Preliminary Results of Clinical Experience with Consolidative High-Dose Thoracic Radiotherapy for Patients with Extensive-Stage Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Scheme
2.3. Surveillance
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
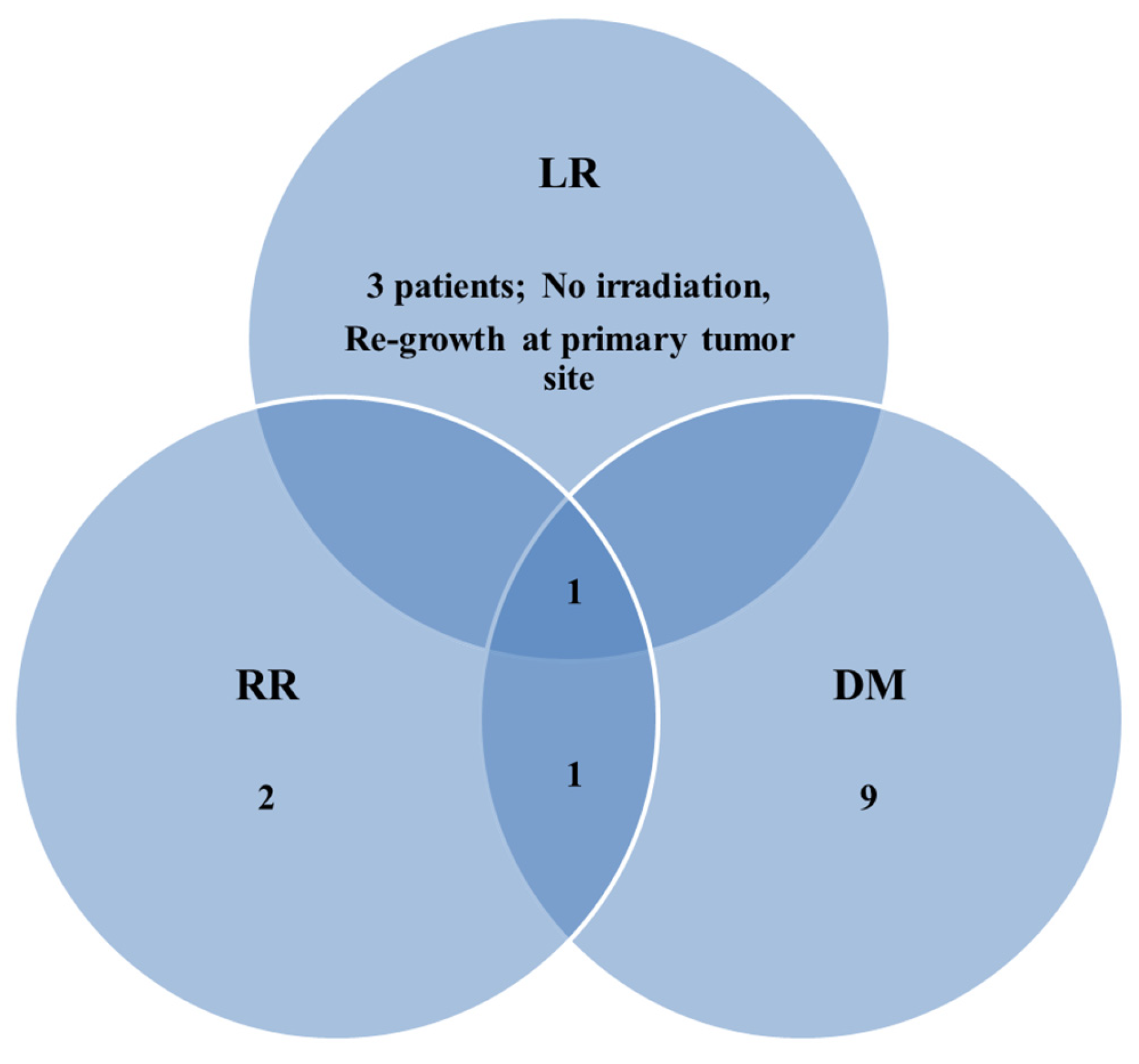
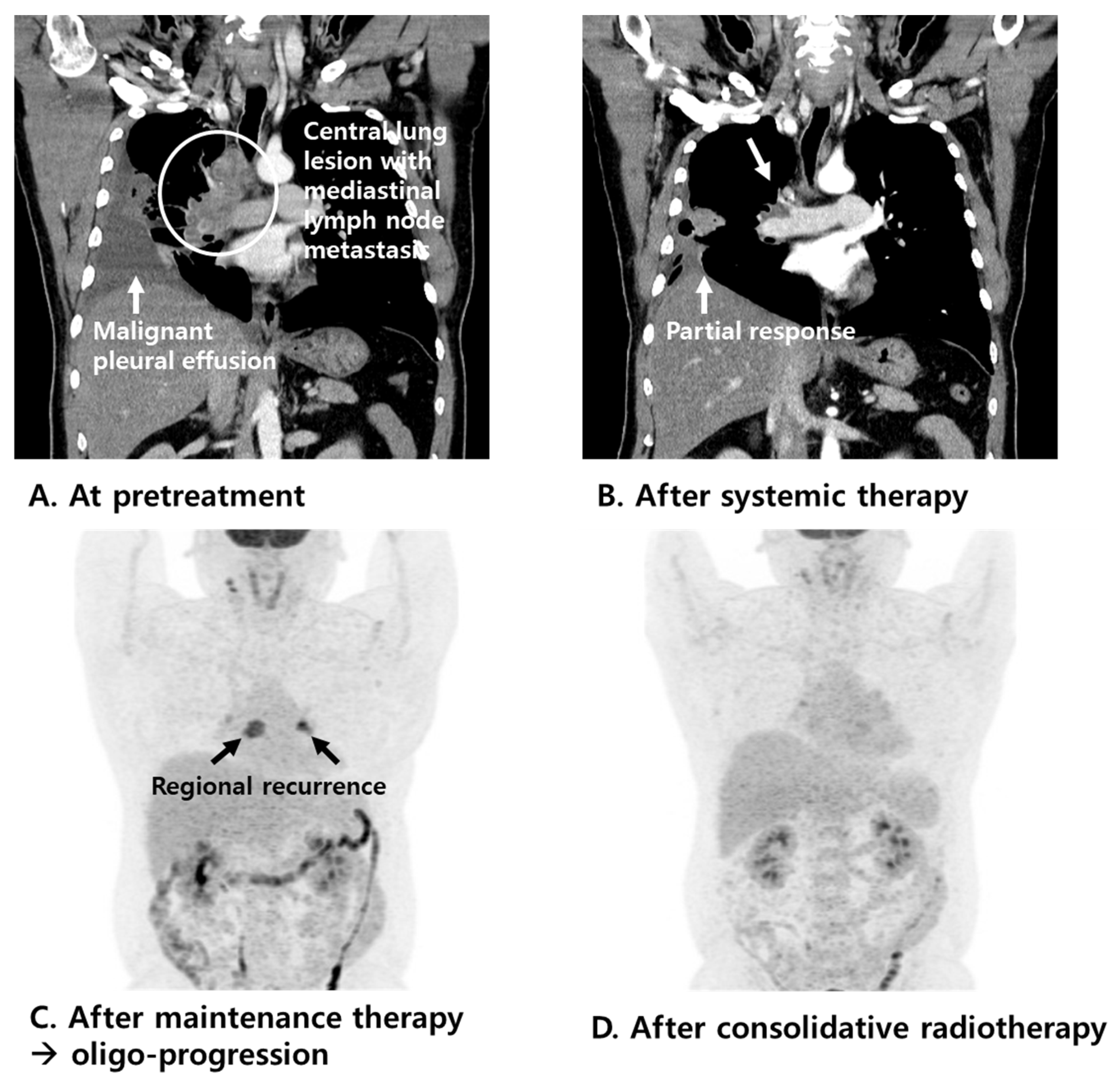
3.2. Patterns of Failure
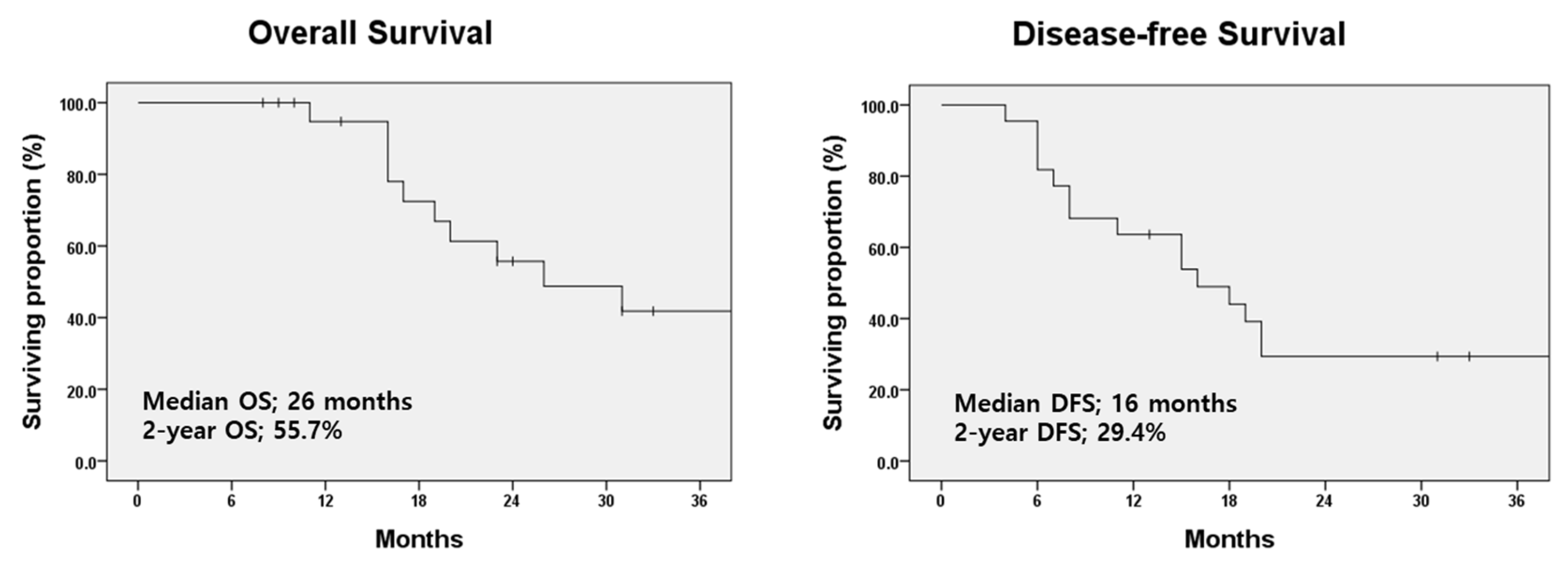
3.3. Treatment-Related Complications and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganti, A.K.P.; Loo, B.W.; Bassetti, M.; Blakely, C.; Chiang, A.; D’Amico, T.A.; D’Avella, C.; Dowlati, A.; Downey, R.J.; Edelman, M.; et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1441–1464. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczesna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Chawla, S.; Chen, Y.; Katz, A.W.; Muhs, A.G.; Philip, A.; Okunieff, P.; Milano, M.T. Stereotactic body radiotherapy for treatment of adrenal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 71–75. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Mendel, E.; Yamada, Y. Radiotherapy and radiosurgery for metastatic spine disease: What are the options, indications, and outcomes? Spine 2009, 34, S78–S92. [Google Scholar] [CrossRef]
- Holy, R.; Piroth, M.; Pinkawa, M.; Eble, M.J. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther. Onkol. 2011, 187, 245–251. [Google Scholar] [CrossRef]
- Mendez Romero, A.; de Man, R.A. Stereotactic body radiation therapy for primary and metastatic liver tumors: From technological evolution to improved patient care. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 603–616. [Google Scholar] [CrossRef]
