Variability of HCC Tumor Diameter and Density Measurements on Dynamic Contrast-Enhanced Computed Tomography
Abstract
1. Introduction
2. Methods
2.1. Study Dataset
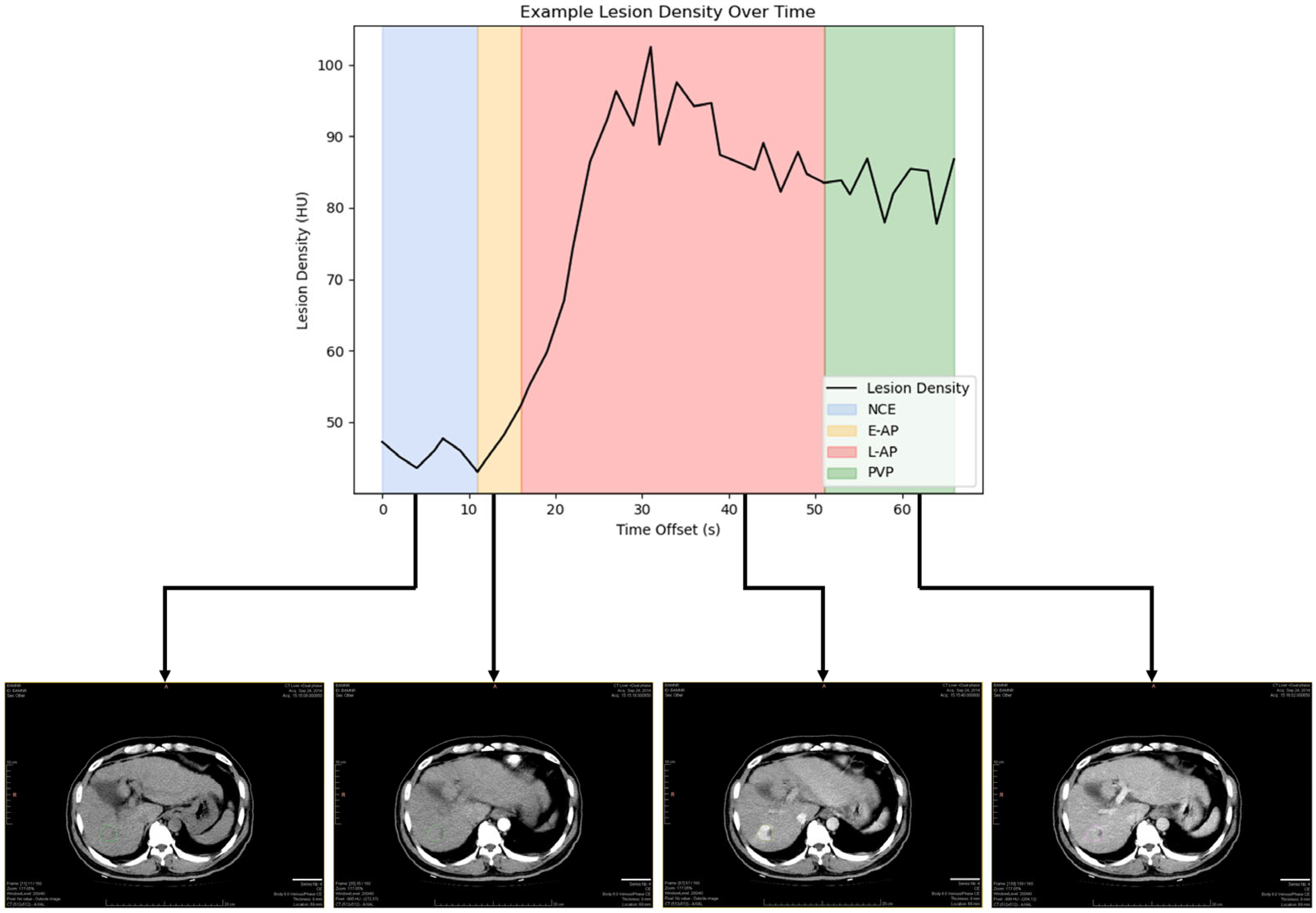
2.2. Reference Standard for Contrast Enhancement Phases
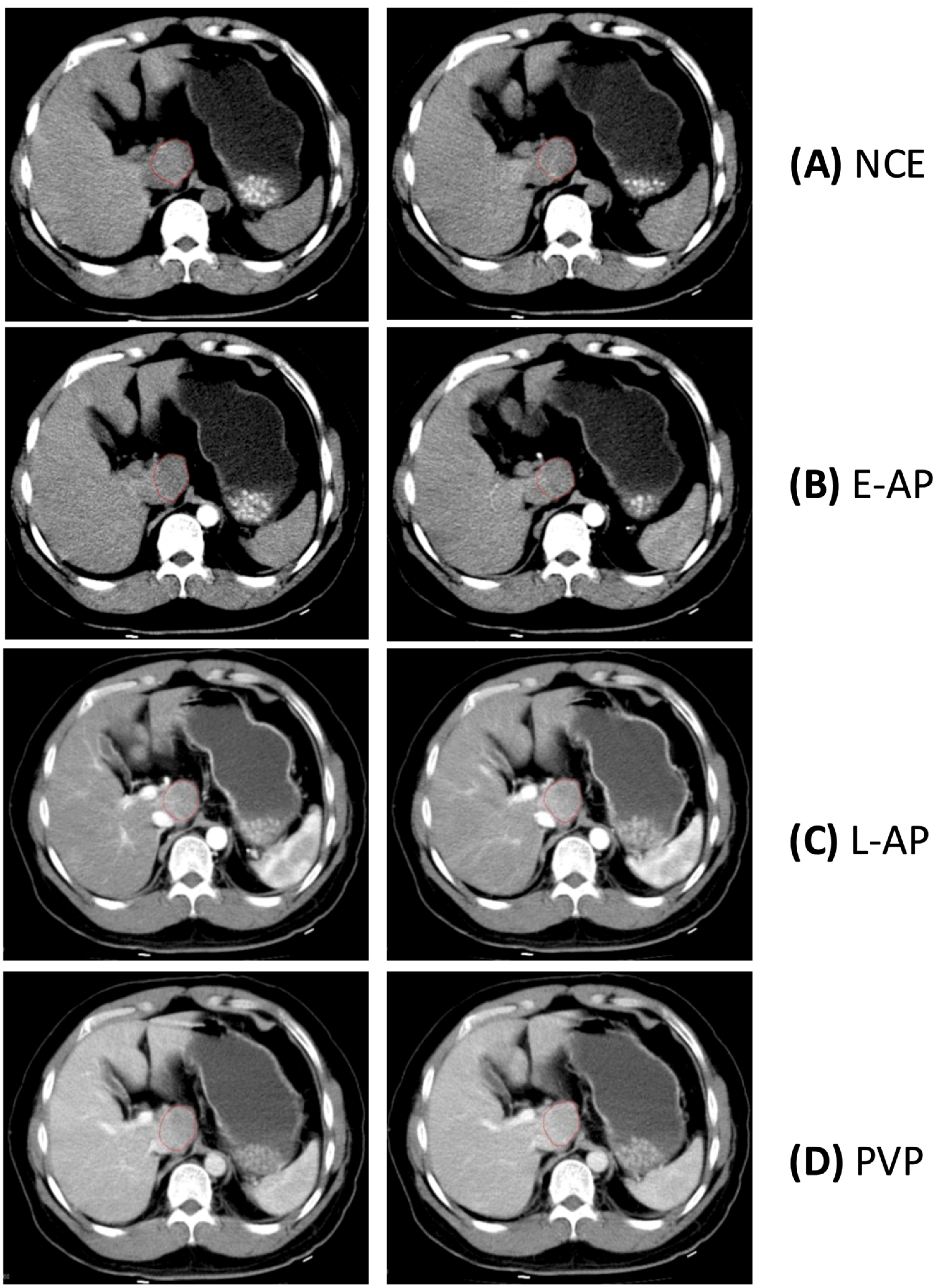
