Ground-Glass Opacities in the Access Route and Biopsy in Highly Perfused Dependent Areas of the Lungs as Risk Factors for Pulmonary Hemorrhage During CT-Guided Lung Biopsy: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
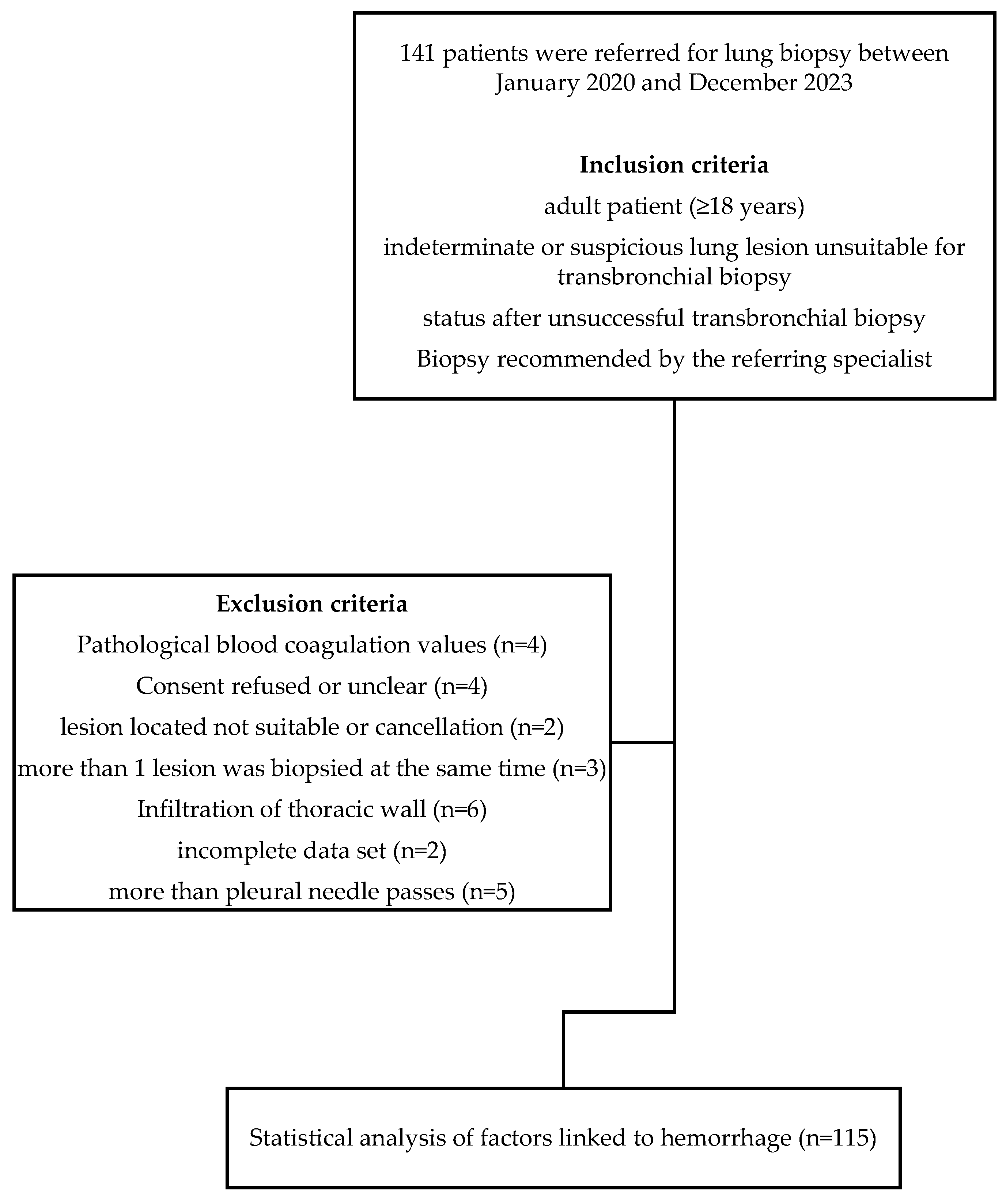
2.1. Study Population
2.2. Initial Assessment
2.3. Biopsy Procedure
2.4. Data Collection and Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Population
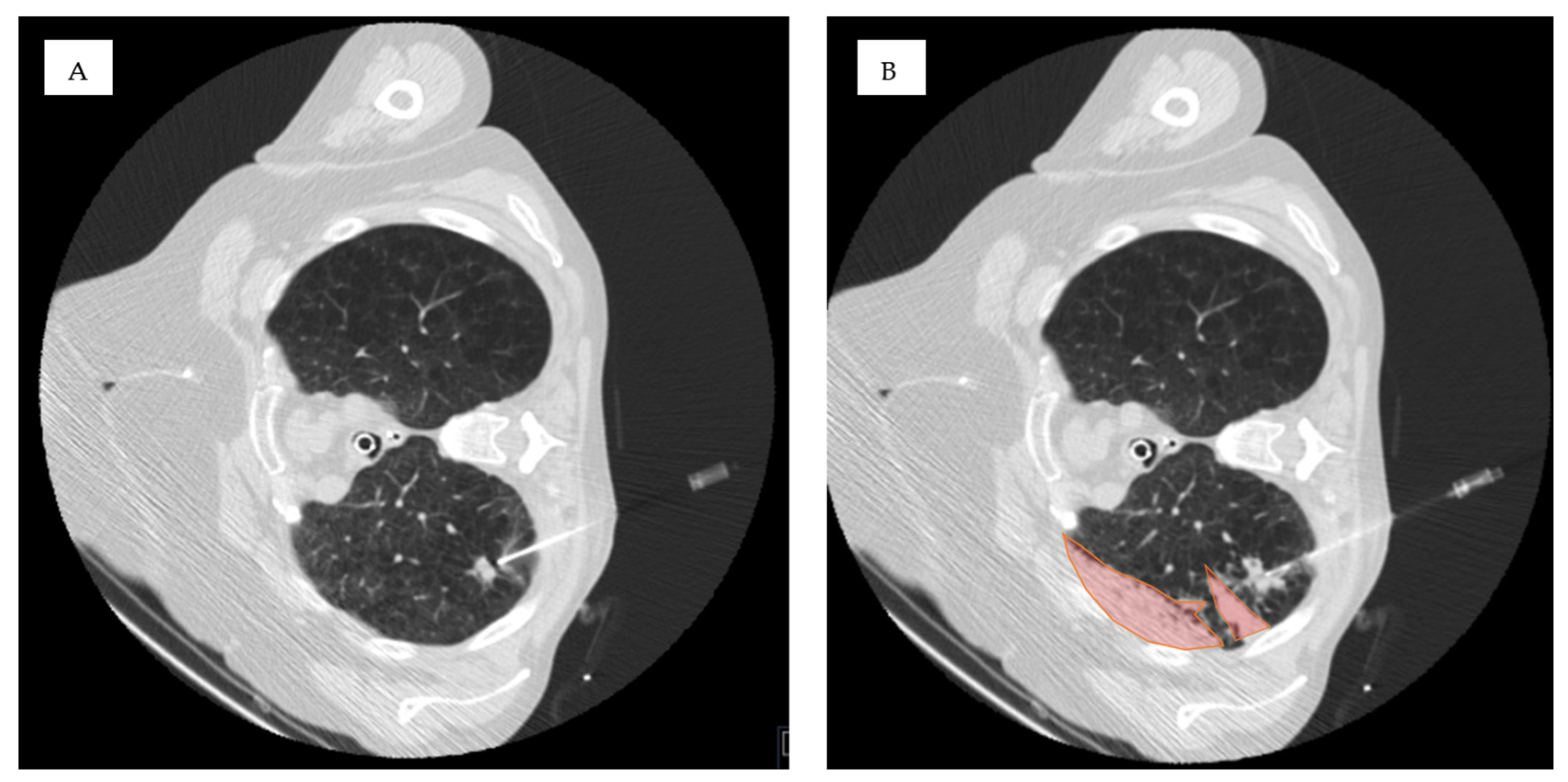
3.2. Hemorrhage After CT-Guided Lung Biopsy
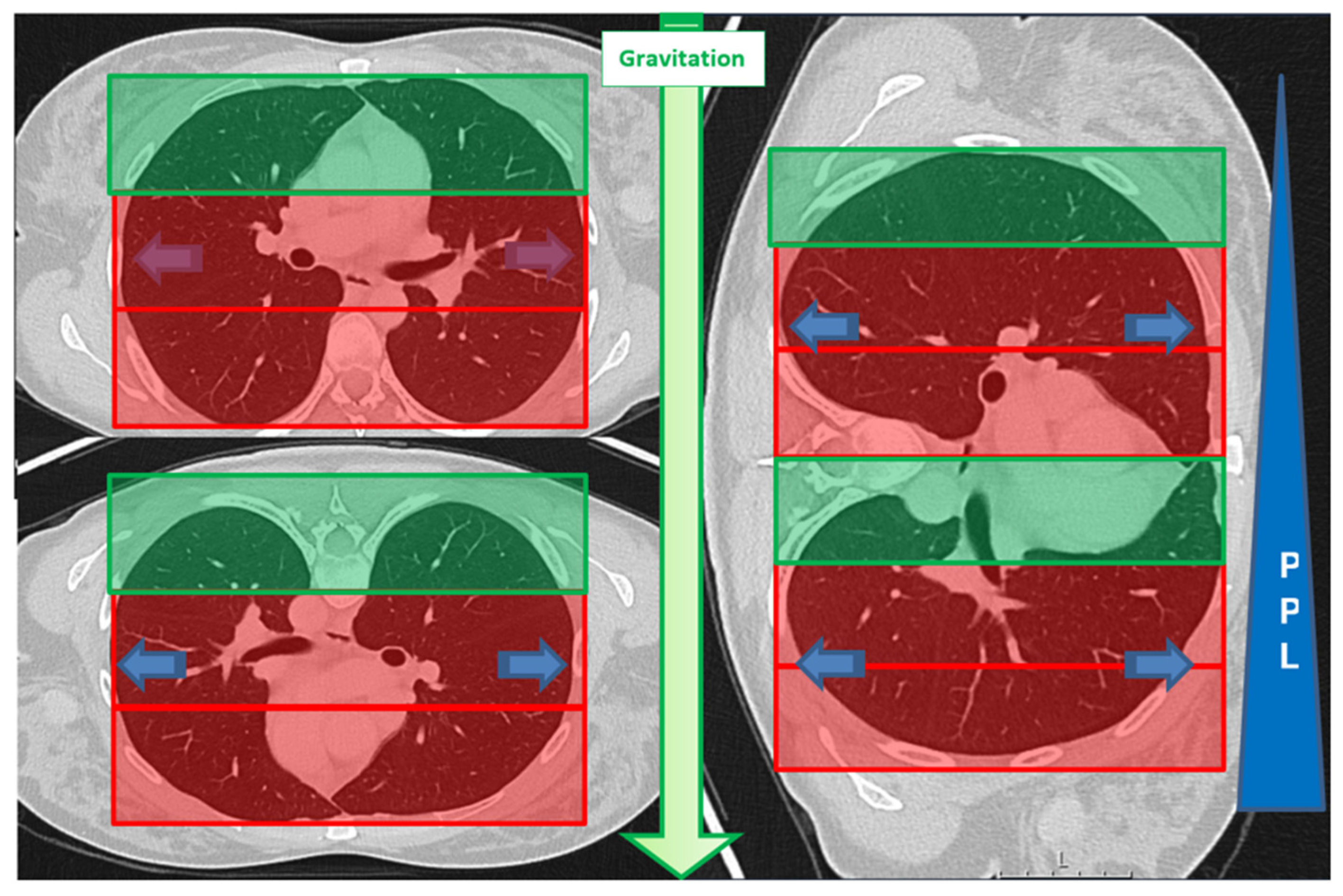
3.3. Association of Lesion Characteristics and Technical Parameters with the Occurrence of Pulmonary Hemorrhage Grade 2 or Higher
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nour-Eldin, N.-E.A.; Alsubhi, M.; Naguib, N.N.; Lehnert, T.; Emam, A.; Beeres, M.; Bodelle, B.; Koitka, K.; Vogl, T.J.; Jacobi, V. Risk factor analysis of pulmonary hemorrhage complicating CT-guided lung biopsy in coaxial and non-coaxial core biopsy techniques in 650 patients. Eur. J. Radiol. 2014, 83, 1945–1952. [Google Scholar] [PubMed]
- Tai, R.; Dunne, R.M.; Trotman-Dickenson, B.; Jacobson, F.L.; Madan, R.; Kumamaru, K.K.; Hunsaker, A.R. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: Single-institution experience of 1175 cases. Radiology 2016, 279, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, N.; Yasuhara, Y.; Nakajima, Y.; Adachi, S.; Arai, Y.; Kusumoto, M.; Eguchi, K.; Kuriyama, K.; Sakai, F.; Noguchi, M. CT-guided needle biopsy of lung lesions: A survey of severe complication based on 9783 biopsies in Japan. Eur. J. Radiol. 2006, 59, 60–64. [Google Scholar] [CrossRef]
- Heerink, W.J.; de Bock, G.H.; de Jonge, G.J.; Groen, H.J.; Vliegenthart, R.; Oudkerk, M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar]