- Mendez Romero, A.; Wunderink, W.; Hussain, S.M.; De Pooter, J.A.; Heijmen, B.J.; Nowak, P.C.; Nuyttens, J.J.; Brandwijk, R.P.; Verhoef, C.; Ijzermans, J.N.; et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006, 45, 831–837. [Google Scholar] [CrossRef]
- Milano, M.T.; Katz, A.W.; Muhs, A.G.; Philip, A.; Buchholz, D.J.; Schell, M.C.; Okunieff, P. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008, 112, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Rusthoven, K.E.; Kavanagh, B.D.; Burri, S.H.; Chen, C.; Cardenes, H.; Chidel, M.A.; Pugh, T.J.; Kane, M.; Gaspar, L.E.; Schefter, T.E. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J. Clin. Oncol. 2009, 27, 1579–1584. [Google Scholar] [CrossRef]
- Siva, S.; MacManus, M.; Ball, D. Stereotactic radiotherapy for pulmonary oligometastases: A systematic review. J. Thorac. Oncol. 2010, 5, 1091–1099. [Google Scholar] [CrossRef]
- Conibear, J.; Chia, B.; Ngai, Y.; Bates, A.T.; Counsell, N.; Patel, R.; Eaton, D.; Faivre-Finn, C.; Fenwick, J.; Forster, M.; et al. Study protocol for the SARON trial: A multicentre, randomised controlled phase III trial comparing the addition of stereotactic ablative radiotherapy and radical radiotherapy with standard chemotherapy alone for oligometastatic non-small cell lung cancer. BMJ Open 2018, 8, e020690. [Google Scholar] [CrossRef]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Palma, D.A.; Salama, J.K.; Lo, S.S.; Senan, S.; Treasure, T.; Govindan, R.; Weichselbaum, R. The oligometastatic state—Separating truth from wishful thinking. Nat. Rev. Clin. Oncol. 2014, 11, 549–557. [Google Scholar] [CrossRef]
