherapy Effect in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2015, 14, 2473–2485. [Google Scholar] [CrossRef]
- Migliaccio, A.R.; Martelli, F.; Verrucci, M.; Migliaccio, G.; Vannucchi, A.M.; Ni, H.; Xu, M.; Jiang, Y.; Nakamoto, B.; Papayannopoulou, T.; et al. Altered SDF-1/CXCR4 axis in patients with primary myelofibrosis and in the Gata1 low mouse model of the disease. Exp. Hematol. 2008, 36, 158–171. [Google Scholar] [CrossRef]
- Slot, S.; Lavini, C.; Zwezerijnen, G.J.C.; Boden, B.J.H.; Marcus, J.T.; Huisman, M.C.; Yaqub, M.; Barbé, E.; Wondergem, M.J.; Zijlstra, J.M.; et al. Characterizing the Bone Marrow Environment in Advanced-Stage Myelofibrosis during Ruxolitinib Treatment Using PET/CT and MRI: A Pilot Study. Tomography 2023, 9, 459–474. [Google Scholar] [CrossRef]
- Robison, T.H.; Solipuram, M.; Heist, K.; Amouzandeh, G.; Lee, W.Y.; Humphries, B.A.; Buschhaus, J.M.; Bevoor, A.; Zhang, A.; Luker, K.E.; et al. Multiparametric MRI to quantify disease and treatment response in mice with myeloproliferative neoplasms. JCI Insight 2022, 7, e161457. [Google Scholar] [CrossRef]
- Robison, T.H.; Lee, W.; Luker, K.E.; Pettit, K.; Talpaz, M.; Chenevert, T.L.; Ross, B.D.; Luker, G.D. Quantitative MRI reveals heterogeneous impacts of treatment on diseased bone marrow in a mouse model of myelofibrosis. Magn. Reson. Med. 2024, 91, 2568–2578. [Google Scholar] [CrossRef]
- Idilman, I.S.; Yildiz, A.E.; Karaosmanoglu, A.D.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Proton density fat fraction: Magnetic resonance imaging applications beyond the liver. Diagn. Interv. Radiol. 2022, 28, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Calabresi, L. The MPL mutation. Int. Rev. Cell Mol. Biol. 2021, 365, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Luker, G.D.; Huong (Marie), N.; Hoff, B.A.; Galbán, C.J.; Hernando, D.; Chenevert, T.L.; Talpaz, M.; Ross, B.D. A Pilot Study of Quantitative MRI Parametric Response Mapping of Bone Marrow Fat for Treatment Assessment in Myelofibrosis. Tomography 2016, 2, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J.P.W. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging 2010, 29, 196–205. [Google Scholar] [CrossRef]
- Shamonin, D.P.; Bron, E.E.; Lelieveldt, B.P.; Smits, M.; Klein, S.; Staring, M. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front. Neuroinformatics 2013, 7, 50. [Google Scholar] [CrossRef]