- Yeow, K.-M.; Su, I.-H.; Pan, K.-T.; Tsay, P.-K.; Lui, K.-W.; Cheung, Y.-C.; Chou, A.S.B. Risk factors of pneumothorax and bleeding: Multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004, 126, 748–754. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, L.; Chen, T.; Zheng, G.; Ye, C.; Jia, W. Factors influencing early recurrence of hepatocellular carcinoma after curative resection. J. Int. Med. Res. 2020, 48, 0300060520945552. [Google Scholar] [PubMed]
- Brönnimann, M.P.; McMurray, M.T.; Heverhagen, J.T.; Christe, A.; Wyss, C.; Peters, A.A.; Huber, A.T.; Dammann, F.; Obmann, V.C. Innovative strategies for minimizing hematoma risk in MRI-guided breast biopsies. Radiol. Oncol. 2025, 59, 91–99. [Google Scholar] [CrossRef]
- Freund, M.C.; Petersen, J.; Goder, K.C.; Bunse, T.; Wiedermann, F.; Glodny, B. Systemic air embolism during percutaneous core needle biopsy of the lung: Frequency and risk factors. BMC Pulm. Med. 2012, 12, 2. [Google Scholar] [CrossRef]
- Lee, D.S.; Bak, S.H.; Jeon, Y.H.; Kwon, S.O.; Kim, W.J. Perilesional emphysema as a predictor of risk of complications from computed tomography-guided transthoracic lung biopsy. Jpn. J. Radiol. 2019, 37, 808–816. [Google Scholar]
- Milner, L.; Ryan, K.; Gullo, J. Fatal intrathoracic hemorrhage after percutaneous aspiration lung biopsy. Am. J. Roentgenol. 1979, 132, 280–281. [Google Scholar] [CrossRef]
- He, C.; Zhao, L.; Yu, H.-L.; Zhao, W.; Li, D.; Li, G.-D.; Wang, H.; Huo, B.; Huang, Q.; Liang, B.; et al. Incidence and risk factors for pulmonary hemorrhage after percutaneous CT-guided pulmonary nodule biopsy: An observational study. Sci. Rep. 2024, 14, 7348. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, C.M.; Park, K.W.; Kim, K.G.; Lee, H.-J.; Shim, M.-S.; Goo, J.M. Does antiplatelet therapy increase the risk of hemoptysis during percutaneous transthoracic needle biopsy of a pulmonary lesion? Am. J. Roentgenol. 