- Schanne, D.H.; Heitmann, J.; Guckenberger, M.; Andratschke, N.H.J. Evolution of treatment strategies for oligometastatic NSCLC patients—A systematic review of the literature. Cancer Treat. Rev. 2019, 80, 101892. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Shibamoto, Y.; Nikolic, N.; Milicic, B.; Milisavljevic, S.; Dagovic, A.; Aleksandrovic, J.; Radosavljevic-Asic, G. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J. Clin. Oncol. 1999, 17, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Slotman, B.J.; van Tinteren, H.; Praag, J.O.; Knegjens, J.L.; El Sharouni, S.Y.; Hatton, M.; Keijser, A.; Faivre-Finn, C.; Senan, S. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: A phase 3 randomised controlled trial. Lancet 2015, 385, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, J.; Wu, M.; Wang, X.; Lu, Y.; Han, C.; Cong, L.; Li, J.; Meng, X. Real-World Efficacy and Safety of Thoracic Radiotherapy after First-Line Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Med. 2023, 12, 3828. [Google Scholar] [CrossRef]
- Hoffmann, E.; De-Colle, C.; Potkrajcic, V.; Baumann, D.; Spengler, W.; Gani, C.; Utz, D. Is consolidative thoracic radiotherapy of extensive-stage small cell lung cancer still beneficial in the era of immunotherapy? A retrospective analysis. Strahlenther. Onkol. 2023, 199, 668–675. [Google Scholar] [CrossRef]
