Time-Intensity Curve Analysis of Contrast-Enhanced Ultrasound for Non-Ossified Thyroid Cartilage Invasion in Laryngeal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Contrast-Enhanced Ultrasound Examination
2.3. Image Analysis
2.4. Histological Examination
2.5. Statistical Analysis
3. Results
3.1. CEUS Imaging Features of Non-Ossified Thyroid Cartilage
3.2. TIC Analysis of Non-Ossified Thyroid Cartilage and Tumor Tissue
3.3. TIC Morphology Analysis of Non-Ossified Thyroid Cartilage and Tumor Tissue
3.4. Logistic Regression Analysis for Non-Ossified Thyroid Cartilage Invasion
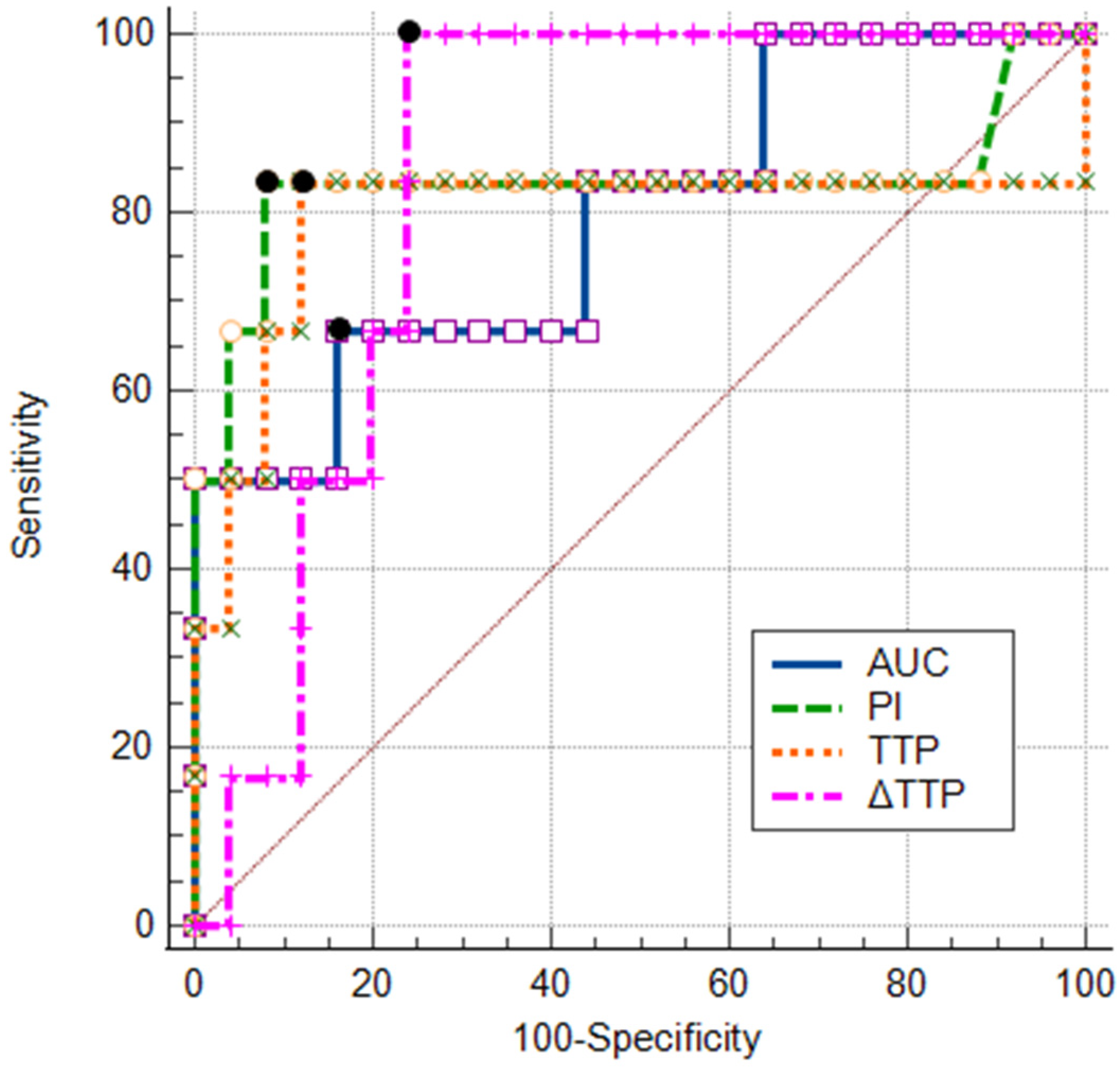
3.5. ROC Analysis of TIC Parameters and Their Combinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCC | Squamous cell carcinoma |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| CEUS | Contrast-enhanced ultrasound |
| TIC | Time–intensity curve |
| CECT | Contrast-enhanced computed tomography |
| ROI | Region of interest |
| TTP | Time to peak |
| PI | Peak intensity |
| WIS | Wash-in slope |
| AUC | Area under the curve |
| DCECT | Dynamic contrast-enhanced computed tomography |
| ROC | Receiver operating characteristic |
References
- Doğan, S.; Vural, A.; Kahriman, G.; İmamoğlu, H.; Abdülrezzak, Ü.; Öztürk, M. Non-squamous cell carcinoma diseases of the larynx: Clinical and imaging findings. Braz. J. Otorhinolaryngol. 2020, 86, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Park, S.G.; Song, E.K.; Cho, S.H.; Park, M.R.; Park, K.U.; Lee, K.-H.; Song, I.-C.; Lee, H.J.; Jo, D.-Y.; et al. Comparison of the therapeutic effects of total laryngectomy and a larynx-preservation approach in patients with T4a laryngeal cancer and thyroid cartilage invasion: A multicenter retrospective review. Head Neck 2016, 38, 1271–1277. [Google Scholar] [CrossRef]
- Connor, S. Laryngeal cancer: How does the radiologist help? Cancer Imaging 2007, 7, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Hermans, R. Staging of laryngeal and hypopharyngeal cancer: Value of imaging studies. Eur. Radiol. 2006, 16, 2386–2400. [Google Scholar] [CrossRef]
- Shoushtari, S.T.; Gal, J.; Chamorey, E.; Schiappa, R.; Dassonville, O.; Poissonnet, G.; Aloi, D.; Barret, M.; Safta, I.; Saada, E.; et al. Salvage vs. Primary Total Laryngectomy in Patients with Locally Advanced Laryngeal or Hypopharyngeal Carcinoma: Oncologic Outcomes and Their Predictive Factors. J. Clin. Med. 2023, 12, 1305. [Google Scholar] [CrossRef]