2013, 200, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, D.; Cavigli, E.; Moroni, C.; Smorchkova, O.; Zantonelli, G.; Pradella, S.; Miele, V. Ground-glass opacity (GGO): A review of the differential diagnosis in the era of COVID-19. Jpn. J. Radiol. 2021, 39, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.S. The CT halo sign. Radiology 2004, 230, 109–110. [Google Scholar] [CrossRef]
- Szarf, G.; Dario, C.F.R.; Chate, R.C.; Yanata, E.; Werebe, E.; Funari, M.B.G. Middle lobe pulmonary torsion after recurrent pleural effusions in a cirrhotic patient. Thorax 2015, 70, 1209–1210. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Liu, J.; Zhu, F.; Liu, Y.; Liu, Y.; Peng, H. Correlation between ground-glass opacity on pulmonary CT and the levels of inflammatory cytokines in patients with moderate-to-severe COVID-19 pneumonia. Int. J. Med. Sci. 2021, 18, 2394. [Google Scholar] [CrossRef] [PubMed]
- Nyren, S.; Mure, M.; Jacobsson, H.; Larsson, S.A.; Lindahl, S.G. Pulmonary perfusion is more uniform in the prone than in the supine position: Scintigraphy in healthy humans. J. Appl. Physiol. 1999, 86, 1135–1141. [Google Scholar] [CrossRef]
- Powers, K.A.; Dhamoon, A.S. Physiology, Pulmonary Ventilation and Perfusion; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Zielinska-Krawczyk, M.; Krenke, R.; Grabczak, E.M.; Light, R.W. Pleural manometry–historical background, rationale for use and methods of measurement. Respir. Med. 2018, 136, 21–28. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, Y.; Wang, X.; Jiang, C.; Mo, J.; Xi, J.; Wen, Z. Risk factors associated with pulmonary hemorrhage and hemoptysis following percutaneous CT-guided transthoracic lung core needle biopsy: A retrospective study of 1090 cases. Quant. Imaging Med. Surg. 2020, 10, 1008. [Google Scholar] [CrossRef]
- Drumm, O.; Joyce, E.A.; de Blacam, C.; Gleeson, T.; Kavanagh, J.; McCarthy, E.; McDermott, R.; Beddy, P. CT-guided lung biopsy: Effect of biopsy-side down position on pneumothorax and chest tube placement. Radiology 2019, 292, 190–196. [Google Scholar] [CrossRef]