2.3. Lesion Segmentation and Measurements
2.4. Biostatistics
3. Results
3.1. Variability of Diameter Measurement by Phase
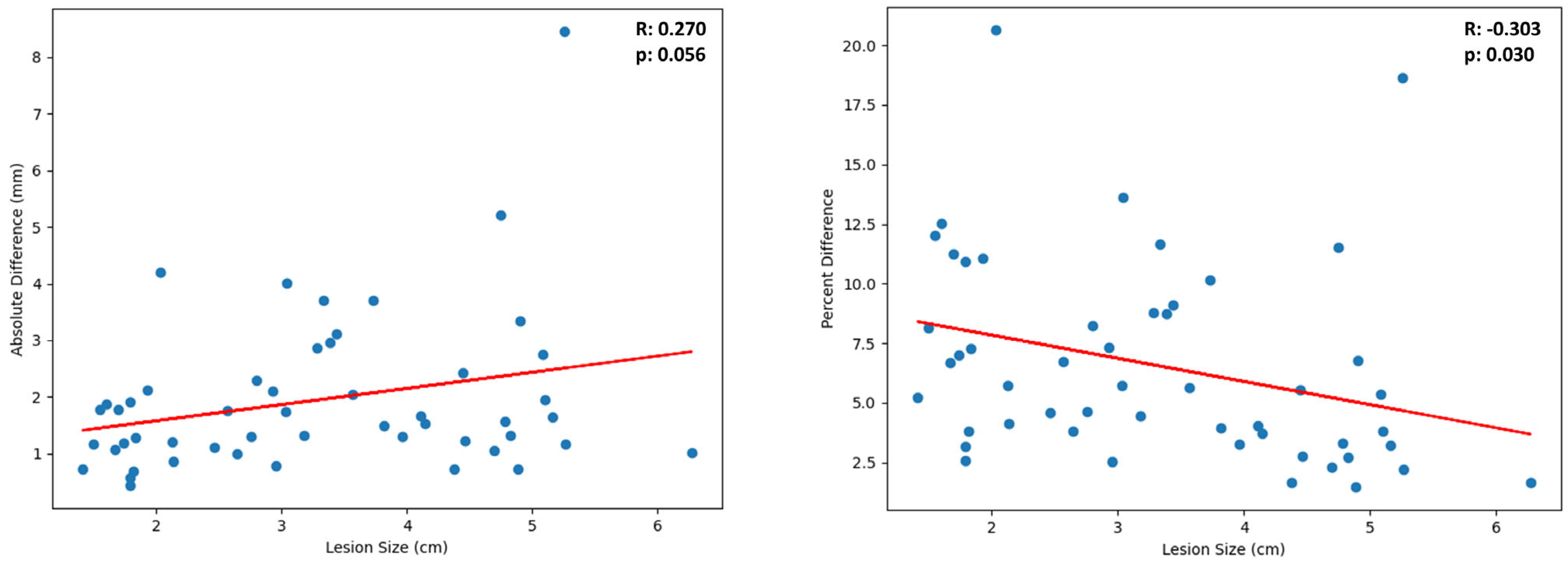
3.2. Variability of Diameter Measurement by Lesion Size
3.3. Variability of Density Measurement by Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kang, H.; Lee, H.Y.; Lee, K.S.; Kim, J.-H. Imaging-Based Tumor Treatment Response Evaluation: Review of Conventional, New, and Emerging Concepts. Korean J. Radiol. 2012, 13, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. 25 January 2021. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics (accessed on 22 September 2022).