- Dadfar, N.; Seyyedi, M.; Forghani, R.; Curtin, H.D. Computed Tomography Appearance of Normal Nonossified Thyroid Cartilage Implication for Tumor Invasion Diagnosis. J. Comput. Assist. Tomogr. 2015, 39, 240–243. Available online: www.jcat.org (accessed on 1 February 2025). [CrossRef]
- Li, B.; Bobinski, M.; Gandour-Edwards, R.; Farwell, D.G.; Chen, A.M. Overstaging of cartilage invasion by multidetector CT scan for laryngeal cancer and its potential effect on the use of organ preservation with chemoradiation. Br. J. Radiol. 2011, 84, 64–69. [Google Scholar] [CrossRef]
- Kuno, H.; Sakamaki, K.; Fujii, S.; Sekiya, K.; Otani, K.; Hayashi, R.; Yamanaka, T.; Sakai, O.; Kusumoto, M. Comparison of MR imaging and dual-energy ct for the evaluation of cartilage invasion by laryngeal and hypopharyngeal squamous cell carcinoma. Am. J. Neuroradiol. 2018, 39, 524–531. [Google Scholar] [CrossRef]
- Cho, S.J.; Lee, J.H.; Suh, C.H.; Kim, J.Y.; Kim, D.; Lee, J.B.; Lee, M.K.; Chung, S.R.; Choi, Y.J.; Baek, J.H. Comparison of diagnostic performance between CT and MRI for detection of cartilage invasion for primary tumor staging in patients with laryngo-hypopharyngeal cancer: A systematic review and meta-analysis. Eur. Radiol. 2020, 30, 3803–3812. [Google Scholar] [CrossRef]
- Becker, M.; Zbären, P.; Casselman, J.W.; Kohler, R.; Dulguerov, P.; Becker, C.D. Neoplastic invasion of laryngeal cartilage: Reassessment of criteria for diagnosis at MR imaging. Radiol. Soc. North Am. 2008, 249, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Monnier, Y.; de Vito, C. MR Imaging of Laryngeal and Hypopharyngeal Cancer. Magn. Reson. Imaging Clin. North Am. 2022, 30, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, I.; Hejleh, T.A.; Qandeel, M.; Al-Hussaini, M.; Koro, S.; Taqash, A.; Almousa, A.; Abuhijla, F.; Abuhijlih, R.; Ajlouni, F.; et al. Concordance between head and neck MRI and histopathology in detecting laryngeal subsite invasion among patients with laryngeal cancer. Cancer Imaging 2023, 23, 99. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: Update 2017 (long version). Ultraschall Der Med. 2018, 39, e2–e44. [Google Scholar]
- Dietrich, C.F.; Nolsoe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver-Update 2020-WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 41, 562–585. [Google Scholar]
- Petrasova, H.; Slaisova, R.; Rohan, T.; Stary, K.; Kyclova, J.; Pavlik, T.; Kovalcikova, P.; Kazda, T.; Valek, V. Contrast-Enhanced Ultrasonography for Differential Diagnosis of Benign and Malignant Thyroid Lesions: Single-Institutional Prospective Study of Qualitative and Quantitative CEUS Characteristics. Contrast Media Mol. Imaging 2022, 2022, 8229445. [Google Scholar] [CrossRef]