- Li, Z.; MacDougald, O.A. Preclinical models for investigating how bone marrow adipocytes influence bone and hematopoietic cellularity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101547. [Google Scholar] [CrossRef]
- Spampinato, M.; Giallongo, C.; Romano, A.; Longhitano, L.; La Spina, E.; Avola, R.; Scandura, G.; Dulcamare, I.; Bramanti, V.; Di Rosa, M.; et al. Focus on Osteosclerotic Progression in Primary Myelofibrosis. Biomolecules 2021, 11, 122. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef]
- Arora, S.; Vachhani, P.; Bose, P. Investigational drugs in early phase trials for myelofibrosis. Expert Opin. Investig. Drugs 2024, 33, 1231–1244. [Google Scholar] [CrossRef]
- Galbán, C.J.; Mukherji, S.K.; Chenevert, T.L.; Meyer, C.R.; Hamstra, D.A.; Bland, P.H.; Johnson, T.D.; Moffat, B.A.; Rehemtulla, A.; Eisbruch, A.; et al. A feasibility study of parametric response map analysis of diffusion-weighted magnetic resonance imaging scans of head and neck cancer patients for providing early detection of therapeutic efficacy. Transl. Oncol. 2009, 2, 184–190. [Google Scholar] [CrossRef][Green Version]
- Galbán, C.J.; Boes, J.L.; Bule, M.; Kitko, C.L.; Couriel, D.R.; Johnson, T.D.; Lama, V.; Telenga, E.D.; Berge, M.v.D.; Rehemtulla, A.; et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1592–1598. [Google Scholar] [CrossRef]
- Rodge, G.A.; Goenka, M.K.; Goenka, U.; Afzalpurkar, S.; Shah, B.B. Quantification of Liver Fat by MRI-PDFF Imaging in Patients with Suspected Non-alcoholic Fatty Liver Disease and Its Correlation with Metabolic Syndrome, Liver Function Test and Ultrasonography. J. Clin. Exp. Hepatol. 2021, 11, 586–591. [Google Scholar] [CrossRef]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef]
- Wu, S.; Pan, J.; Song, M.; Zhao, Y.-C.; Chen, W.; Huang, H.; Zhu, Y.; Chen, F. Performance of Magnetic Resonance Imaging and Ultrasound for Identifying the Different Degrees of Hepatic Steatosis: A Systematic Review and Meta-analysis. Acad. Radiol. 2025. [Google Scholar] [CrossRef]

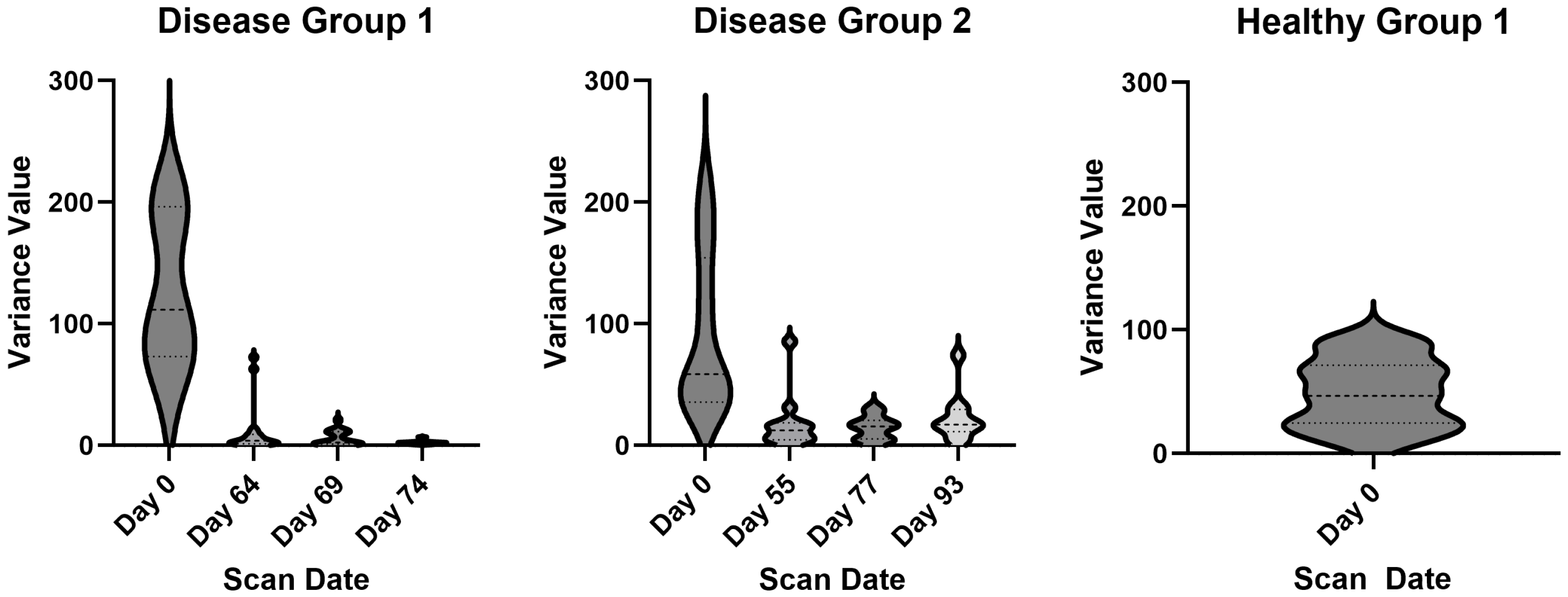

| Healthy | Disease 1 | Disease 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | Day 0 | Mouse | Day 0 | Day 64 | Day 69 | Day 74 | Mouse | Day 0 | Day 55 | Day 77 | Day 93 |
| Mouse 1 | 65.89 | Mouse 1 | 82.12 | 1.05 | 1.26 | Mouse 1 | 31.93 | 9.5 | 18.89 | 19.77 | |
| Mouse 2 | 14.32 | Mouse 2 | 111.74 | 1.57 | 20.78 | 1.65 | Mouse 2 | 57.44 | 31.09 | ||
| Mouse 3 | 34.97 | Mouse 3 | 214.96 | 1.4 | 10.82 | 6.71 | Mouse 3 | 163.22 | 1.41 | 1.6 | 3.29 |