- Cassel, D.; Birnberg, F. Preventing pneumothorax after lung biopsy: The roll-over technique. Radiology 1990, 174, 282. [Google Scholar] [CrossRef] [PubMed]
- Brönnimann, M.P.; Manser, L.; Gebauer, B.; Auer, T.A.; Schnapauff, D.; Collettini, F.; Pöllinger, A.; Komarek, A.; Krokidis, M.E.; Heverhagen, J.T. Enhancing safety in CT-guided lung biopsies: Correlation of MinIP imaging with pneumothorax risk prediction. Insights Imaging 2025, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Brönnimann, M.P.; Manser, L.; Maurer, M.H.; Gebauer, B.; Auer, T.A.; Schnapauff, D.; Collettini, F.; Nguyen, T.-L.; Komarek, A.; Krokidis, M.E. Enhanced Positioning Strategies to Reduce Pneumothorax in CT-Guided Lung Biopsies. Diagnostics 2024, 14, 2639. [Google Scholar] [CrossRef] [PubMed]
- Covey, A.M.; Gandhi, R.; Brody, L.A.; Getrajdman, G.; Thaler, H.T.; Brown, K.T. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J. Vasc. Interv. Radiol. 2004, 15, 479–483. [Google Scholar] [CrossRef]
- Leger, T.; Jerjir, N.; Gregory, J.; Bennani, S.; Freche, G.; Revel, M.-P.; Chassagnon, G. Does ipsilateral-dependent positioning during percutaneous lung biopsy decrease the risk of pneumothorax? Am. J. Roentgenol. 2019, 212, 461–466. [Google Scholar] [CrossRef]
- Najafi, A.; Al Ahmar, M.; Bonnet, B.; Delpla, A.; Kobe, A.; Madani, K.; Roux, C.; Deschamps, F.; de Baère, T.; Tselikas, L. The PEARL approach for CT-guided lung biopsy: Assessment of complication rate. Radiology 2022, 302, 473–480. [Google Scholar] [CrossRef]
- Zidulka, A.; Braidy, T.F.; Rizzi, M.C.; Shiner, R.J. Position may stop pneumothorax progression in dogs. Am. Rev. Respir. Dis. 1982, 126, 51–53. [Google Scholar]