- Wu, M.; Hu, Y.; Hang, J.; Peng, X.; Mao, C.; Ye, X.; Li, A. Qualitative and Quantitative Contrast-Enhanced Ultrasound Combined with Conventional Ultrasound for Predicting the Malignancy of Soft Tissue Tumors. Ultrasound Med. Biol. 2022, 48, 237–247. [Google Scholar] [CrossRef]
- Badiu, M.S.; Gheorghe, E.C.; Nicolau, C.; Săftoiu, A. Quantitative time intensity curve analysis of contrast-enhanced ultrasound (CEUS) examinations for the assessment of focal liver lesions. Med. Ultrason. 2024, 26, 63–71. [Google Scholar] [CrossRef]
- Schwarz, S.; Clevert, D.A.; Ingrisch, M.; Geyer, T.; Schwarze, V.; Rübenthaler, J.; Armbruster, M. Quantitative analysis of the time–intensity curve of contrast-enhanced ultrasound of the liver: Differentiation of benign and malignant liver lesions. Diagnostics 2021, 11, 1244. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Liu, J.; Wang, B.; Zhang, H. Value of Qualitative and Quantitative Contrast-Enhanced Ultrasound Analysis in Preoperative Diagnosis of Cervical Lymph Node Metastasis From Papillary Thyroid Carcinoma. J. Ultrasound Med. 2020, 39, 73–81. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Danišovič, L.; Varga, I.; Zamborský, R.; Böhmer, D. The tissue engineering of articular cartilage: Cells, scaffolds and stimulating factors. Exp. Biol. Med. 2012, 237, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhu, S.Y.; Liu, R.C.; Zheng, H.Y.; Lun, H.M.; Wei, H.M.; Weng, J.J. Contrast-enhanced ultrasound for the preoperative assessment of laryngeal carcinoma: A preliminary study. Acta Radiol. 2021, 62, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Pucėtaitė, M.; Farina, D.; Ryškienė, S.; Mitraitė, D.; Tarasevičius, R.; Lukoševičius, S.; Padervinskis, E.; Vaitkus, S. The Diagnostic Value of CEUS in Assessing Non-Ossified Thyroid Cartilage Invasion in Patients with Laryngeal Squamous Cell Carcinoma. J. Clin. Med. 2024, 13, 891. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C.H. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Chichester, UK, 2017; pp. 25–36. [Google Scholar]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.-A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef]
- Jung, E.M.; Weber, M.A.; Wiesinger, I. Contrast-enhanced ultrasound perfusion imaging of organs. Radiologe 2021, 61, 19–28. Available online: https://link.springer.com/article/10.1007/s00117-021-00891-7 (accessed on 28 December 2024). [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Olszewski, E. Blood Vascular System in Cancer of the Larynx. Available online: http://archotol.jamanetwork.com/ (accessed on 1 February 2025).