| Mouse 4 | 61.81 | Mouse 4 | 199.62 | 4.41 | 4.83 | 1.96 | Mouse 4 | 38.51 | 3.59 | 7.05 | 13.59 |
| Mouse 5 | 25.33 | Mouse 5 | 66.81 | 7.55 | 0.74 | 2.04 | Mouse 5 | 52.02 | 5.98 | 20.52 | 28.64 |
| Mouse 6 | 70.4 | Mouse 6 | 177.22 | 5.16 | 1.65 | 2.37 | Mouse 6 | 127.93 | 9.52 | 15.55 | 17.99 |
| Mouse 7 | 46.29 | Mouse 7 | 72.02 | 10.33 | 1.42 | 0.93 | Mouse 7 | 97.35 | 16.15 | 14.07 | 4.62 |
| Mouse 8 | 72.34 | Mouse 8 | 111.65 | 72.55 | 2.75 | 2.83 | Mouse 8 | 59.87 | 19.28 | 5.45 | |
| Mouse 9 | 26.59 | Mouse 9 | 213.76 | 2.53 | 9.85 | 0.87 | Mouse 9 | 34.51 | 16.19 | 27.19 | 74.25 |
| Mouse 10 | 86.88 | Mouse 10 | 169.2 | 3.93 | 1.18 | 2.26 | Mouse 10 | 186.73 | 4.13 | 3.3 | 16.2 |
| Mouse 11 | 16.88 | Mouse 11 | 118.98 | 2.35 | Mouse 11 | 31.97 | 15.13 | 31.33 | 15.4 | ||
| Mouse 12 | 23.8 | Mouse 12 | 24.07 | 13.27 | 2.05 | 2.11 | Mouse 12 | 213.79 | 85.38 | 16.48 | 32.83 |
| Mouse 13 | 88.29 | Mouse 13 | 74.3 | 7.85 | 0.68 | 3.62 | |||||
| Mouse 14 | 46.58 | Mouse 14 | 193.03 | 0.83 | 13.17 | 2.38 | |||||
| Mouse 15 | 93.63 | Mouse 15 | 394.17 | 1.86 | 0.59 | 0.62 | |||||
| Mouse 16 | 90.85 | 62.97 | 11.41 | 2.86 | |||||||
| Mouse 17 | 57.7 | 0.6 | 12.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenner, L.; Robison, T.H.; Johnson, T.D.; Pettit, K.; Talpaz, M.; Chenevert, T.L.; Ross, B.D.; Luker, G.D. Fat Fraction MRI for Longitudinal Assessment of Bone Marrow Heterogeneity in a Mouse Model of Myelofibrosis. Tomography 2025, 11, 82. https://doi.org/10.3390/tomography11080082
Brenner L, Robison TH, Johnson TD, Pettit K, Talpaz M, Chenevert TL, Ross BD, Luker GD. Fat Fraction MRI for Longitudinal Assessment of Bone Marrow Heterogeneity in a Mouse Model of Myelofibrosis. Tomography. 2025; 11(8):82. https://doi.org/10.3390/tomography11080082
Chicago/Turabian StyleBrenner, Lauren, Tanner H. Robison, Timothy D. Johnson, Kristen Pettit, Moshe Talpaz, Thomas L. Chenevert, Brian D. Ross, and Gary D. Luker. 2025. "Fat Fraction MRI for Longitudinal Assessment of Bone Marrow Heterogeneity in a Mouse Model of Myelofibrosis" Tomography 11, no. 8: 82. https://doi.org/10.3390/tomography11080082
APA StyleBrenner, L., Robison, T. H., Johnson, T. D., Pettit, K., Talpaz, M., Chenevert, T. L., Ross, B. D., & Luker, G. D. (2025). Fat Fraction MRI for Longitudinal Assessment of Bone Marrow Heterogeneity in a Mouse Model of Myelofibrosis. Tomography, 11(8), 82. https://doi.org/10.3390/tomography11080082