- Stenqvist, O.; Gattinoni, L.; Hedenstierna, G. What’s new in respiratory physiology? The expanding chest wall revisited! Intensive Care Med. 2015, 41, 1110–1113. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Muller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Brookes, M.; MacVicar, D.; Husband, J. Metastatic carcinoma of the breast: The appearances of metastatic spread to the abdomen and pelvis as demonstrated by CT. Br. J. Radiol. 2007, 80, 284–292. [Google Scholar] [CrossRef]
- Oliver, J.H., 3rd; Baron, R.L.; Federle, M.P.; Jones, B.C.; Sheng, R. Hypervascular liver metastases: Do unenhanced and hepatic arterial phase CT images affect tumor detection? Radiology 1997, 205, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Paulson, E.K.; McDermott, V.G.; Keogan, M.T.; DeLong, D.M.; Frederick, M.G.; Nelson, R.C. Carcinoid metastases to the liver: Role of triple-phase helical CT. Radiology 1998, 206, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Evans, J.M.; McCullough, A.E.; Jatoi, M.A.; Vargas, H.E.; Hara, A.K. MR imaging of hypervascular liver masses: A review of current techniques. Radiographics 2009, 29, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Som, P.M. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am. J. Roentgenol. 1992, 158, 961–969. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: Berlin/Heidelberg, Germany, 2001; Volume 608. [Google Scholar]
- Cohen, J. Quantitative methods in psychology: A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Nemec, S.F.; Bankier, A.A.; Eisenberg, R.L. Lower lobe—Predominant diseases of the lung. Am. J. Roentgenol. 2013, 200, 712–728. [Google Scholar] [CrossRef]
- Glenny, R.W.; Bernard, S.; Robertson, H.T.; Hlastala, M.P. Gravity is an important but secondary determinant of regional pulmonary blood flow in upright primates. J. Appl. Physiol. 1999, 86, 623–632. [Google Scholar] [CrossRef]