- Wangaryattawanich, P.; Agarwal, M.; Rath, T. Imaging features of cartilaginous tumors of the head and neck. J. Clin. Imaging Sci. 2021, 11, 66. [Google Scholar] [CrossRef]
- Yousefzadeh, D.K.; Doerger, K.; Sullivan, C. The blood supply of early, late, and nonossifying cartilage: Preliminary gray-scale and Doppler assessment and their implications. Pediatr. Radiol. 2008, 38, 146–158. [Google Scholar] [CrossRef]
- Citil, S.; Dogan, S.; Atilgan, H.I.; Menzilcioglu, M.S.; Sahin, T.; Abdulrezzak, U.; Duymus, M.; Ozturk, M. Comparison of dynamic contrast-enhanced MRI and PET/CT in the evaluation of laryngeal cancer after inadequate CT results. Pol. J. Radiol. 2015, 80, 428–432. [Google Scholar] [CrossRef]
- Trojanowska, A.; Trojanowski, P.; Drop, A.; Jargiełło, T.; Klatka, J. Head and neck cancer: Value of perfusion CT in depicting primary tumor spread. Med. Sci. Monit. 2012, 18, CR112–CR118. [Google Scholar] [CrossRef]
- Yu, J.; Xu, W.; Wang, L.; Jiang, N.; Dou, W.; Li, C.; Sun, L. The clinical value of DCE-MRI for differentiating secondary laryngeal cartilage lesions. Medicine 2023, 102, E33352. [Google Scholar] [CrossRef] [PubMed]
- Dankbaar, J.W.; Oosterbroek, J.; Jager, E.A.; de Jong, H.W.; Raaijmakers, C.P.; Willems, S.M.; Terhaard, C.H.; Philippens, M.E.; Pameijer, F.A. Detection of cartilage invasion in laryngeal carcinoma with dynamic contrast-enhanced CT. Laryngoscope Investig. Otolaryngol. 