- Karrison, T.G.; Maitland, M.L.; Stadler, W.M.; Ratain, M.J. Design of Phase II Cancer Trials Using a Continuous Endpoint of Change in Tumor Size: Application to a Study of Sorafenib and Erlotinib in Non-Small-Cell Lung Cancer. J. Natl. Cancer Inst. 2007, 99, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Burzykowski, T.; Coart, E.; Saad, E.D.; Shi, Q.; Sommeijer, D.W.; Bokemeyer, C.; Díaz-Rubio, E.; Douillard, J.-Y.; Falcone, A.; Fuchs, C.S.; et al. Evaluation of Continuous Tumor-Size-Based End Points as Surrogates for Overall Survival in Randomized Clinical Trials in Metastatic Colorectal Cancer. JAMA Netw. Open 2019, 2, e1911750. [Google Scholar] [CrossRef]
- Anagnostou, V.; Yarchoan, M.; Hansen, A.R.; Wang, H.; Verde, F.; Sharon, E.; Collyar, D.; Chow, L.Q.M.; Forde, P.M. Immuno-Oncology Trial Endpoints: Capturing Clinically Meaningful Activity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4959–4969. [Google Scholar] [CrossRef]
- Ko, C.-C.; Yeh, L.-R.; Kuo, Y.-T.; Chen, J.-H. Imaging biomarkers for evaluating tumor response: RECIST and beyond. Biomark. Res. 2021, 9, 52. [Google Scholar] [CrossRef]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Zhao, B.; Sima, C.S.; Ginsberg, M.S.; James, L.P.; Lefkowitz, R.A.; Guo, P.; Kris, M.G.; Schwartz, L.H.; Riely, G.J. Variability of lung tumor measurements on repeat computed tomography scans taken within 15 minutes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3114–3119. [Google Scholar] [CrossRef]
- Zhao, B.; James, L.P.; Moskowitz, C.S.; Guo, P.; Ginsberg, M.S.; Lefkowitz, R.A.; Qin, Y.; Riely, G.J.; Kris, M.G.; Schwartz, L.H. Evaluating Variability in Tumor Measurements from Same-Day Repeat CT Scans of Patients with Non-Small Cell Lung Cancer. Radiology 2009, 252, 263–272. [Google Scholar] [CrossRef]
- McErlean, A.; Panicek, D.M.; Zabor, E.C.; Moskowitz, C.S.; Bitar, R.; Motzer, R.J.; Hricak, H.; Ginsberg, M.S. Intra- and Interobserver Variability in CT Measurements in Oncology. Radiology 2013, 269, 451–459. [Google Scholar] [CrossRef]
- Tirkes, T.; Hollar, M.A.; Tann, M.; Kohli, M.D.; Akisik, F.; Sandrasegaran, K. Response Criteria in Oncologic Imaging: Review of Traditional and New Criteria. RadioGraphics 2013, 33, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 052–060. [Google Scholar] [CrossRef]
- Weng, Z.; Ertle, J.; Zheng, S.; Lauenstein, T.; Mueller, S.; Bockisch, A.; Gerken, G.; Yang, D.; Schlaak, J.F. Choi criteria are superior in evaluating tumor response in patients treated with transarterial radioembolization for hepatocellular carcinoma. Oncol. Lett. 2013, 6, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Patchett, N.; Furlan, A.; Marsh, J.W. Decrease in tumor enhancement on contrast-enhanced CT is associated with improved survival in patients with hepatocellular carcinoma treated with Sorafenib. Jpn. J. Clin. Oncol. 2016, 46, 839–844. [Google Scholar] [CrossRef]
- Dercle, L.; Lu, L.; Lichtenstein, P.; Yang, H.; Wang, D.; Zhu, J.; Wu, F.; Piessevaux, H.; Schwartz, L.H.; Zhao, B. Impact of Variability in Portal Venous Phase Acquisition Timing in Tumor Density Measurement and Treatment Response Assessment: Metastatic Colorectal Cancer as a Paradigm. JCO Clin. Cancer Inform. 2017, 1, CCI.17.00108. [Google Scholar] [CrossRef]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- Baba, Y. Early Arterial Phase|Radiology Reference Article|Radiopaedia.org. Radiopaedia. Available online: https://radiopaedia.org/articles/early-arterial-phase?lang=us (accessed on 22 September 2022).