- Glenny, R.W.; Lamm, W.J.; Bernard, S.L.; An, D.; Chornuk, M.; Pool, S.L.; Wagner Jr, W.W.; Hlastala, M.P.; Robertson, H.T. Selected contribution: Redistribution of pulmonary perfusion during weightlessness and increased gravity. J. Appl. Physiol. 2000, 89, 1239–1248. [Google Scholar] [CrossRef]
- Hopkins, S.R.; Henderson, A.C.; Levin, D.L.; Yamada, K.; Arai, T.; Buxton, R.B.; Prisk, G.K. Vertical gradients in regional lung density and perfusion in the supine human lung: The Slinky effect. J. Appl. Physiol. 2007, 103, 240–248. [Google Scholar] [CrossRef]
- Glaister, D. The effect of positive centrifugal acceleration upon the distribution of ventilation and perfusion within the hum and lung, and its relation to pulmonary arterial and intraoesophageal pressures. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1967, 168, 311–334. [Google Scholar]
- Jiang, L.; Jiang, S.; Lin, Y.; Yang, H.; Zhao, Z.; Xie, Z.; Lin, Y.; Long, H. Modified inflammation-based score as an independent malignant predictor in patients with pulmonary focal ground-glass opacity: A propensity score matching analysis. Sci. Rep. 2016, 6, 19105. [Google Scholar]
- Misura, T.; Drakopoulos, D.; Mitrakovic, M.; Loennfors, T.; Primetis, E.; Hoppe, H.; Obmann, V.C.; Huber, A.T.; Ebner, L.; Christe, A. Avoiding the intercostal arteries in percutaneous thoracic interventions. J. Vasc. Interv. Radiol. 2022, 33, 416–419.e2. [Google Scholar] [PubMed]

| Survey of Lung Biopsies | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | All (n = 115) | None or Grade 1 Hemorrhage (n = 85) | Grade 2 Hemorrhage or Higher (n = 30) | p Value | |||
| Sex | |||||||
| Female | 48 | 42% | 35 | 41% | 13 | 43% | 1 |
| Male | 67 | 58% | 50 | 59% | 17 | 57% | |
| Age (y) | 67.23 | ±12.24 | 66.67 | ±13.05 | 68.83 | ±9.556 | 1 |
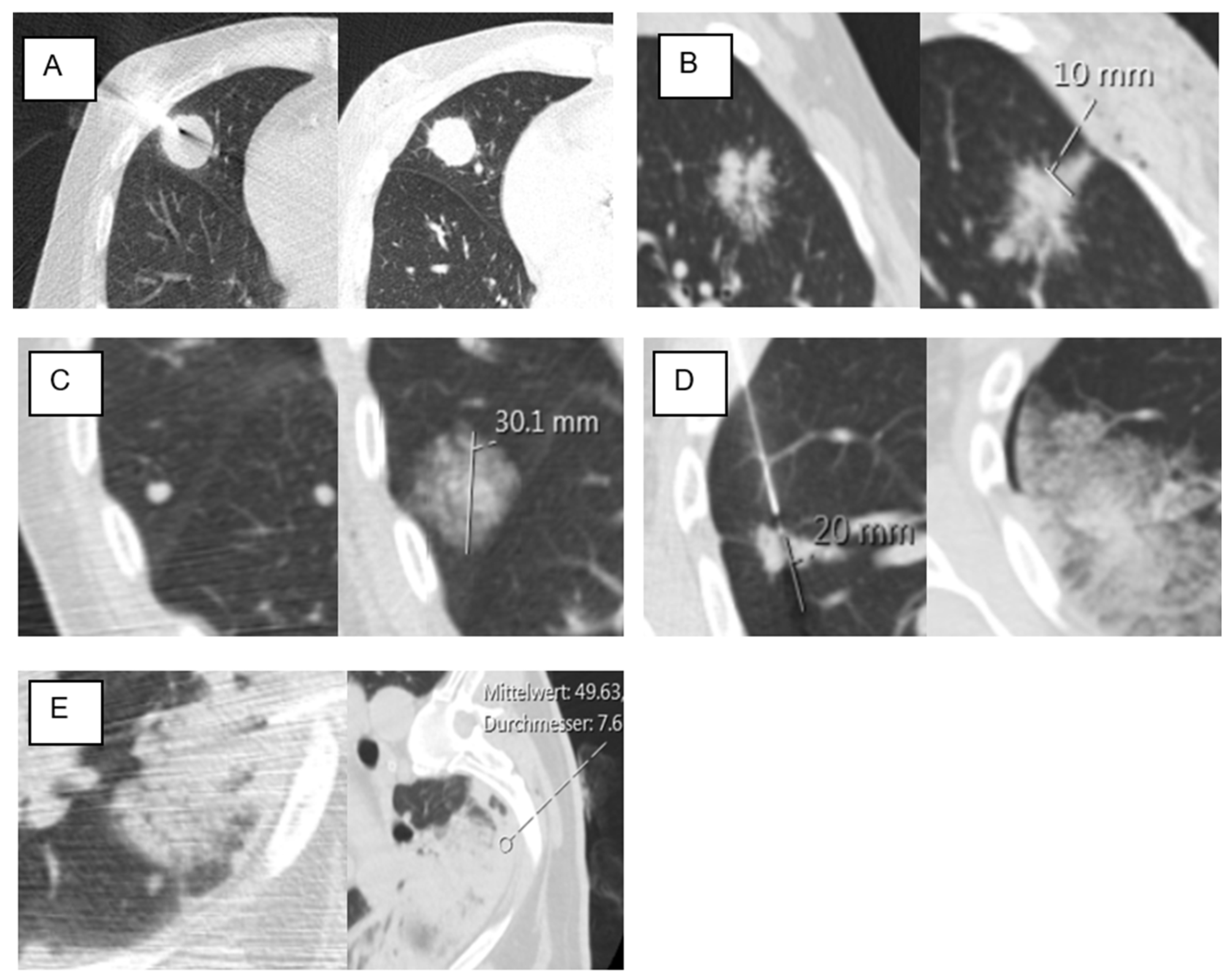
| Lesion size (mm) | 25.03 | ±18.81 | 26.8 | ±20.99 | 20.03 | ±8.83 | 0.18 |
| Needle size | 1 | ||||||
| 18 G | 70 | 61% | 52 | 61% | 18 | 60% | |
| 20 G | 45 | 39% | 33 | 39% | 12 | 40% | |
| Biopsy system | 0.638 | ||||||