2017, 2, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, Y.; Liu, X.; Chen, Y.; Xu, L.; Ren, W.; Chen, W.; Chan, Q. Early response to chemoradiotherapy for nasopharyngeal carcinoma treatment: Value of dynamic contrast-enhanced 3.0 T MRI. J. Magn. Reson. Imaging 2015, 41, 1528–1540. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, Y.; Chen, Y.; Xu, L.; Chen, W.; Yao, Y.; Du, Z.; Deng, X.; Chan, Q. Dynamic contrast-enhanced MRI of nasopharyngeal carcinoma: A preliminary study of the correlations between quantitative parameters and clinical stage. J. Magn. Reson. Imaging 2014, 39, 940–948. [Google Scholar] [CrossRef]
- Huang, B.; Wong, C.S.; Whitcher, B.; Kwong, D.L.W.; Lai, V.; Chan, Q.; Khong, P.-L. Dynamic contrast-enhanced magnetic resonance imaging for characterising nasopharyngeal carcinoma: Comparison of semiquantitative and quantitative parameters and correlation with tumour stage. Eur. Radiol. 2013, 23, 1495–1502. [Google Scholar] [CrossRef]
- Zhang, C.C.; Yan, Z.; Giddabasappa, A.; Lappin, P.B.; Painter, C.L.; Zhang, Q.; Li, G.; Goodman, J.; Simmons, B.; Pascual, B.; et al. Comparison of dynamic contrast-enhanced MR, ultrasound and optical imaging modalities to evaluate the antiangiogenic effect of PF-03084014 and sunitinib. Cancer Med. 2014, 3, 462–471. [Google Scholar] [CrossRef]
- Baba, A.; Kurokawa, R.; Rawie, E.; Kurokawa, M.; Ota, Y.; Srinivasan, A. Normalized Parameters of Dynamic Contrast-Enhanced PerfusionMRI and DWI-ADC for Differentiation between Posttreatment Changes and Recurrence in Head and Neck Cancer. Am. J. Neuroradiol. 2022, 43, 1184–1189. [Google Scholar] [CrossRef]