- Baba, Y. Late Arterial Phase|Radiology Reference Article|Radiopaedia.org. Radiopaedia. Available online: https://radiopaedia.org/articles/late-arterial-phase?lang=us (accessed on 22 September 2022).
- Baba, Y. Portal Venous Phase|Radiology Reference Article|Radiopaedia.org. Radiopaedia. Available online: https://radiopaedia.org/articles/portal-venous-phase?lang=us (accessed on 22 September 2022).
- Choi, J.-Y.; Lee, J.-M.; Sirlin, C.B. CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part II. Extracellular Agents, Hepatobiliary Agents, and Ancillary Imaging Features. Radiology 2014, 273, 30–50. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- European Association for the Study of The Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Kudo, M. Real practice of hepatocellular carcinoma in Japan: Conclusions of the Japan Society of Hepatology 2009 Kobe Congress. Oncology 2010, 78 (Suppl. S1), 180–188. [Google Scholar] [CrossRef]
- Omata, M.; Lesmana, L.A.; Tateishi, R.; Chen, P.-J.; Lin, S.-M.; Yoshida, H.; Kudo, M.; Lee, J.M.; Choi, B.I.; Poon, R.T.P.; et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010, 4, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Aslam, A.; Mubarak, E.; Hofley, C.; Lala, K.; Arora, S.; Madoff, D.C.; Smith, E.; Owen, D.; Gabr, A.; et al. Imaging after liver-directed therapy: Evidenced-based update of the LI-RADS treatment response algorithm. Hepatoma Res. 2023, 9, 21. [Google Scholar] [CrossRef]
- Jones, M.A.; Islam, W.; Faiz, R.; Chen, X.; Zheng, B. Applying artificial intelligence technology to assist with breast cancer diagnosis and prognosis prediction. Front. Oncol. 2022, 12, 980793. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Widaatalla, Y.; Refaee, T.; Primakov, S.; Miclea, R.L.; Oecal, O.; Fabritius, M.P.; Ingrisch, M.; Ricke, J.; Hustinx, R.; et al. Reproducibility of CT-based hepatocellular carcinoma radiomic features across different contrast imaging phases: A proof of concept on SORAMIC trial data. Cancers 2021, 13, 4638. [Google Scholar] [CrossRef]
- Ibrahim, A.; Lu, L.; Yang, H.; Akin, O.; Schwartz, L.H.; Zhao, B. The impact of image acquisition parameters and ComBat harmonization on the predictive performance of radiomics: A renal cell carcinoma model. Appl. Sci. 2022, 12, 9824. [Google Scholar] [CrossRef]
- Alkhafaji, H.; Ibrahim, A. Effects of Contrast Enhancement Phase on the Reproducibility and Predictivity of CT-Based Renal Lesions Radiomic Features. Appl. Sci. 2022, 12, 12599. [Google Scholar] [CrossRef]



| Number of patients | 51 |
| Age (years) ± standard deviation | 54 ± 13 |
| Male | 45 (0.88) |
| Female | 6 (0.12) |
| Cirrhosis cause Alcohol | 9 (0.18) |
| Hepatitis B | 45 (0.88) |
| Hepatitis C | 1 (0.02) |
| HIV | 0 (0.0) |
| Hemochromatosis | 0 (0.0) |
| NASH | 0 (0.0) |
| Unreported | 1 (0.02) |