| side-cut | 32 | 28% | 25 | 29% | 7 | 23% | |
| full-core | 83 | 72% | 60 | 71% | 23 | 77% | |
| Number of samples | 0.217 | ||||||
| 1 and 2 | 22 | 19% | 17 | 20% | 5 | 17% | |
| 3 | 55 | 48% | 39 | 46% | 16 | 53% | |
| 4 | 24 | 21% | 19 | 22% | 5 | 17% | |
| 5 and 6 | 14 | 12% | 10 | 12% | 4 | 13% | |
| Biopsy angle (degree) | 64.4 | ±18.10 | 65.02 | ±17.65 | 62.67 | ±19.54 | 0.593 |
| Distance SL (mm) | 63.11 | ±21.32 | 61 | 21.15 | 69.1 | ±21.01 | 0.074 |
| Distance PL (mm) | 16.57 | ±14.33 | 15.26 | ±14.84 | 20.3 | ±12.24 | 0.035 * |
| Lesion location | 0.09 | ||||||
| UL | 53 | 46% | 35 | 41% | 8 | 27% | |
| LL | 62 | 54% | 50 | 59% | 12 | 40% | |
| Lesion location in D area | 53 | 46% | 34 | 40% | 19 | 63% | 0.034 * |
| Emphysema | 22 | 19% | 14 | 16% | 8 | 27% | 0.28 |
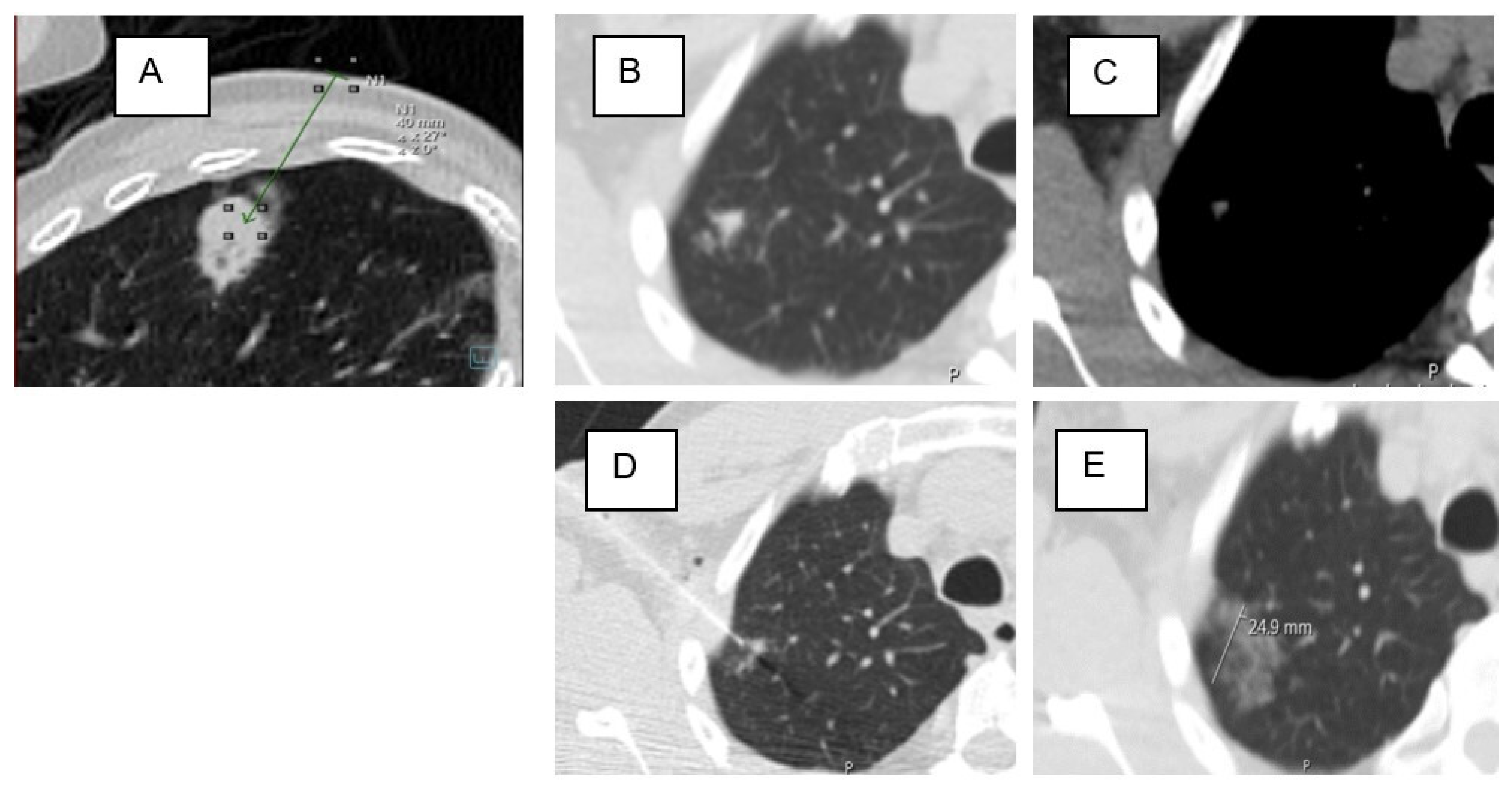
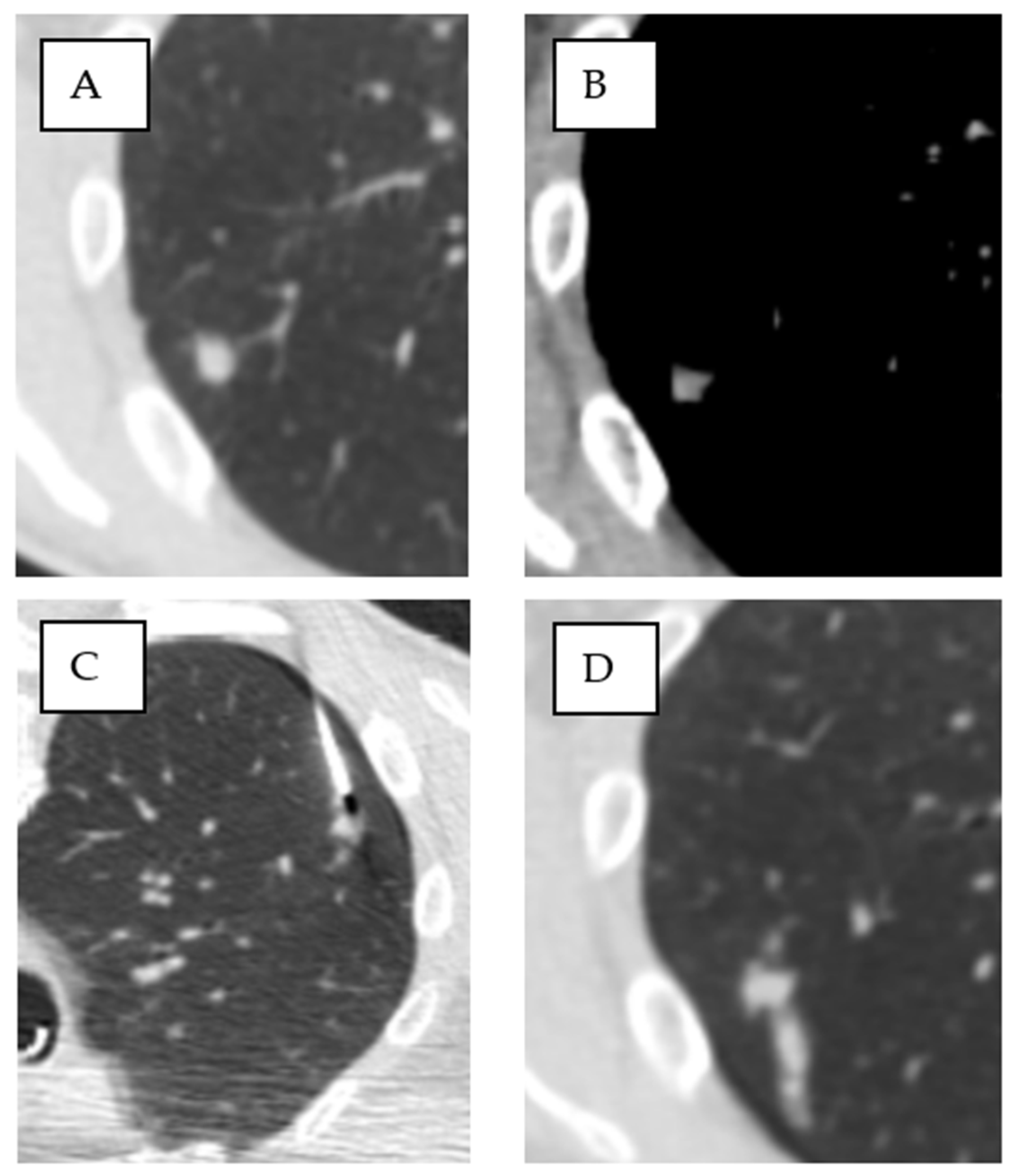
| Ground-glass in the access route | 46 | 40% | 26 | 31% | 20 | 67% | 0.001 * |
| Pathological findings | |||||||
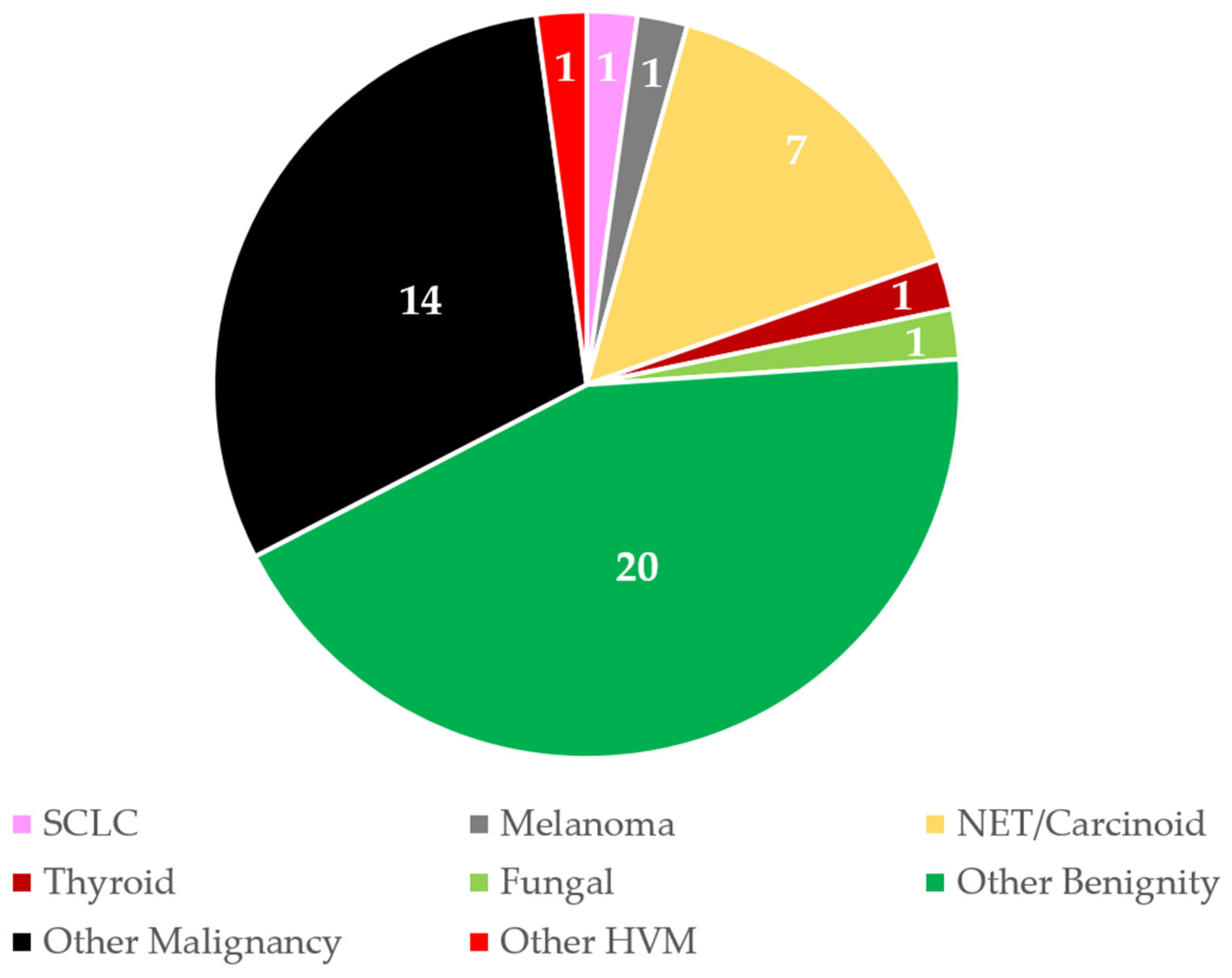
| Hypervascular lesions | 12 | 10% | 11 | 13% | 1 | 3% | 0.18 |
| Other malignancy | 38 | 33% | 29 | 34% | 9 | 30% | 0.822 |
| NSCLC | 34 | 30% | 25 | 29% | 9 | 30% | 1 |
| Adenocarcinoma | 33 | 29% | 21 | 25% | 12 | 40% | 0.161 |
| Metastasis | 44 | 38% | 37 | 44% | 7 | 23% | 0.079 |
| Benign | 30 | 26% | 23 | 27% | 7 | 23% | 0.811 |
| B | S.E. | Wald Test | df | p Value | Odds Ratio | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Variable | − | + | ||||||
| Lesion location in D area | 1.402 | 0.517 | 7.369 | 1 | 0.007 * | 4.064 | 1.477 | 11.186 |
| LL | −0.98 | 0.505 | 3.765 | 1 | 0.052 | 0.375 | 0.139 | 1.01 |
| Lesion size | −0.027 | 0.023 | 1.63 | 1 | 0.244 | 0.974 | 0.931 | 1.018 |
| GG in the access route | 1.643 | 0.514 | 10.233 | 1 | 0.001 * | 5.169 | 1.889 | 14.144 |
| Distance PL | 0.027 | 0.019 | 2.012 | 1 | 0.156 | 1.027 | 0.99 | 1.066 |