- Ng, S.H.; Liao, C.T.; Lin, C.Y.; Chan, S.C.; Lin, Y.C.; Yen, T.C.; Li, G.; Goodman, J.; Simmons, B.; Pascual, B.; et al. Dynamic contrast-enhanced MRI, diffusion-weighted MRI and 18F-FDG PET/CT for the prediction of survival in oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiation. Eur Radiol. 2016, 26, 4162–4172. [Google Scholar] [CrossRef]
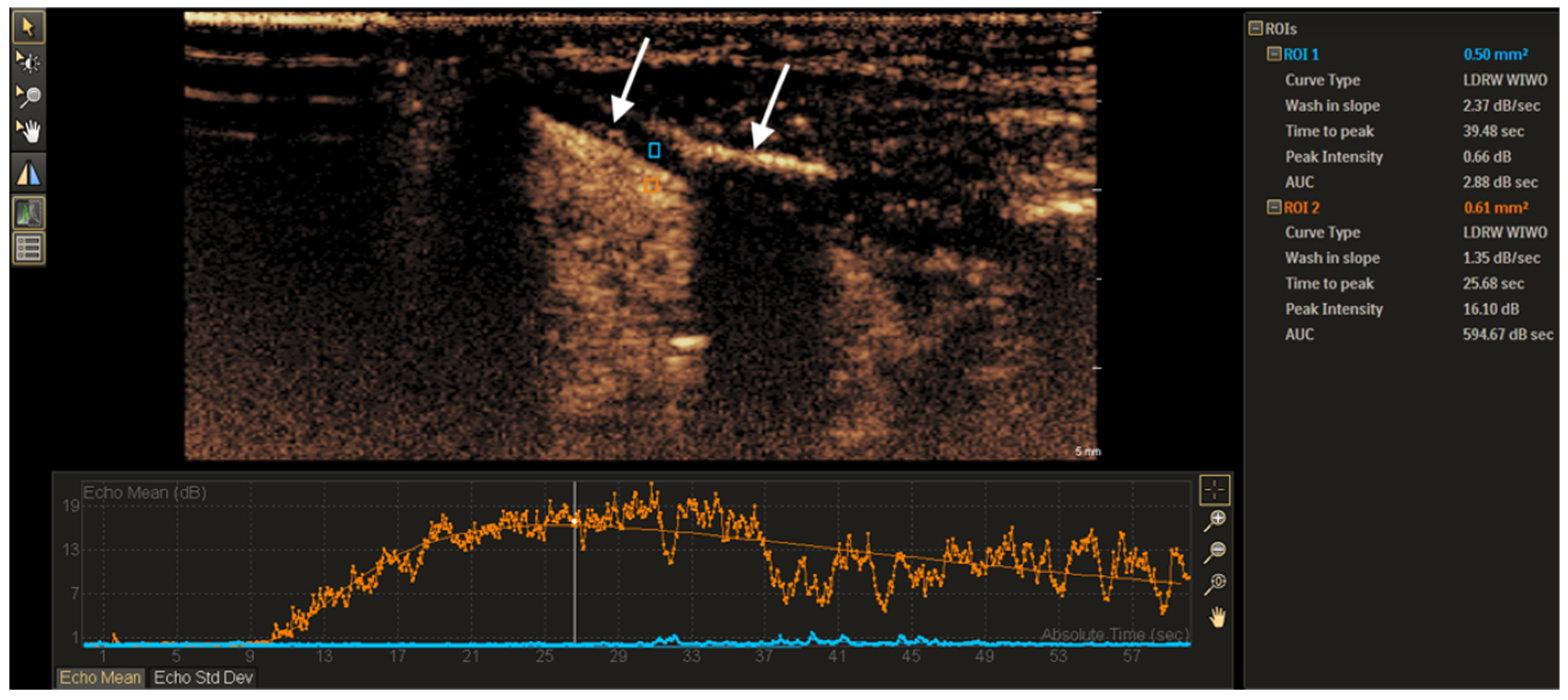
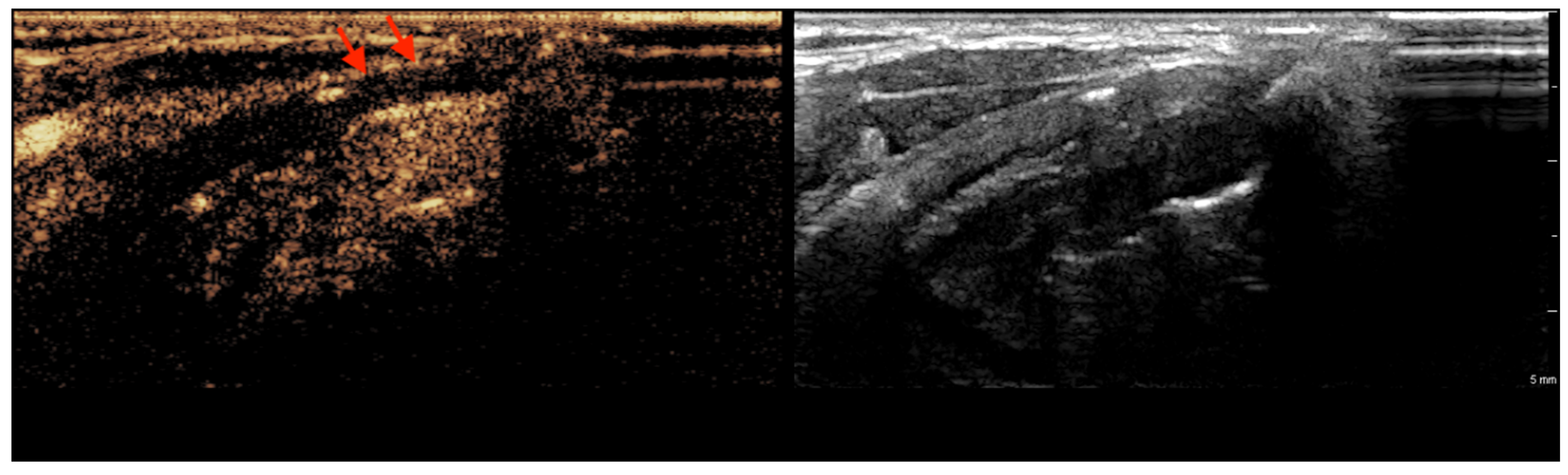
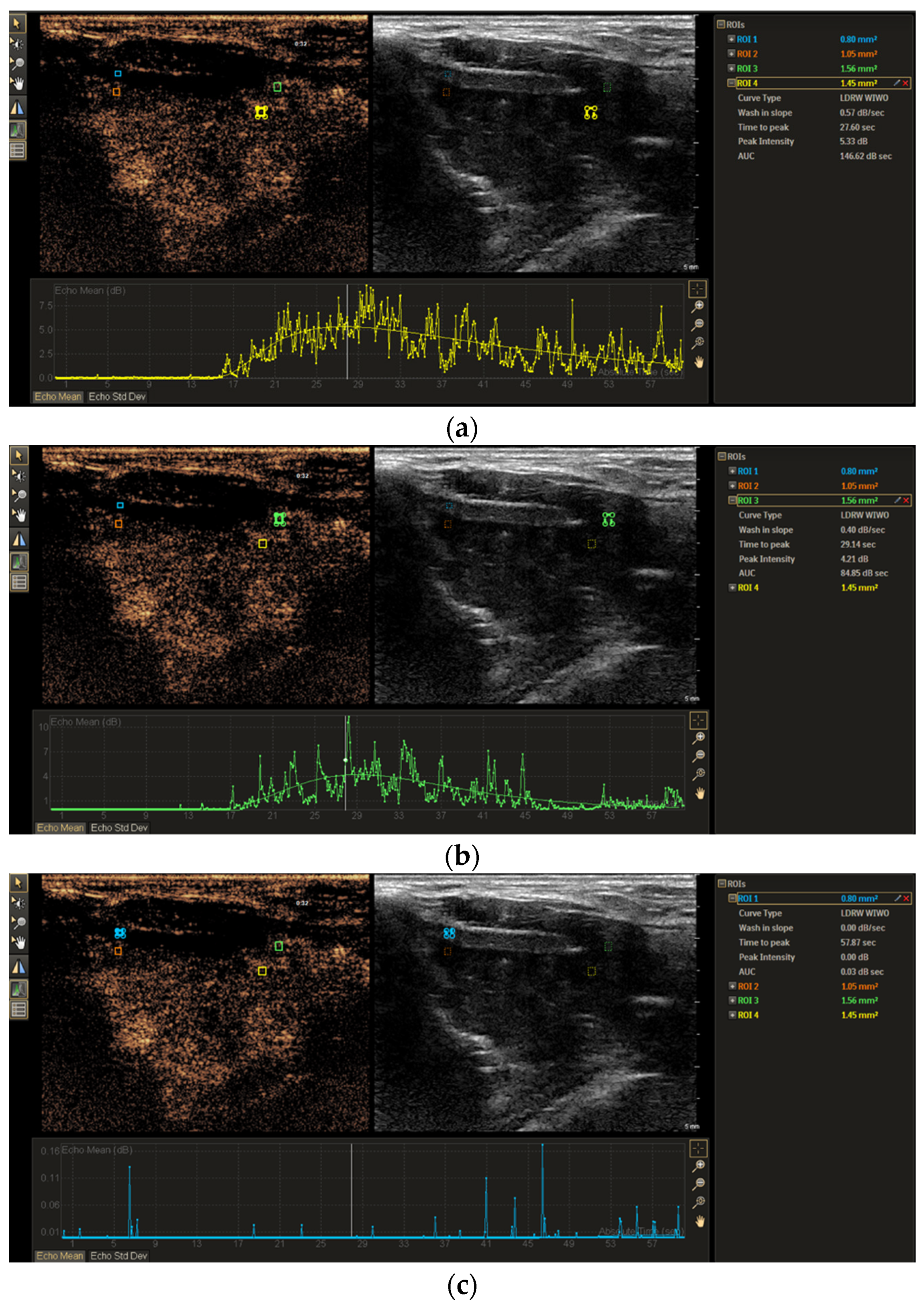
| Feature | Thyroid Cartilage Without Invasion (n = 26) | Thyroid Cartilage with Invasion (n = 6) | p Value |
|---|---|---|---|
| No enhancement | 22 (84.6) | 1 (16.7) | <0.05 |
| Visible enhancement | 4 (15.4) | 5 (83.3) |
| Parameter | Thyroid Cartilage Without Invasion | Thyroid Cartilage with Invasion | p Value 1 | Tumor | p Value 2 |
|---|---|---|---|---|---|
| TTP, s | 44.4 (30.99–58.31) | 32.83 (29.02–58.70) | <0.05 | 28.44 (10.95–49.80) | <0.05 n <0.05 i |
| PI, dB | 1.85 (0.00–7.22) | 12.27 (0.30–21.80) | <0.05 | 13.59 (4.65–46.41) | <0.05 n <0.05 i |
| AUC, dB × s | 10.30 (0.30–58.54) | 69.05 (3.85–330.12) | <0.05 | 313.00 (61.05–657.89) | <0.05 n >0.05 i |
| WIS, dB/s | 3.64 (0.00–19.51) | 11.89 (0.60–46.77) | >0.05 | 1.74 (0.48–10.06) | <0.05 n >0.05 i |
| Parameter | Thyroid Cartilage Without Invasion | Thyroid Cartilage with Invasion | p Value |