- Li, Y.; Jing, W.; Jing, X.; Sun, Y.; Tang, X.; Guo, J.; Zhang, Y.; Zhu, H. Role of consolidative thoracic radiation in extensive-stage small-cell lung cancer with frst-line chemoimmunotherapy: A retrospective study from a single cancer center. Discov. Oncol. 2023, 14, 55. [Google Scholar] [CrossRef]
| Characteristics | Number | % |
|---|---|---|
| Age [years; median (range)] | 66 (51–79) | |
| Sex | ||
| Female | 4 | 18.2% |
| Male | 18 | 81.8% |
| Smoking Status | ||
| Never smoker | 2 | 9.1% |
| Current or Ex-smoker | 20 | 90.9% |
| ECOG performance status | ||
| 0–1 | 22 | 100% |
| 2 | 0 | 0% |
| Underlying pulmonary disease | ||
| None | 14 | 63.6% |
| COPD | 7 | 31.8% |
| IPF | 1 | 4.6% |
| Number of metastatic sites at diagnosis | ||
| Single | 12 | 54.5% |
| Multiple | 10 | 45.5% |
| M Stage_8th Edition, AJCC | ||
| M1a | 9 | 40.9% |
| M1b | 3 | 13.6% |
| M1c | 10 | 45.5% |
| Immuno-oncologic agent treatment | ||
| No | 2 | 9.1% |
| Yes | 20 | 90.9% |
| Response to prior systemic treatment | ||
| Partial response/Stable disease | 12 | 54.5% |
| Oligo-progressive disease | 10 | 45.5% |
| No. | Age/Sex | Smoking | Underlying Disease | Metastatic Site(s) | Chemotherapy Regimen | Time to Radiotherapy (Months) | Radiotherapy Site(s) | Current Status |
|---|---|---|---|---|---|---|---|---|
| 1 | 67/M | Ex- | COPD | Pleura, Bone | Atezo + EP | 5 | Lung, MLN | LRR (SCN), AWD |
| 2 | 72/M | Current- | None | Lung, Liver | Atezo + EP | 8 | MLN, SCN | DM (lung), DOD |
| 3 | 51/M | Ex- | COPD | Pleura | Atezo + EP | 14 | MLN | AWD |
| 4 | 62/M | Ex- | COPD | Brain, Adrenal gland | Atezo + EP | 28 | Lung, MLN | AWD |
| 5 | 75/M | Current- | None | Pleura | Atezo + EP | 15 | Lung, MLN | DM (lung), DOD |