| Metastasis | −0.706 | 0.543 | 1.689 | 1 | 0.194 | 0.494 | 0.17 | 1.431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brönnimann, M.P.; Manser, L.; Christe, A.; Heverhagen, J.T.; Gebauer, B.; Auer, T.A.; Schnapauff, D.; Collettini, F.; Schroeder, C.; Dorn, P.; et al. Ground-Glass Opacities in the Access Route and Biopsy in Highly Perfused Dependent Areas of the Lungs as Risk Factors for Pulmonary Hemorrhage During CT-Guided Lung Biopsy: A Retrospective Study. Tomography 2025, 11, 35. https://doi.org/10.3390/tomography11030035
Brönnimann MP, Manser L, Christe A, Heverhagen JT, Gebauer B, Auer TA, Schnapauff D, Collettini F, Schroeder C, Dorn P, et al. Ground-Glass Opacities in the Access Route and Biopsy in Highly Perfused Dependent Areas of the Lungs as Risk Factors for Pulmonary Hemorrhage During CT-Guided Lung Biopsy: A Retrospective Study. Tomography. 2025; 11(3):35. https://doi.org/10.3390/tomography11030035
Chicago/Turabian StyleBrönnimann, Michael P., Leonie Manser, Andreas Christe, Johannes T. Heverhagen, Bernhard Gebauer, Timo A. Auer, Dirk Schnapauff, Federico Collettini, Christophe Schroeder, Patrick Dorn, and et al. 2025. "Ground-Glass Opacities in the Access Route and Biopsy in Highly Perfused Dependent Areas of the Lungs as Risk Factors for Pulmonary Hemorrhage During CT-Guided Lung Biopsy: A Retrospective Study" Tomography 11, no. 3: 35. https://doi.org/10.3390/tomography11030035
APA StyleBrönnimann, M. P., Manser, L., Christe, A., Heverhagen, J. T., Gebauer, B., Auer, T. A., Schnapauff, D., Collettini, F., Schroeder, C., Dorn, P., Gassenmaier, T., Ebner, L., & Huber, A. T. (2025). Ground-Glass Opacities in the Access Route and Biopsy in Highly Perfused Dependent Areas of the Lungs as Risk Factors for Pulmonary Hemorrhage During CT-Guided Lung Biopsy: A Retrospective Study. Tomography, 11(3), 35. https://doi.org/10.3390/tomography11030035