|---|---|---|---|
| ∆TTP, s | 15.19 (2.02–36.54) | 5.10 (1.83–8.90) | <0.05 |
| ∆PI, dB | 11.60 (3.33–37.11) | 4.15 (1.60–24.61) | >0.05 |
| ∆AUC, dB × s | 333.75 (67.18–650.42) | 199.55 (85.39–303.46) | >0.05 |
| ∆WIS, dB/s | 5.11 (0.24–15.92) | 11.18 (0.27–45.87) | >0.05 |
| Univariate Logistic Regression | Odds Ratio | 95% CI | p Value | |
|---|---|---|---|---|
| TTP (1 s) | 0.88 | 0.76–1.01 | 0.067 | |
| PI (1 dB) | 1.59 | 0.97–2.62 | 0.067 | |
| AUC (1 dB × s) | 1.03 | 0.99–1.07 | 0.103 | |
| ∆TTP (1 s) | 0.80 | 0.64–1.00 | 0.047 | |
| Multivariate logistic regression models | ||||
| Model 1 | TTP (1 s) | 1.04 | 0.87–1.25 | 0.64 |
| ∆TTP (1 s) | 0.76 | 0.56–1.05 | 0.09 | |
| Model 2 | PI (1 dB) | 1.66 | 0.87–3.14 | 0.12 |
| ∆TTP (1 s) | 0.82 | 0.64–1.06 | 0.12 | |
| Parameter | AUCROC | 95% CI | Sensitivity (%) | Specificity (%) | Accuracy (%) | Cut-Off | p Value |
|---|---|---|---|---|---|---|---|
| TTP (s) | 0.80 | 0.62–0.92 | 83.3 | 88.5 | 87.5 | ≤36.3 | 0.047 |
| PI (dB) | 0.83 | 0.65–0.94 | 83.3 | 88.0 | 87.1 | ≥3.65 | 0.015 |
| AUC (dB × s) | 0.79 | 0.61–0.92 | 66.7 | 80.0 | 77.4 | ≥18.36 | 0.008 |
| ∆TTP (s) | 0.85 | 0.68–0.95 | 100.0 | 76.9 | 81.3 | ≤8.9 | <0.001 |
| Model 1 (TTP + ∆TTP) | 0.82 | 0.65–0.93 | 88.5 | 76.9 | 87.5 | – | <0.0001 |