| Pathology ** | |
| Well-differentiated HCC | 3 (0.06) |
| Well-differentiated to moderately differentiated HCC | 6 (0.12) |
| Moderately differentiated HCC | 23 (0.45) |
| Moderately to poorly differentiated HCC | 14 (0.27) |
| Poorly differentiated HCC | 5 (0.10) |
| Vendors | Model | X-Ray Tube Current (mA) | Exposure Time (ms) | kVP | CTDVol (mGy) | Reconstruction Kernel | Slice Thickness (mm) | Pixel Spacing (mm2) |
|---|---|---|---|---|---|---|---|---|
| Toshiba | Aquillon | 50–250 | 500–4000 | 120 | 9.6–14.4 | FC02 | 2, 5, 8 | 0.56 × 0.56–1.0 × 1.0 |
| FC04 | ||||||||
| FL03 |
| Size of Tumor (cm) | Coefficient of Variance (CV) | Standard Deviation (cm) | Example Tumor | ||
|---|---|---|---|---|---|
| Size (cm) * | Range as a Result of Variability (cm) ** | % Change as a Result of Variability | |||
| 1–2 | 5.80% (4.50–7.10%) | 0.15 | 1.7 | 1.4–2.0 | ±18 |
| 2–3 | 5.60% (2.66–8.54%) | 0.34 | 2.5 | 1.9–3.1 | ±23 |
| 3–5 | 4.65% (3.39–5.90%) | 0.65 | 4.0 | 3.5–4.5 | ±12 |
| 5–7 | 4.45% (−0.87–9.77%) | 0.46 | 5.4 | 4.6–6.2 | ±14 |
| Phase | Coefficient of Variance (CV) | Standard Deviation (HU) | Example Tumor | ||
|---|---|---|---|---|---|
| Mean Density (HU) | Range as a Result of Variability (HU) | % Change as a Result of Variability | |||
| All | 26.19% (24.66–27.72%) | 15.57 | 76.41 | 34.8–118.0 | ±54 |
| NCE | 9.62% (7.15–12.09%) | 9.73 | 45.03 | 36.4–53.6 | ±19 |
| E-AP | 7.58% (6.08–9.09%) | 10.59 | 44.25 | 37.1–51.5 | ±16 |
| L-AP | 22.84% (21.48–24.20%) | 18.93 | 77.96 | 41.6–114.4 | ±47 |
| PVP | 7.83% (6.76–8.89%) | 16.68 | 88.01 | 73.6–102.4 | ±16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guha, S.; Ibrahim, A.; Geng, P.; Wu, Q.; Chou, Y.; Akin, O.; Schwartz, L.H.; Xie, C.-M.; Zhao, B. Variability of HCC Tumor Diameter and Density Measurements on Dynamic Contrast-Enhanced Computed Tomography. Tomography 2025, 11, 36. https://doi.org/10.3390/tomography11030036
Guha S, Ibrahim A, Geng P, Wu Q, Chou Y, Akin O, Schwartz LH, Xie C-M, Zhao B. Variability of HCC Tumor Diameter and Density Measurements on Dynamic Contrast-Enhanced Computed Tomography. Tomography. 2025; 11(3):36. https://doi.org/10.3390/tomography11030036
Chicago/Turabian StyleGuha, Siddharth, Abdalla Ibrahim, Pengfei Geng, Qian Wu, Yen Chou, Oguz Akin, Lawrence H. Schwartz, Chuan-Miao Xie, and Binsheng Zhao. 2025. "Variability of HCC Tumor Diameter and Density Measurements on Dynamic Contrast-Enhanced Computed Tomography" Tomography 11, no. 3: 36. https://doi.org/10.3390/tomography11030036
APA StyleGuha, S., Ibrahim, A., Geng, P., Wu, Q., Chou, Y., Akin, O., Schwartz, L. H., Xie, C.-M., & Zhao, B. (2025). Variability of HCC Tumor Diameter and Density Measurements on Dynamic Contrast-Enhanced Computed Tomography. Tomography, 11(3), 36. https://doi.org/10.3390/tomography11030036