| 6 | 65/F | Current- | COPD | Lung, lymph node | Atezo + EP | 10 | Lung | DM (lung), DOD |
| 7 | 56/M | Ex- | None | Pleura | Atezo + EP | 6 | MLN, SCN | LRR (primary lung), AWD |
| 8 | 55/F | Never- | None | Brain | Atezo + EP | 8 | Lung | RR (MLN), DOD |
| 9 | 65/M | Ex- | COPD | Pleura, Bone | Atezo + EP | 10 | MLN | DM (lung), DOD |
| 10 | 69/M | Ex- | None | Brain, lymph node | Atezo + EP | 5 | MLN | DM (spinal cord), AWD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Hwang, J.; Kim, S.M.; Yang, D.S. Preliminary Results of Clinical Experience with Consolidative High-Dose Thoracic Radiotherapy for Patients with Extensive-Stage Small Cell Lung Cancer. Tomography 2025, 11, 55. https://doi.org/10.3390/tomography11050055
Kim H, Hwang J, Kim SM, Yang DS. Preliminary Results of Clinical Experience with Consolidative High-Dose Thoracic Radiotherapy for Patients with Extensive-Stage Small Cell Lung Cancer. Tomography. 2025; 11(5):55. https://doi.org/10.3390/tomography11050055
Chicago/Turabian StyleKim, Hakyoung, Jeongeun Hwang, Sun Myung Kim, and Dae Sik Yang. 2025. "Preliminary Results of Clinical Experience with Consolidative High-Dose Thoracic Radiotherapy for Patients with Extensive-Stage Small Cell Lung Cancer" Tomography 11, no. 5: 55. https://doi.org/10.3390/tomography11050055
APA StyleKim, H., Hwang, J., Kim, S. M., & Yang, D. S. (2025). Preliminary Results of Clinical Experience with Consolidative High-Dose Thoracic Radiotherapy for Patients with Extensive-Stage Small Cell Lung Cancer. Tomography, 11(5), 55. https://doi.org/10.3390/tomography11050055