| Model 2 (PI + ∆TTP) | 0.93 | 0.77–0.99 | 83.3 | 100 | 96.8 | – | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucėtaitė, M.; Mitraitė, D.; Tarasevičius, R.; Farina, D.; Ryškienė, S.; Lukoševičius, S.; Padervinskis, E.; Šarauskas, V.; Vaitkus, S. Time-Intensity Curve Analysis of Contrast-Enhanced Ultrasound for Non-Ossified Thyroid Cartilage Invasion in Laryngeal Squamous Cell Carcinoma. Tomography 2025, 11, 57. https://doi.org/10.3390/tomography11050057
Pucėtaitė M, Mitraitė D, Tarasevičius R, Farina D, Ryškienė S, Lukoševičius S, Padervinskis E, Šarauskas V, Vaitkus S. Time-Intensity Curve Analysis of Contrast-Enhanced Ultrasound for Non-Ossified Thyroid Cartilage Invasion in Laryngeal Squamous Cell Carcinoma. Tomography. 2025; 11(5):57. https://doi.org/10.3390/tomography11050057
Chicago/Turabian StylePucėtaitė, Milda, Dalia Mitraitė, Rytis Tarasevičius, Davide Farina, Silvija Ryškienė, Saulius Lukoševičius, Evaldas Padervinskis, Valdas Šarauskas, and Saulius Vaitkus. 2025. "Time-Intensity Curve Analysis of Contrast-Enhanced Ultrasound for Non-Ossified Thyroid Cartilage Invasion in Laryngeal Squamous Cell Carcinoma" Tomography 11, no. 5: 57. https://doi.org/10.3390/tomography11050057
APA StylePucėtaitė, M., Mitraitė, D., Tarasevičius, R., Farina, D., Ryškienė, S., Lukoševičius, S., Padervinskis, E., Šarauskas, V., & Vaitkus, S. (2025). Time-Intensity Curve Analysis of Contrast-Enhanced Ultrasound for Non-Ossified Thyroid Cartilage Invasion in Laryngeal Squamous Cell Carcinoma. Tomography, 11(5), 57. https://doi.org/10.3390/tomography11050057






